Determine the shape around each central atom in each molecule, and explain any deviation from ideal bond angles:
(b) N₂O₄ (O₂NNO₂)
Answers
The shape around each central atom in each molecule of N₂O₄ (O₂NNO₂) is bent (V-shape).
What is the explanation?Three single N-O bonds and one double N-O bond are all present on the nitrogen atom, which is the core atom of N2O4 (O2NNO2). There are no solitary pairings. The steric number, according to the VSEPR theory, is the total of the bonds (ignoring multiplicity) and lone pairs that are present on the central atom. The total number of bonds in N is 4, and there are no lone pairs. We do have two lone pairings, though. This indicates that the molecule is bent at an angle of approximately and that the tetrahedral form has been lost. Due to the existence of lone pairs that resist more powerfully than the shared electrons, the angle is substantially smaller than it would be in a tetrahedral structure.
To learn more about this problem visit:
https://brainly.com/question/14089750
#SPJ4
Related Questions
The average atomic mass of an element is 39.95 amu. What is the identity of the element? 1. Potassium 2. Yttrium 3. Argon 4. Calcium Enter the answer choice number.
Answers
Answer:
3. Argon
Explanation:
The average atomic mass of an element with a value of 39.95 amu is Argon (Ar). The answer choice number is 3.
NaCl express your answer as an Ion
Does anyone know what the answer for this would be
Answers
Answer:
iconic compound
Explanation:
why are some voltaic cells called dry cells even though their chemistry involves water
Answers
Hope this helps!
Please give Brainliest!
Some voltaic cells are called dry because they do not have liquids in them.
Dry voltaic cellsThey are called dry because their electrolytes are usually in paste forms instead of aqueous form in which the electrolytes of wet cells are usually are.
The pastes in dry cells are made moist enough for the electric current to be able to flow through them.
Dry cells are advantageous because they are less heavy and one does not have to worry about the electrolyte spilling, unlike wet cells.
More on dry voltaic cells can be found here: https://brainly.com/question/2674687
#SPJ2
I only need the answers to B and C:
B) What has to be done to the reaction mixture to recover solid silver nitrate?
C: why must this process be done in a well ventilated area?

Answers
Answer:
B
Explanation:
becouse is a valintede area
8. Consider a single crystal of nickel oriented such that a tensile stress is applied along a 1011 direction. If slip occurs on a (111) plane and in a (101) direction and is initiated at an applied tensile stress of 13.9 MPa (2020 psi), compute the critical resolved shear stress. (5pts) o=cas-1 [g [ 000) + 1012 (1210²+1²) (12+0 24 1²) 2
Answers
When slip occurs in a material, the critical resolved shear stress (CRSS) is used to determine the minimum shear stress required to start the slip.
Given that a tensile stress is applied on a nickel crystal along a 1011 direction, and slip occurs on a (111) plane and in a (101) direction, the critical resolved shear stress will be computed as follows: Calculation for g[000] Since the tensile stress is applied along the 1011 direction, g[000] = 0.Calculation for g[1012]:The direction of slip (101) lies in the (1012) plane. Therefore, g[1012] = 1.Calculation for .
From the direction of the applied tensile stress and the direction of the slip plane, we can use the expression given as o = cas-1 [g [000) + 1012 (1210²+1²) (12+0 24 1²) 2 to determine o. Substituting the values of g[000], g[1012], and other parameters gives us.
To know more about material visit:
https://brainly.com/question/27403649
#SPJ11
ILL GIVE BRAINLY!!!!!!
How many different elements are involved in the reaction shown below?
