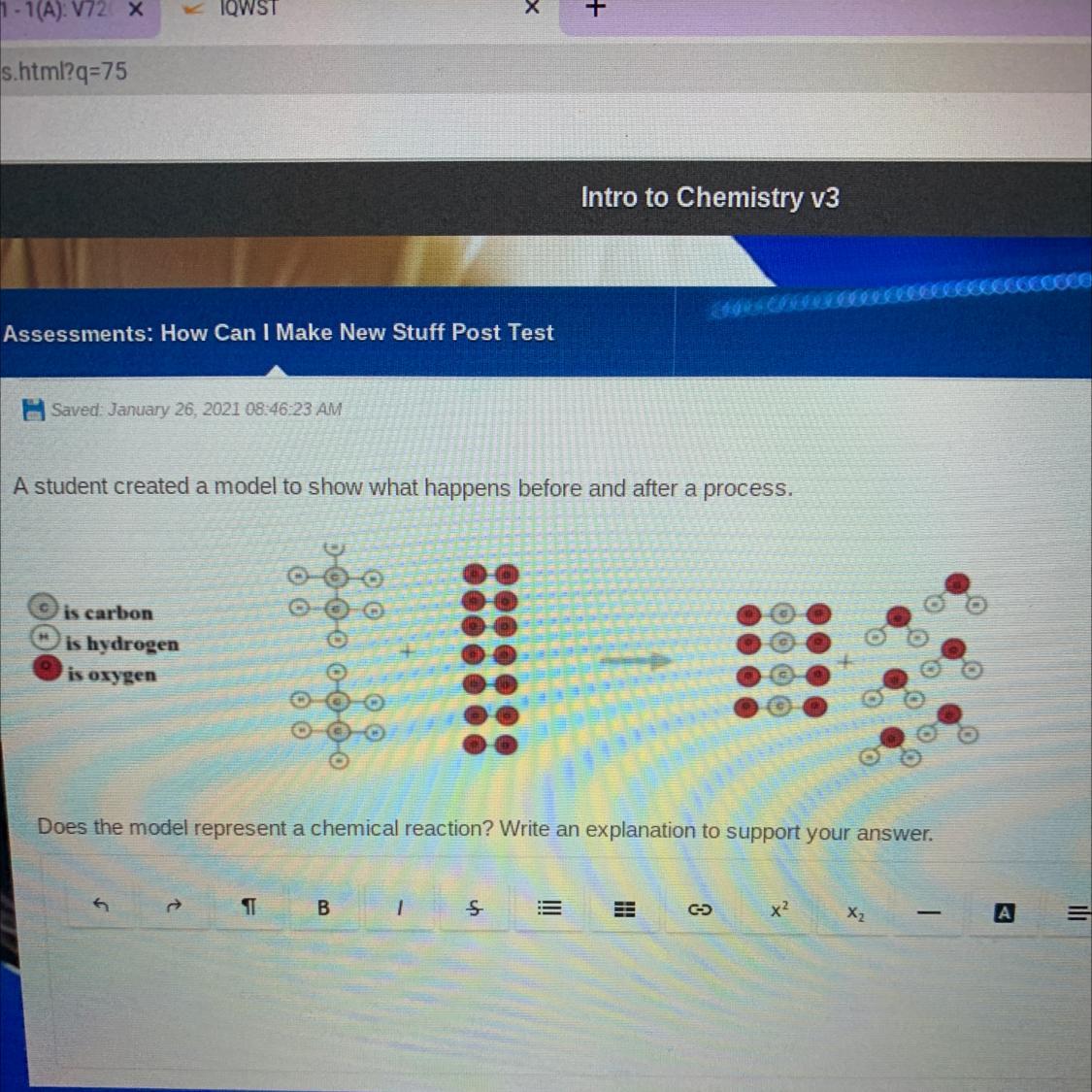
Answers
Answer:
yes it is a chemical reaction
Explanation:
because the substances combined and made something new
Related Questions
how to separate water and cooking oil
Answers
Explanation:
Separatory funnels can be used to separate immiscible liquids. Immiscible liquids are those which won't mix to give a single phase. Oil and water are examples of immiscible liquids. This is because water is more dense than oil, and two layers will thus form. A water layer, and above that, an oil layer.
A separatory funnel has two main components:
A cone shaped glass funnel with hemispherical ends and a neck for a stoppera stopcock at the bottom (a valve which can be opened and closed)A separatory funnel is also usually held above a beaker or flask, with a retort stand and ring clamp.
Consider the separation of water and oil.
Step 1:
The solution is poured into the separatory funnel with the stopcock CLOSED. Due to the immiscible nature and density of the two liquids, they will eventually separate and form two layers.
Step 2:The stopcock is slowly and carefully opened, allowing the bottom layer to flow out. Make sure stopcock is closed JUST before the end of the bottom layer, to ensure there is no contamination of oil with the water in the beaker.
Step 3:Stopcock is opened again, with a new beaker underneath, to allow the little bit of water and a little bit of oil out, to ensure ONLY oil remains in the separatory funnel. Stopcock is closed after this.
Step 4:Stopcock is opened again, with a third clean beaker underneath, to allow ALL of the oil to pass through.
Therefore, you have separated water and oil. See the attached image for a diagram of the setup.
To learn more about the separation of mixtures of immiscible liquids:
https://brainly.com/question/31032868

