Directions: Read the paragraph carefully and identify the correct words that fit in the given sentences inside the box. Write your answer on a separate sheet.
Analyze the results
Dependent Hypothesis Test the hypothesis Draw a conclusion Question/Problem Observation Six Scientific step will lead you to an educated guess called (5) where you can have tentative answer to your question. In order for you to prove your educated guess you need to (6) by designing and conducting an experiment. In the experiment you need to identify the variables present and these are the (7) and (8) variables. The data from the experiment will be collected to (9) The summarized results from the experiment will determine whether the hypothesis is accepted or rejected and that is where you (10)Lu

Answers
Answer:
Scientific method, six, observation, question/ problem, hypothesis, test the hypothesis, depedent, independent, analyze the results, draw a conclusion
Related Questions
the third law of thermodynamics describes the entropy of a: select the correct answer below: solid liquid gas all of the above
Answers
The third law of thermodynamics describes the entropy of a: solid.
The third law of thermodynamics states that the entropy of a pure crystalline substance approaches zero as the temperature approaches absolute zero (0 Kelvin or -273.15 degrees Celsius). This law implies that at absolute zero, a perfectly ordered and pure crystalline solid will have zero entropy.
The third law of thermodynamics is not specific to liquids or gases but applies to solids. In a solid, the molecules are highly ordered and have fixed positions in a regular lattice structure. As the temperature decreases towards absolute zero, the thermal motion of the molecules reduces, and the system becomes more ordered, resulting in a decrease in entropy.
In contrast, liquids and gases have higher entropy compared to solids at absolute zero because their molecules have more freedom of movement and are not as tightly arranged. Therefore, the third law of thermodynamics specifically addresses the entropy of solids and does not apply to liquids or gases.
To learn more about law of thermodynamics, here
https://brainly.com/question/1368306
#SPJ4
A. The pendulum would accelerate to the left
B. The pendulum would accelerate downward
C. The pendulum would accelerate to the right
D. The pendulum would accelerate upward
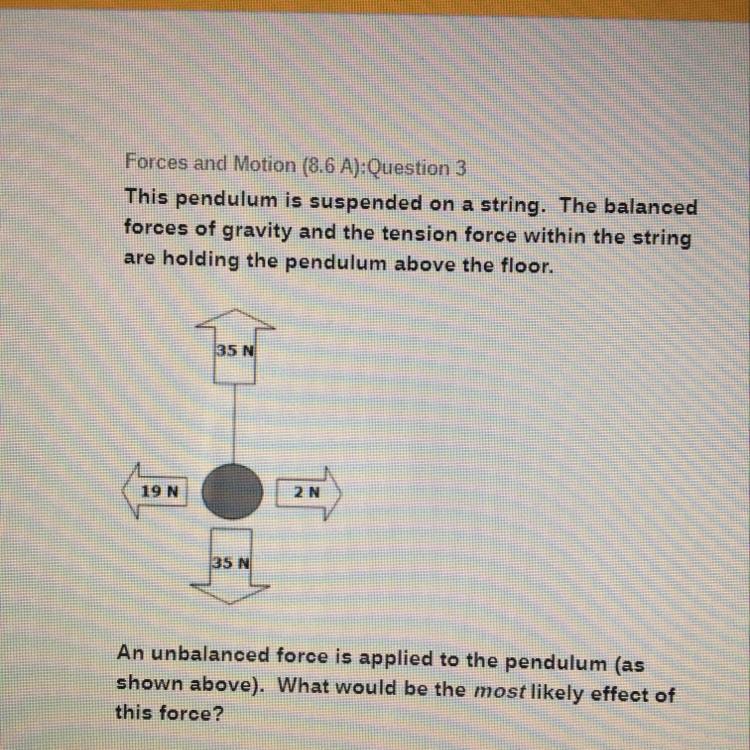
Answers
Answer:
The pendulum would accelerate to the left
Explanation:
hope this helps
When stomach acid helps to break down food into smaller particles this is
an example of a change.*
O Physical
Chemical
O
Elemental
о
Atomic
Answers
Answer:
chemical, is the answer your looking for
After the HCl and NaOH react, Fernando measures the
mass again. Using the mass before the reaction in the
diagram, what is the mass after the reaction?