CH4 + 2 O2 → CO2 + 2 H2O
Answers
Answer:
four
Explanation:
There is four
CH 4 methane
O2 oxygen
CO2 carbon dioxide
H20 water
Calculate the densities of the objects using the volumes found by displacement. Record your data
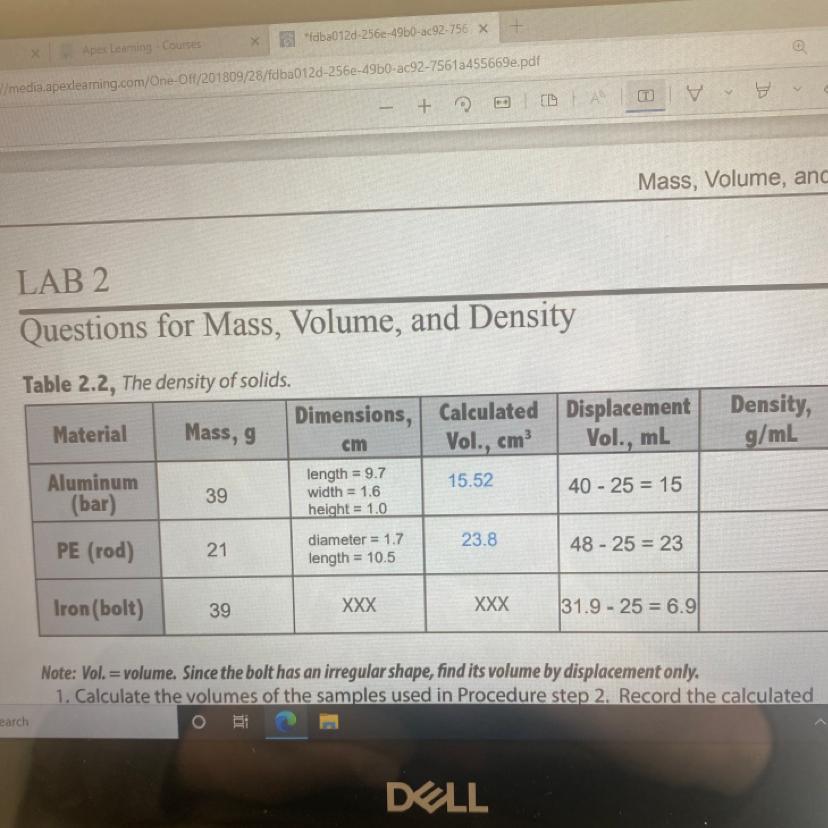
Answers
The densities of the substances are;
Aluminum bar = 2.6 g/ml
PE rod = 0.91 g/mL
Iron bolt = 5.65 g/mL
What is density?The term density is defined as the ratio of the mass to the volume of the object. We know that mass is an intrinsic property. This means that the density of the object can be used to identify what the object that is under study is. Let us now try to find the density of each of the objects.
In this case, we have the mass and the volumes of the objects as they have been shown in the table that have here. We can now be able to find the volume of each of the objects.
Density of aluminum bar = 39 g/ 15 g/mL = 2.6 g/ml
Density of the PE rod = 21 g/23 mL = 0.91 g/mL
Density of iron bolt = 39 g/6.9 mL = 5.65 g/mL
Learn more about density:https://brainly.com/question/15164682
#SPJ1
Iron reacts with chlorine gas, Cl2, to form iron(III) chloride, FeCl3
Write the balanced equation for this synthesis reaction.
Answers
Answer:
2Fe + 3Cl2 --> 2 FeCl3
Explanation:
Fe + Cl2 --> FeCl3
iron is balanced but chlorine isn't so we find what number can make each one equal
Fe +3Cl2 --> 2FeCl3
now we need to balance iron
2Fe + 3Cl2 --> 2 FeCl3
Calculate the standard reaction enthalpy for the reaction below:
3Fe2O3(s) → 2Fe3O4(s) + ½O2(g)
Answers
The standard reaction enthalpy for the given reaction is +235.8 kJ/mol.
What is the standard reaction enthalpy of reaction?The standard reaction enthalpy (ΔH°) for the given reaction is determined as follows:
Equation of reaction: 3 Fe₂O₃ (s) → 2 Fe₃O₄ (s) + ½ O₂ (g)
The standard enthalpy of formation values for Fe₂O₃ (s), Fe₃O₄(s), and O₂(g) is used to calculate the standard reaction enthalpy.