The following series of reactions were carried out.
PbCO3(s) + 2HNO3(aq) → Pb(NO3)2(aq) + H₂O(1) + CO₂(g)
Pb(NO3)2(aq) + 2HBr(aq) → 2HNO3(aq) + PbBr2(s)
(a) If a student starts with 2.457 g of lead(II) carbonate for the first reaction and all
other reagents are added in excess, what is the theoretical yield of lead(II) bromide
solid?
Answers
First, let's calculate the molar mass of PbCO3:
PbCO3: Pb (207.2 g/mol) + C (12.0 g/mol) + 3O (16.0 g/mol) = 267.2 g/mol
Next, we can calculate the number of moles of PbCO3:
moles = mass / molar mass = 2.457 g / 267.2 g/mol ≈ 0.00919 mol
From the balanced equation, we can see that the mole ratio between PbCO3 and PbBr2 is 1:1. Therefore, the moles of PbBr2 formed will be the same as the moles of PbCO3 used.
The molar mass of PbBr2 is:
PbBr2: Pb (207.2 g/mol) + 2Br (2 × 79.9 g/mol) = 366.0 g/mol
Now, we can calculate the theoretical yield of PbBr2:
theoretical yield = moles of PbBr2 × molar mass of PbBr2
= 0.00919 mol × 366.0 g/mol
≈ 3.36 g
Therefore, the theoretical yield of lead(II) bromide (PbBr2) solid is approximately 3.36 grams.
If the theoretical yield of a reaction is 332.5 g and the percent yield for the reaction is 38 percent, what's the actual yield of product in grams?
A)
126.4 g
B)
8.74 g
C)
116.3 g
D)
12616 g
Answers
Q)A certain mass of gas occupies a volume 2.5 L at 90atm. What pressure would the gas exert if it were placed in a 10 L container at the same temperatur?
Answers
Answer:
23 atm
Explanation:
Step 1: Given data
Initial volume (V₁): 2.5 LInitial pressure (P₁): 90 atmFinal volume (V₂): 10 LFinal pressure (P₂): ?Step 2: Calculate the final pressure of the gas
If we assume constant temperature and ideal behavior, we can calculate the final pressure of the gas using Boyle's law.
P₁ × V₁ = P₂ × V₂
P₂ = P₁ × V₁ / V₂
P₂ = 90 atm × 2.5 L / 10 L = 23 atm
As expected, since the volume increased, the pressure decreased.
What is the density of the marble?
0.22 g/mL
1.67 g/mL
60 g/mL.
460 g/mL

Answers
Explanation:
option B is the correct answer, 1.67g/mL.
hope this helps you.
Who knows how to do this please help