Remember, It is in a closed system.
A. 5.00 grams
OB. 10.00 grams
O C. 15.00 grams
OD. 20.00 grams
Answers
Answer:c
Explanation:
As the combined mass of the HCl and NaOH is 15 grams before the reaction. Therefore the mass after the reaction will be 15 grams according to the law of conservation of mass. Therefore, option (C) is correct.
What is law of conservation of matter?Matter can be transformed form via physical changes and chemical changes from one form to another form, during any of these changes, the total mass is conserved. The same quantity of matter exists before and after the chemical or physical as none of the matter is created or destroyed.
The balanced equation between the reaction of HCl and NaOH:
\(HCl +NaOH \longrightarrow H_2O +NaCl\)
According to the law of conservation of mass, the mass of HCl and NaOH will be equal to the mass of the products water and NaCl.
As mentioned in the question the combined mass of HCl and NaOH measured before the reaction is 15 grams. Therefore, the mass of the products in the closed container will be equal to 15 grams as well.
Learn more about law conservation of matter, here:
brainly.com/question/23910777
#SPJ5
Your question was incomplete, most probably the complete question was,
Fernando places 15 ml of HCl and 50 ml of NaOH in 100 ml of a beaker. He places them on a scale together and measures the combined mass of 15 grams.
After the HCl and NaOH react, Fernando measures the mass again. Using the mass before the reaction, what is the mass after the reaction? Remember, It is in a closed system.
A. 5.00 grams
B. 10.00 grams
C. 15.00 grams
D. 20.00 grams
What is the pH of a solution if the pOH is 2.96
Answers
The pH of a solution if the pOH is 2.96 will be 11.04. As the sum of the pH and pOH is always 14.
A pH can be regarded as a scale that is used to measure acids and bases. The scale measures from 0 to 14. A litmus paper is used to indicate if the substance is an acid or a base and then the color of the litmus paper is used to match the numbers on the pH scale in order to indicate what kind of substance is it. The term PH is widely used in the fields of biology, chemistry, and agronomy. In chemistry, it depicts the hydrogen potentials. It shows us the concentration of hydrogen ions in a solution.To know more about, pH levels, visit :
https://brainly.com/question/2288405
The molar mass of iron (iii) oxide (fe2o3) is 159.7 g/mol. what is the correct way to write the molar mass of iron oxide as a conversion factor? startfraction 159.7 grams upper f e subscript 2 upper o subscript 3 over 159.7 moles upper f e subscript 2 upper o subscript 3 endfraction. startfraction 1 gram upper f e subscript 2 upper o subscript 3 over 159.7 moles upper f e subscript 2 upper o subscript 3 endfraction. startfraction 159.7 grams upper f e subscript 2 upper o subscript 3 over 1 mole upper f e subscript 2 upper o subscript 3 endfraction. startfraction 1 gram upper f e subscript 2 upper o subscript 3 over 1 mole upper f e subscript 2 upper o subscript 3 endfraction.
Answers
The molar mass of iron (iii) oxide (Fe₂O₃) is 159.7 g/mol. the correct way to write the molar mass of iron oxide as a conversion factor is :
159.7 g Fe₂O₃/ 1 mol
The molar mass of Fe₂O₃ = 159.7 g/mol
molar mass of the substance is the mass in grams per mole of a substance.
the molar mass of Fe = 55.8 g/mol
the molar mass of O = 16 g/mol
the molar mass of Fe₂O₃ = 2 × 55.8 + 3 × 16
= 159.7 g/mol
the molar mass of Fe₂O₃ = 159.7 g/mol
the correct way of write the molar mass of Fe₂O₃ = 159.7 g Fe₂O₃/ 1 mol.
To learn more about molar mass here
https://brainly.com/question/5566319
#SPJ4
what is the identity of the unknown white solid? what is the identity of the unknown white solid? barium chloride calcium carbonate magnesium chloride
Answers
According to the claim, the substance in question is barium chloride, also known as BaCl₂.