ΔH° = [2 × ΔH°f(Fe₂O₃)] + [½ × ΔH°f(O₂)] - [3 × ΔH°f(Fe₃O₄)]
where;
ΔH°f(Fe₂O₃) = -824.2 kJ/mol
ΔH°f(Fe₃O₄) = -1118.4 kJ/mol
ΔH°f(O₂) = 0 kJ/mol
ΔH° = [2 × (-1118.4 kJ/mol)] + [½ × 0 kJ/mol] - [3 × (-824.2 kJ/mol)]
ΔH° = -2236.8 kJ/mol + 0 kJ/mol + 2472.6 kJ/mol
ΔH° = 235.8 kJ/mol
Learn more about standard reaction enthalpy at: https://brainly.com/question/15174388
#SPJ1
The carbon dioxide concentration in the atmosphere was 282 parts per million (ppm) in the year 1750. In the year 2010, this increased to 387 ppm. Calculate the percentage increase in carbon dioxide concentration over this period of time. Round your answer to the nearest whole number.
Answers
37 percent is the increase in percentage.
The air bubbles locked in ice sheets as well as glaciers over Earth's previous three glacial cycles are used in the second graph to depict carbon dioxide (CO2) levels during those periods. Human activities had boosted atmospheric CO2 approximately 50% since the start of the industrial era (in the 18th and 19th centuries), bringing it to 150% of it's own value in 1750.
Actually 282 ppm means 282 gm of CO2 is in 1 million gm of air similarly 382 ppm means 387 gm is in 1 million gm of air . Increase in mass of CO2 387-282 = 105 gm .
So percentage increase= (105× 100)÷282= 37%
To know more do carbon dioxide
https://brainly.com/question/28810601
#SPJ1
Manganese (IV) perbromate please put into formula form
Answers
Answer
The formula form of Manganese (IV) perbromate is
\(Mn(BrO_4)_4\)Explanation
The formula of Manganese is Mn
The formula for perbromate is BrO₄⁻
Oxidation number of Manganese (IV) = +4, That is Manganese (IV) is Mn⁺⁴
Therefore, multiply the charge of manganese by 1 and perchlorate by 4 t
Which solution has the higher boiling point: 26.4 g c2h6o2 in 0.500 kg of h2o or 22.0 g nacl in 0.500 kg of h2o?
Answers
The NaCl solution will have higher boiling point.
The temperature at which the vapour pressure equals the typical air pressure at sea level is known as the normal boiling point.
A molecular compound is \(C_{2} H_{6} O_{2}\). Meaning that it will continue to be in this state after dissolving:
\(C_{2} H_{6} O_{2}\) ⇒ \(C_{6} H_{12} O_{6}\) (aq)
Ionic materials include sodium chloride (NaCl). In other words, it will produce ions when it dissolves:
NaCl(s) ⇒ \(Na^{+}\)(aq) + \(Cl^{-}\) (aq)
\(C_{2} H_{6} O_{2}\) is a molecule and NaCl is an ionic substance. The boiling points of ionic compounds are higher than those of molecular molecules. NaCl will therefore have a higher boiling point.
Learn more about boiling point here:
https://brainly.com/question/2153588
#SPJ4
Place an O for the element in this reaction that is being oxidized and place an R for the element that is being reduced. 5Fe2+(aq) + Mn*'04 (aq) + 8H* → 5Fe3+ (aq) + Mn2+(ag) + 4H20
Answers
In this reaction, Fe2+ (O) is being oxidized to Fe3+ and MnO4- (R) is being reduced to Mn2+.
To identify the elements being oxidized and reduced, we first need to look at the changes in oxidation states of the elements involved. In this reaction, Fe2+ (iron) has an oxidation state of +2, and it becomes Fe3+ (iron) with an oxidation state of +3.
This increase in oxidation state indicates that Fe2+ is being oxidized (O). On the other hand, MnO4- (manganese) has an oxidation state of +7 for manganese, and it becomes Mn2+ (manganese) with an oxidation state of +2. This decrease in oxidation state indicates that MnO4- is being reduced (R).
Learn more about oxidation states here:
https://brainly.com/question/31688257
#SPJ11
En la siguiente ecuación se observa la obtención de oxígeno y cloruro de potasio a partir del clorato de potasio 2KClO3------>2KCl + 3O2 de la ecuación puede deducirse
Answers
Answer:
Ver respuesta mas abajo
Explanation:
Tu pregunta no es muy clara pero te expondré lo que se puede deducir de esa ecuación solo con esos datos.
Primero, anotemos nuevamente la ecuación:
2KClO₃ --------> 2KCl + 3O₂
Asi como está escrita la ecuación se deduce lo siguiente:
1. Es una ecuación de descomposición. Esto significa que un compuesto o molécula se descompuso o se separó, en sus moléculas o átomos elementales para producir 2 o más átomos y/o moléculas. En esta ecuación claramente vemos que el clorato de potasio, se separó en dos moléculas distintas, pero conservando sus mismos elementos, por un lado el cloruro de potasio (KCl) y por el otro oxígeno molecular. Ahora bien, una reacción de descomposición no se da por si solo; generalmente este tipo de reacciones se dan a lugar en presencia de calor o electricidad. Y esto da a lugar a la siguiente deducción.
2. Es una reacción de tipo endotérmica. Es decir, una reacción que absorbe calor para llevarse a cabo. El KClO₃ por si solo y colocado en un simple beaker o recipiente cualquiera no se va a descomponer, si no tiene influencia de algún tipo de catalizador o agente externo que permita su descomposición. En ese sentido, el calor o la electricidad son dos agentes que influyen mucho en la descomposición de este compuesto, y al usar el calor como agente para que la reacción ocurra, el compuesto lo absorbe y se descompone. Por ende es una reacción endotérmica.
3. El radio de moles entre reactivo y un producto es el mismo. En este caso podemos observar como 2 moles de reactivo inicial (KClO₃) se descompone y genera otros 2 moles de producto, en este caso, KCl, lo que significa que el radio molar entre estos dos compuestos es 2:2 o 1:1, lo que significa que para efectos de calcular moles prácticos a partir de cierta masa, este dato nos puede ayudar a determinar los moles de los productos y posteriormente la masa.
Si necesitas alguna otra deducción o completar la pregunta si hace falta algo, posteala en una nueva pregunta.
Espero te ayude.
help me please!
What pressure will be required for neon at 30°C to have the same density as nitrogen at 20°C and 1.0 atm?
Answers
Ideal gas law is valid only for ideal gas not for vanderwaal gas. Therefore, the pressure that will be required for neon at 30°C to have the same density as nitrogen at 20°C and 1.0 atm is 1.44atm.
What is ideal gas equation?
Ideal gas equation is the mathematical expression that relates pressure volume and temperature. There is no force of attraction between the particles.
Mathematically the relation between Pressure and temperature can be given as
P₁M₁=DRT₁
P₂M₂=DRT₂
Rearranging the two equation we get
P₁M₁÷T₁=P₂M₂÷T₂
where,
P = pressure
M= Molarity
D=density
T =temperature
R = Gas constant = 0.0821 L.atm/K.mol
Substituting all the given values, we get
(P₁×M₁)÷T₁=(P₂×M₂)÷T₂
(P₁×20)÷303=(1.0 ×28)÷293
P₁=1.44atm
Therefore, the pressure that will be required for neon at 30°C to have the same density as nitrogen at 20°C and 1.0 atm is 1.44atm.
To learn more about ideal gas equation, here:
https://brainly.com/question/14826347
#SPJ2
which of the molecular orbital diagrams is correctly filled for the diatomic molecule r2? (each atom of r has six valence electrons in ns and np orbitals.)