Why is solid so crucial?For the purpose of overcoming these unfavorable design patterns, the SOLID tenets were created. Reducing dependencies allows engineers to alter one component of software without affecting others, which is the main objective of the SOLID principles. They are also meant to make designs simpler to comprehend, update, and expand.
A solid thing is what?A solid is distinguished by its rigidity and tolerance to external forces. A solid substance, unlike a liquid, doesn't quite flow to conform to the form of its containers or, like a gas, expand to cover the entire space.
To know more about Solid visit:
https://brainly.com/question/225975
#SPJ4
The complete question is-
You are presented with a white solid and told that due to carless labeling it is not clear if the substance is barium chloride, lead chloride or zinc chloride. When you transfer the solid to a beaker and add water the solid dissolves to give a clear solution. Next a Na2SO4 (aq) solution is added and a white precipitate forms. What is the identity of the unknown white solid?
a). BaCl₂
b). CaCO₃
c). MgCl₂
When mercury oxide is heated, it decomposes into mercury and oxygen. If 28. 4 g of mercury oxide decomposes, producing 2. 0 g of oxygen, what is the percent by mass of mercury in mercury oxide?.
Answers
In mercuric oxide percent by mass of mercury is 93 %.
The mercuric oxide molecular formula is HgO. When mercury is reacted with oxygen, then it forms the product mercuric oxide. Mercuric oxide has a molecular mass of 216 g/mol. It has a melting point of 500°C.
The balance equation for the reaction of decomposition of mercury oxide into mercury and oxygen is,
\(2HgO (s)→ 2Hg (l) + O _{2}(g)\)
Mass of mercury oxide = 28.4 g
Mass of the oxygen = 2 g
In mercuric oxide percent by mass of mercury is,
\(Mass ℅= \frac{Mass \: of \: Mercury }{Mass \: of \: Mercury \: oxide} \times 100\)
\(= \frac{28.4 - 2}{28.4} \times 100\)
= 93 %
Therefore, in mercuric oxide percent by mass of mercury is 93 %.
To know more about mercury, refer to the below link:
https://brainly.com/question/2279846
#SPJ4
The bond angle of the molecule h2o is less than the tetrahedral bond angle of 109.5˚ because:
Answers
Answer:
The bond angle of the molecule H₂0 is less than the tetrahedral bond angle of 109.5˚ because of the electron repulsion that exists between the lone pairs.
Explanation:
Methane's H—C—H bond has a tetrahedral angle of 109.5°. When all four pairs of outer electrons repel one another equally, this angle is produced.
Due to the increased electron repulsion shown by the lone pairs of electrons in ammonia and water, the bond angles are less than 109.5°.
The lone pair of electrons on the oxygen atom cause the bond angle of H₂O to be less than 109.5°. The bond angle is a little bit smaller because these electrons occupy more space than those in a bond. Further distorting the binding angle is the electron pair repulsion between the bonding pairs of electrons and the lone pair. In molecules having a core atom that has more than two bonding partners, this is known as "angular hybridization" and is a frequent occurrence.
In molecule, H20, the angle between H-O-H is 104.5 degrees due to lone pair and bond pair repulsion.
This deviation is due to repulsion between lone pair- lone pair and bond pair-bond pair and lone pair-bond pair as the oxygen atom has an extra lone pair electron which causes slight distortion in bond angle from 109.5 degrees to 104.50 degrees. In the H2O molecule, the oxygen is sp3 hybridised and thus tetrahedral configuration comes into existence.
To learn more visit https://brainly.in/question/14325022#:~:text=Explaination%3A,with%20bond%20angle%20104.5%20degrees
What is a cell? Give me detail and answer

Answers
Answer:
A cell is a phone or a component in a circuit.
Not sure if correct
Concentrations-
How many moles of HCI are present in 562.0 ml of a 6.17 M HCI solution?
If possible, show work.
Answers
Work shown on photo

as carbon bonds with atoms of increasingly higher electronegativities, the polarity of the bond
Answers
As carbon bonds with atoms of increasingly higher electronegativities, the polarity of the bond increases.
Electronegativity refers to the ability of an atom to attract electrons towards itself. When carbon bonds with atoms that have higher electronegativities than itself, such as oxygen or nitrogen, the electrons in the bond are more strongly attracted to the higher electronegative atom.