Answers
Atomic orbitals are the areas to the left and right of the dashed lines. The possible molecular orbitals that they can form are indicated by the dashed lines.
Normally, in diatomic molecular orbitals, the atomic orbitals with the closest energy level can overlap with each other and form molecular orbitals. Therefore, the atomic orbitals generally tend to overlap one by one from the lowest potential energy to the highest potential energy. For example, in a homonuclear diatomic molecule, which means that both atoms are the same element, the same orbitals will overlap together and form molecular orbitals.
Learn more about atomic orbitals here:
https://brainly.com/question/14571416
#SPJ4
What is the periodic table?
Answers

Answer:
The periodic table, also known as the periodic table of chemical elements, is a tabular display of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of chemistry.
Explanation:
The periodic table is a tabular arrangement of chemical elements that classifies elements according to repeating qualities and is structured by increasing atomic number. Metals are located on the left side of the table, whereas nonmetals are located on the right. The columns are referred to as groupings.
in pic
____________________________________________________
(Hope this helps can I pls have brainlist (crown)

a 12.00 ml sample of an ammonia solution is titrated with 1.499 m hno3solution. a total of 19.48 ml of acid is required to reach the equivalencepoint. what is the molarity of the ammonia solution?
Answers
The molarity of the ammonia solution in this problem is 2.42 M. This titration problem requires the use of stoichiometry to determine the molarity of the ammonia solution.
The balanced chemical equation is used to determine the mole ratio between ammonia and nitric acid, which is 1:1. Knowing the volume and molarity of the nitric acid used, the moles of nitric acid can be calculated. Since the mole ratio between the two reactants is 1:1, the moles of ammonia in the 12.00 ml sample is equal to the moles of nitric acid used in the titration. Finally, the molarity of the ammonia solution is calculated by dividing the moles of ammonia by the volume of the ammonia solution used. The molarity of the ammonia solution in this problem is 2.42 M.
To find the molarity of the ammonia solution, we need to use the balanced chemical equation of the reaction between ammonia and nitric acid:
NH₃ + HNO₃ → NH₄NO₃
From the equation, we can see that the mole ratio between ammonia and nitric acid is 1:1. This means that the moles of ammonia in the 12.00 ml sample is the same as the moles of nitric acid used in the titration, which is:
moles of HNO₃ = (1.499 mol/L) x (19.48 mL/1000 mL) = 0.02899 mol
Therefore, the molarity of the ammonia solution is:
Molarity of NH₃ = moles of NH₃ / volume of NH₃ solution
Molarity of NH₃ = 0.02899 mol / 12.00 mL = 2.42 M
Learn more about titration here:
https://brainly.com/question/30140546
#SPJ11
a 1) How would you make 1 liter of a 10% NaCl solution from a solid stock? Provide details of what kind of containers you would use.
Answers
To make 1 liter of a 10% NaCl solution from a solid stock, you will require the following materials and containers.MaterialsSolid NaClDistilled water1-Liter volumetric flask250-mL volumetric flask 2-beakersProcedureTo prepare 1 liter of a 10% NaCl solution, the following procedure should be followed:Measure out 100g of NaCl using a balance.
Measure the weight of an empty 250-mL volumetric flask.Add the NaCl to a 250-mL beaker and add a small amount of distilled water to it to dissolve the NaCl.Carefully pour the dissolved NaCl solution into the 250-mL volumetric flask. Add distilled water to the mark on the flask to make up the volume. Stopper the flask and invert it several times to mix the solution.Measure the weight of the 1-Liter volumetric flask.Add the 250-mL volumetric flask solution to a 1-Liter volumetric flask.Add distilled water to the mark on the flask to make up the volume.
Stopper the flask and invert it several times to mix the solution.The final volume of the solution will be 1 liter of a 10% NaCl solution.PrecautionsEnsure the NaCl has completely dissolved before adding more water to avoid making a less concentrated solution.Measure the weight of the volumetric flask before and after adding the solution to calculate the volume of solution that was added.Use distilled water to prepare the solution.
To know more about volumetric flask visit:-
https://brainly.com/question/28997155
#SPJ11
What is the name of this molecule?

Answers
Answer:
The name of this molecule structure is 1-Butyne
The name of the given molecule according to IUPAC is 1-Butyne. Thus, option C is correct.
What is IUPAC nomenclature?The IUPAC nomenclature is given as the standard naming technique that is used to name a chemical compound. It is named with the following main rules.
The triple bond compound is an alkyne with the suffix -yne. The development of the name to the given structure is therefore given by counting the number of carbon atoms, and the location of the triple bond.
There are 4 carbon atoms in the structure with the triple bond at carbon 1, therefore the name will be 1-Butyne. Hence, option C is correct.
Learn more about IUPAC nomenclature, here:
https://brainly.com/question/11587934
#SPJ1
At what rate is hydrogen gas being produced by this reaction? explain your reasoning
Answers
Hydrogen gas is being produced at a rate that depends on the specific conditions of the reaction, such as the temperature, pressure, and concentration of reactants.
In order to determine the rate of hydrogen gas production, we would need to know these specific conditions and use them to calculate the rate using the rate law for the reaction. The rate law is an equation that relates the rate of a reaction to the concentration of the reactants and the rate constant, k.
Hence, the rate law for a reaction can be determined experimentally and is specific to the reaction. Once the rate law is known, we can plug in the specific conditions of the reaction to determine the rate of hydrogen gas production.
Learn more about Hydrogen gas at https://brainly.com/question/14235627
#SPJ11
The isotopes K-37 and K-42 have the same...
1) number of neutrons
2) number of protons
3) mass number for their atoms
Answers
Answer:
WHEA O IN AN EREM STATO. PETUA TOT low 6 EN 6ACY STATE ... Determine both the total number of protons and the total number of neutrons in an atom of the naturally occurring carbon isotope with the largest mass number.
Explanation:
ok
This chemical equation represents one of the reactions that form acid rain.
Which two options list the bonds that break in the reaction?,
A. The bonds between H and O in H20
B. The bonds between N and O in NO2
C. The bonds between N and O and between Hand O in HNO2
D. The bonds between N and O and between Hand O in HNO3
Answers
When sulfur dioxide (SO2) and nitrogen oxides (NOX) are released into the atmosphere and carried by wind and air currents, acid rain is the volcanoes.
Thus, Nitric and sulfuric acids are created when the SO2 and NOX react with water, oxygen, and other substances. Then, before hitting the ground, they combine with water and other substances.
The majority of the SO2 and NOX that contribute to acid rain originates from burning fossil fuels, however a tiny amount comes from natural sources like volcanoes.
Thus, When sulfur dioxide (SO2) and nitrogen oxides (NOX) are released into the atmosphere and carried by wind and air currents, acid rain is the volcanoes.
Learn more about sulphur dioxide, refer to the link:
https://brainly.com/question/31142164
#SPJ1
Zn + Cu SO 4 —>
What’s the reaction
Answers
Answer:
Single displacement reaction
Explanation:
You have an element (Zinc) + a compound that consists of an element (Copper) and a polyatomic ion (Sulfate)
Since there's one element and one compound, the metals in both trade places, giving us Zinc Sulfate and Copper as our products.
This should be the complete balanced equation:
Zn + CuSO4 -------> ZnSO4 + Cu
]
What are typical characteristics of metals?
lonization energy
Electron affinity
A
low
low
B.
high
high
C.
high
low
D.
low
high
Answers
Answer:
A
Explanation:
Metals has low Ionization energy and low electron affinity. (Option A)
Characteristics of Metals (Ionization energy/Electron Affinity)Metals has low ionization energy because the valence electrons in alkali metals, are very away from the nucleus. Hence, they can easily lose electrons with low energy and become cationic.