This results in an unequal sharing of electrons between the two atoms, creating a polar covalent bond where one atom has a slightly negative charge (the more electronegative atom) and the other has a slightly positive charge (the carbon atom). Therefore, as the electronegativity of the atom carbon is bonding with increases, the polarity of the bond also increases.
To know more about atoms visit:
https://brainly.com/question/1566330
#SPJ11
PLEASE HELP!!!!! I'LL YOU BRAINIEST!!!!



Answers
Answer:
I believe 11 is B 12 is C 13 is B and 14 is C
A rigid vessel containing a 3:1 mol ratio of carbon dioxide and water vapor is held at 200oC where it has a total pressure of 2.00 atm. If the vessel is cooled to 10oC so that all of the water vapor condenses, what is the pressure of carbon dioxide? Neglect the volume of the liquid water that forms on cooling.
Answers
After the water vapor condenses, the pressure of carbon dioxide will be 1.5 atm.
How to find the pressure of the carbon dioxide?To find the pressure of the carbon dioxide after the water vapor condenses, we can use the idea of partial pressures. The total pressure of the vessel is 2.00 atm, and the ratio of moles of CO2 to moles of water vapor is 3:1. Therefore, the partial pressure of the CO2 is 3/4 of the total pressure, and the partial pressure of the water vapor is 1/4 of the total pressure.
So,
P(CO2) = (3/4) * 2.00 atm = 3/4* 2 atm = 1.5 atm
Learn more about carbon dioxide in https://brainly.com/question/3049557
#SPJ1
(URGENT!!!!!) the electron configuration of some elements are given. Based on the electron configuration which ELEMENTS can likely form ions with multiple charges?
a) zinc [Ar] 3d¹⁰4s²
b) iron [Ar] 3d⁶4s²
c) sodium [Ne] 3s¹
d) cobalt [Ar] 3d⁷ 4s²
e) silver [Kr] 4d¹⁰ 5s¹
Answers
Answer:
iron (Ar) 3d^6 4s^2 and cobalt (Ar) 3d^7 4s^2
Based on the electron configuration of elements Fe and Co can likely form ions with multiple charges.
What is electron configuration?Electron configuration of an element describes how electrons are distributed in its atomic orbitals. Electron configurations of atoms follow a standard notation in which all electron-containing atomic subshells (with the number of electrons they hold written in superscript) are placed in a sequence.
Electron configuration can be written according to the following rules-
1. Aufbau Principle
2. Pauli's exclusion principle
3. Hund's rule
Electrons will be filled according to the increasing order of the energy as per the n+l rule.
Given,
a) zinc [Ar] 3d¹⁰4s²
b) iron [Ar] 3d⁶4s²
c) sodium [Ne] 3s¹
d) cobalt [Ar] 3d⁷ 4s²
e) silver [Kr] 4d¹⁰ 5s¹
Except Co and Fe, all the elements when loose 1 or 2 electrons form full filled electrons, Fe and Co show +2, +3, +4 etc oxidation states.
Therefore, Based on the electron configuration of elements Fe and Co can likely form ions with multiple charges.
Learn more about electron configuration , here:
https://brainly.com/question/29757010
#SPJ7
A gas contains a mixture of He and Ne. The gas is at a temperature of 592 K, with 0.55 mol of He and 3 mol of Ne. What is the average ki
Answers
In a gas mixture containing 0.55 mol of He and 3 mol of Ne at a temperature of 592 K, the average kinetic energy can be calculated using the equation E_avg = (3/2)kT.
The average kinetic energy of a gas is related to its temperature through the Boltzmann constant. The equation to calculate the average kinetic energy is given by:
E_avg = (3/2)kT,
where
E_avg is the average kinetic energy,
k is the Boltzmann constant (approximately 1.38 x 10⁻²³ J/K), and
T is the temperature in Kelvin.
In this case, we are given the temperature T as 592 K. To calculate the average kinetic energy, we need to consider the individual contributions of He and Ne. Since He and Ne are monatomic gases, they have the same degrees of freedom, and thus each contributes (3/2)kT to the total average kinetic energy.