Metals have a low electron affinity because they want to give up their valence electrons rather than gain electrons, which require more energy than necessary.
Read more on Characteristics of Metals:
https://brainly.com/question/24662549
#SPJ2
What is 191 days to seconds?
Answers
Answer:
191 days =16,502, 400 seconds
Which is the correct theory about the solar system: sun-centered or Earth-centered? Why?
Answers
Answer:i dont know thats why imon her
Explanation:
For n=1, ∫Ψ* Ψ d3x = 1
Show that the groundstate hydrogen wavefunction is properly normalized
Answers
For n=1, ∫Ψ* Ψ d³x = 1
The ground state wavefunction of hydrogen satisfies the normalization condition ∫Ψ* Ψ d³x = 1.
To show that the ground state hydrogen wavefunction is properly normalized, we need to calculate the integral of the wavefunction squared, Ψ², over all space and demonstrate that it equals 1.
The ground state wavefunction of hydrogen is given by:
Ψ(r) = (1/√πa₀³) \(e^(^-^r^/^a^_0)\)
where a₀ is the Bohr radius.
To show normalization, we evaluate the integral:
∫ Ψ*(r) Ψ(r) d³r
where Ψ*(r) represents the complex conjugate of Ψ(r).
Substituting the expression for Ψ(r), we have:
∫ (1/√πa₀³) \(e^(^-^r^/^a^_0)\) (1/√πa₀³) \(e^(^-^r^/^a^_0)\) d³r
Expanding the product and rearranging the terms, we get:
(1/π²a₀⁶) ∫ \(e^(^-^2^r^/^a^_0)\) d³r
The integral represents the volume integral over all space, so we can rewrite it as:
(1/π²a₀⁶) ∫∫∫ \(e^(^-^2^r^/^a^_0)\) dxdydz
Since the wavefunction is spherically symmetric, we can use spherical coordinates for the integral. The volume element in spherical coordinates is given by r² sin(θ) dr dθ dφ.
Therefore, the integral becomes:
(1/π²a₀⁶) ∫∫∫ \(e^(^-^2^r^/^a^_0)\) r² sin(θ) dr dθ dφ
To solve the integral, we perform the integration in each coordinate:
∫∫∫ \(e^(^-^2^r^/^a^_0)\) r² sin(θ) dr dθ dφ = ∫ [0,∞] \(e^(^-^2^r^/^a^_0)\) r² dr ∫ [0,π] sin(θ) dθ ∫ [0,2π] dφ
The φ integral gives 2π, and the θ integral gives 2.
∫ [0,∞] \(e^(^-^2^r^/^a^_0)\) r² dr = (\(-a_0^3^/^8\)) [ \(e^(^-^2^r^/^a^_0)\) (2r² + 2r\(a_0\) + \(a_0^2\))]
Evaluating this integral from 0 to ∞ gives a_0^3/8.
Thus, the integral becomes:
(1/π²a₀⁶) (\(-a_0^3^/^8\)) (2)(2π)
Simplifying, we get:
(1/π²a₀⁶) (\(a_0^3\)/4π)
The π terms cancel out, and we are left with:
1/(2\(a_0^3\) )
This value is equal to 1, confirming that the ground state hydrogen wavefunction is properly normalized.
Therefore, the ground state wavefunction of hydrogen satisfies the normalization condition ∫Ψ* Ψ d³x = 1, demonstrating that it is properly normalized.
To know more about ground state here
https://brainly.com/question/1314094
#SPJ4
The time for one cycle of a periodic process is called the:.
Answers
Answer:
Period
Explanation:
I believe your answer should be period.
I hope it helps! Sorry if it didn’t… Have a great day!
Layla~
Plutonium-242 (242Pu) undergoes alpha decay to form what daughter isotope and decay particle?
Answers
Answer:
Decay. Plutonium-242 primarily decays into uranium-238 via alpha decay, before continuing along the uranium series.
Explanation:
please mark as brainlist