For He, we have 0.55 mol, and for Ne, we have 3 mol. We can calculate the total average kinetic energy by summing the contributions from each gas:
E_avg = [(0.55 mol) * (3/2)kT] + [(3 mol) * (3/2)kT].
By plugging in the values of k and T, we can evaluate the equation and find the average kinetic energy of the gas mixture at 592 K.
To calculate the average kinetic energy of the gas mixture at 592 K, we need to plug in the values of k (Boltzmann constant) and T (temperature) into the equation:
E_avg = (3/2)kT.
The Boltzmann constant, k, is approximately 1.38 x 10⁻²³ J/K, and the temperature, T, is given as 592 K.
Plugging in these values, we have:
E_avg = (3/2) * (1.38 x 10⁻²³ J/K) * (592 K).
Evaluating this expression, we find:
E_avg = 1.38 x 10⁻²³ J/K * 3/2 * 592 K.
Simplifying further:
E_avg = 3.3 x 10⁻²¹ J.
Therefore, the average kinetic energy of the gas mixture at 592 K is approximately 3.3 x 10⁻²¹ Joules.
To know more about kinetic , click here-
brainly.com/question/20005035
#SPJ11
The solubility of a gas is 2.0 g/L at 50.0 kPa of pres-
sure. How much gas will dissolve in 1 L at a pressure
of 10.0 kPa?
our of
Answers
Answer:
That means, under 10.0kPa of pressure, 0.4g of gas can be dissolved in 1L
Explanation:
Based on Henry's law, the solubility of the gas is directly proportional to the pressure. The equation is:
P1S2 = P2S1
Where P is pressure and S solubility of 1, initial state and 2, final state of the gas.
Replacing:
P1 = 50.0kPa
S1 = 2.0g/L
P2 = 10.0kPa
S2 = ??
50.0kPa*S2 = 10.0kPa*2.0g/L
S2 = 0.4g/L
That means, under 10.0kPa of pressure, 0.4g of gas can be dissolved in 1L
Answer: 0.4 g/L
Explanation:
S2 = S1 x P2 / P1
S2= 2 g/L x 10 kPa / 50 kPa
CROSS OUT
S2= 2g/L x 10 / 50
S2= 20 g/L/50
S2= .4 g/L
Learning Task 3: Why can a car run faster on a smooth
rood than on a rough roade (5points).
Answers
Answer:
it is easy to push a car on smooth road than on a rough road because friction is less in smooth road than on rough roads.
Answer:
Why can a car run faster on a smooth
rood than on a rough road it is because friction is less in smooth roads than in rough road....
Explanation:
hope it helps..stay safe!
why is sulfuric acid considered as a positive catalysts in esterification
Answers
Answer:
Because it is a strong acid
Explanation:
All strong acids are consideredpositive
Identify the type of energy this object possesses. A girl roller-skating Kinetic energy Potential energy
Answers
A girl roller-skating has kinetic energy.
Kinetic energy is the energy of motion. It is the energy possessed by an object due to its movement. In this case, the girl roller-skating has kinetic energy because she is moving.
Potential energy is the energy an object possesses due to its position or configuration. It is the energy an object has stored within it, ready to be released. An object at rest has potential energy because it has the potential to be set in motion and does work.
So, in this case, the girl roller-skating has kinetic energy because she is moving, and not potential energy because she is not at rest.
To learn more about kinetic energy, check out https://brainly.com/question/24933254
what is the real gas pressure exerted by 1.00 mol of o2 at 300. k in 2.41 l if the ideal pressure is 10.0 atm?
Answers
the real gas pressure for above question was 91.4atm
what is van der waals equatio?
The Van der Waals equation (or Van der Waals equation of state) is an equation of state used in chemistry and thermodynamics that extends the ideal gas law to take into account the effects of interactions between molecules in a gas as well as accounting for the finite size of the molecules.
The ideal gas law views gas molecules as point particles that interact only with their surroundings and not with one another, which means that when they collide, they do not occupy any space or change their kinetic energy.
The volume V occupied by n moles of any gas has a pressure P at a temperature T determined by the following relationship, according to the ideal gas law, where R is the gas constant:
PV=nRT
(pxn2a/v2) (v-nb)=nRt
p 10x1.36/2.41x2.41-10x0.0318
p 13.6/2.41x2.092
nRt=p2.69
10x0.08206x300/2.69
=91.4atm
therefore the real gas pressure =91.4atm
to learn more about real gas pressure follow the given link: https://brainly.com/question/13313291
#SPJ4
Air is cooling at night. The frost point (temperature at which RH with respect to ice reaches 100%) is reached at T = -10 degree Celsius. a) What is the RH (normal RH with respect to liquid water) at this point? b) Upon further cooling the air reaches a temperature of T =-11 degree Celsius Kaolinite particles of 200 nm diameter are present. Do you expect ice particles to form? If yes, do they form via deposition nucleation or condensation of droplets followed by freezing? Briefly explain your answer. c) Upon even further cooling the air reaches a temperature of T = -12 degree Celsius. Same question as before: Do you expect ice particles to form now? If yes, do they form via deposition nucleation or condensation of droplets followed by freezing? Briefly explain your answer. Equilibrium vapor pressures may be calculated or taken from the table below. t/°C 0 -1 -2 -3 -4 -5 -6 -7 -8 -9 - 10 -11 -12 -13 T/ Keow /Pa 273.15 611.2 272.15 568.2 271.15 527.9 270.15 490.2 269.15 454.8 268.15 421.8 267.15 390.9 266.15 362.1 265.15 335.1 264.15 310.0 263.15 286.5 262.15 264.7 261.15 244.3 260.15 225.4 259.15 207.8 258.15 191.4 e oi/Pa 611.2 562.7 517.7 476.1 437.5 401.8 368.7 338.2 310.0 283.9 259.9 237.7 217.3 198.5 181.2 165.3 - 14 - 15 Equilibrium vapor pressures with respect to water (eow) and with respect to ice (coi).
Answers
The equilibrium vapor pressure with respect to water (eow) is 259.9 Pa. assume that saturation vapor pressure is same as equilibrium vapor pressure.
Therefore, the RH at the frost point is
RH = (eow / saturation vapor pressure) × 100
= (259.9 Pa / 259.9 Pa) × 100
= 100%
b) At T = -11 °C, we need to compare the equilibrium vapor pressure with respect to water (eow) and the equilibrium vapor pressure with respect to ice (coi) to determine if ice particles will form. From the given table, at T = -11 °C, the equilibrium vapor pressure with respect to water (eow) is 237.7 Pa, and the equilibrium vapor pressure with respect to ice (coi) is 165.3 Pa.
The air is supersaturated with respect to ice, and the presence of Kaolinite particles can provide surfaces for water droplets to condense onto, leading to the formation of ice particles.
c) At T = -12 °C, we compare the equilibrium vapor pressure with respect to water (eow) and the equilibrium vapor pressure with respect to ice (coi). From the given table, at T = -12 °C, the equilibrium vapor pressure with respect to water (eow) is 217.3 Pa, and the equilibrium vapor pressure with respect to ice (coi) is 181.2 Pa.
Learn more about equilibrium vapor here
https://brainly.com/question/15629887
#SPJ11
When people talk about the phrase “blood sugar,” they really mean?
Answers
Answer:
Specific chemical sugar glucose
Explanation:
When we say “blood sugar,” we mean the specific chemical sugar glucose. Diabetes is all about glucose. There lots of sugars….table sugar is sucrose, malt sugar is maltose, milk sugar is lactose, and you’ve probably heard of fructose.
Blood sugar, or glucose, is the main sugar found in your blood. It comes from the food you eat, and is your body's main source of energy. Your blood carries glucose to all of your body's cells to use for energy.
Pls mark me brainiest and my name is Misty.
What are the coefficients in front of NO3 -(aq) and Mg(s) when the following redox equation is balanced in an acidic solution:
___ NO3 -(aq) + ___ Mg(s) → ___ NO(g) + ___ Mg 2+(aq)?
Answers
The coefficients in front of NO₃⁻(aq) and Mg(s) when the given redox equation is balanced in an acidic solution are 2 and 1, respectively.
To balance the redox equation in an acidic solution, we need to first determine the half-reactions and balance them separately. Then, we'll combine the balanced half-reactions.
Oxidation half-reaction (Mg to Mg²⁺):
Mg(s) → Mg²⁺(aq) + 2e-
Reduction half-reaction (NO₃⁻ to NO):
2H⁺(aq) + NO₃⁻(aq) + e- → NO(g) + H₂O(l)
Now, to balance the electrons, we multiply the oxidation half-reaction by 1 and the reduction half-reaction by 2:
Oxidation: Mg(s) → Mg²⁺(aq) + 2e-
Reduction: 4H⁺(aq) + 2NO₃⁻(aq) + 2e- → 2NO(g) + 2H₂O(l)
Combining the balanced half-reactions, we get:
Mg(s) + 4H⁺(aq) + 2NO₃⁻(aq) → Mg²⁺(aq) + 2NO(g) + 2H₂O(l)
So, the coefficients in front of NO₃⁻(aq) and Mg(s) are 2 and 1, respectively.
Learn more about redox equation here: https://brainly.com/question/27907895
#SPJ11
The coefficients in front of NO₃⁻(aq) and Mg(s) when the given redox equation is balanced in an acidic solution are 2 and 1, respectively.
To balance the redox equation in an acidic solution, we need to first determine the half-reactions and balance them separately. Then, we'll combine the balanced half-reactions.
Oxidation half-reaction (Mg to Mg²⁺):
Mg(s) → Mg²⁺(aq) + 2e-
Reduction half-reaction (NO₃⁻ to NO):
2H⁺(aq) + NO₃⁻(aq) + e- → NO(g) + H₂O(l)
Now, to balance the electrons, we multiply the oxidation half-reaction by 1 and the reduction half-reaction by 2:
Oxidation: Mg(s) → Mg²⁺(aq) + 2e-
Reduction: 4H⁺(aq) + 2NO₃⁻(aq) + 2e- → 2NO(g) + 2H₂O(l)
Combining the balanced half-reactions, we get:
Mg(s) + 4H⁺(aq) + 2NO₃⁻(aq) → Mg²⁺(aq) + 2NO(g) + 2H₂O(l)
So, the coefficients in front of NO₃⁻(aq) and Mg(s) are 2 and 1, respectively.
Learn more about redox equation here: https://brainly.com/question/27907895
#SPJ11
what is on the left side ?

Answers
Answer:
reactants
Explanation:
What is the difference between an aqueous solution and a liquid (simple definition)
Answers
Answer:
The primary distinction between liquid and aqueous is that liquid refers to any fluid that is almost incompressible, whereas aqueous refers to liquids that contain water as the solvent.
Explanation:
1.Which form of energy is due to the motion of an object's particles .
A. thermal energy, B.chemical energy C.mechanical energy D.electromagnetic energy
Answers
Answer:
thermal
Explanation:
Thermal energy is due to the motion of an object's particles, due to the random movement of molecules in a system, thermal energy also known as random or internal kinetic energy is produced, hence option A is correct.
Atoms or molecules in motion are the source of thermal energy. An object's thermal energy rises as its temperature rises because the movement of its molecules or atoms accelerates. It is possible to transmit an object's thermal energy to another.
Thermal energy, also known as random or internal kinetic energy, is created when molecules in a system move randomly, as a result of the motion of an object's particles.
Learn more about thermal energy, here:
https://brainly.com/question/31631845
#SPJ2
a total of 1.4 moles of sodium nitrate is dissolved in enough water to make 2.0 liters of an aqueous solution. the gram-formula mass of sodim notrate is 85 grams per mole. determine the molarity of the solution
Answers
Answer:
1.4 moles/ 2.0 L= 0.7 M
Explanation: Molarity= moles of solute/ Liters of solution
therefore just plug the numbers in and you'll find the molarity to equal. 0.7
5. Water, wind, ice, and gravity are important agents of ___ which is a destructive force
Answers
What would be the volume, in liters measured at STP, of 0.56 moles of carbon monoxide?
Answers
Answer:
"This conversions relies on the fact that a mole of gas at STP has a volume of 22.4 L. It is important to note, however, that if the conditions of the gas are different this conversion will NOT work. Under those conditions you must use the ideal gas law to convert between moles and liters."