Answers
Answer: You forgot to zero the balance
Explanation:
which 2 criteria are the most important of engineers to consider when developing a procsses to produce
Answers
Two key criteria that engineers must prioritize are efficiency and safety. By emphasizing efficiency and safety during process development, engineers can create robust and reliable processes that not only maximize productivity but also prioritize the well-being of personnel and the environment.
When developing a process, engineers need to consider several important criteria. Two key criteria that engineers must prioritize are efficiency and safety.
Efficiency is crucial in process development to ensure optimal use of resources, time, and energy. Engineers strive to design processes that maximize productivity, minimize waste, and reduce costs. This involves optimizing reaction conditions, streamlining workflow, and implementing automation where possible. Efficiency considerations also extend to energy consumption, raw material utilization, and overall process sustainability.
Safety is another critical aspect that engineers must prioritize. They need to identify and mitigate potential hazards associated with the process, ensuring the safety of both personnel and the environment. This involves conducting thorough risk assessments, implementing safety protocols, and designing equipment and systems with safety features. Engineers must also consider the safe handling and storage of materials, as well as potential risks during transportation and disposal.
By emphasizing efficiency and safety during process development, engineers can create robust and reliable processes that not only maximize productivity but also prioritize the well-being of personnel and the environment.
For more question on environment
https://brainly.com/question/1186120
#SPJ8
Which simple machine is best used to split apart an object
A. A screw
B. A lever
C. A wedge
D. A pulley
Answers
Answer:
its A screw :))) your welcome
HELPPP PLEASEE w/ all

Answers
The covalent bond is present in the compound C₃H₈. The reactant C is 3, product C is 6, reactant H is 8, product H is 10, Reactant O is 2, product O is 9.
What is covalent bond ?
Atoms share electron pair between them in covalent bonds. H-H or C-H are examples of nonpolar covalent bonds between atoms with similar or identical electronegativity, whereas polar covalent bonds are formed when unequal electronegativity is shared between atoms (e.g., H–O).
What is reactant ?
Raw materials known as reactants combine to create products. When the right factors, such as temperature, time, or pressure, come into play, the chemical bonds between the reactants are broken, allowing the atoms to form new bonds that lead to various combinations.
Therefore, covalent bond is present in the compound C₃H₈. The reactant C is 3, product C is 6, reactant H is 8, product H is 10, Reactant O is 2, product O is 9.
Learn more about covalent bond from the given link.
https://brainly.com/question/3447218
#SPJ1
For alkyl halides used in SN1 and SN2 mechanisms, rank the leaving groups in order of reaction rate. You are currently in a ranking module. Turn off browse mode or quick nav, Tab to move, Space or Enter to pick up, Tab to move items between bins, Arrow Keys to change the order of items, Space or Enter to drop.
Answers
Answer:
Iodide> Bromide > chloride > flouride
Explanation:
During a nucleophilic substitution reaction, a nucleophilie replaces another in a molecule.
This process may occur via an ionic mechanism (SN1) or via a concerted mechanism (SN2).
In either case, the ease of departure of the leaving group is determined by the nature of the C-X bond. The stronger the C-X bond, the worse the leaving group will be in nucleophilic substitution. The order of strength of C-X bond is F>Cl>Br>I.
Hence, iodine displays the weakest C-X bond strength and it is thus, a very good leaving group in nucleophillic substitution while fluorine displays a very high C-X bond strength hence it is a bad leaving group in nucleophilic substitution.
Therefore, the ease of the use of halide ions as leaving groups follows the trend; Iodide> Bromide > chloride > flouride
Which statements best describe half lives of radioactive isotopes
Answers
Answer:
The half-life varies depending on the isotope.
Half-lives range from fractions of a second to billions of years.
The half-life of a particular isotope is constant.
Explanation:
Make sure you add the options
Which energy source is a nonrenewable resource?
Answers
Answer:
Fossil fuels.
Explanation:
Non-renewable energy comes from sources that will run out or will not be replenished in our lifetimes—or even in many, many lifetimes. Most non-renewable energy sources are fossil fuels: coal, petroleum, and natural gas. Carbon is the main element in fossil fuels.
Answer:
Fossil fuel
Explanation:
I searched this up since I forgot.
HELP ME PLEASE. I REALLY WANT TO KNOW CHEMISTRY BUT I NEED HELP. SOME KIND SOUL PLEASE.

Answers
The limiting reactant is CH4. This is because the number of moles of CH4 is 4.44mol, while the number of moles of water is 0.86mol. The number of moles of hydrogen produced from the reaction is 8.88mol.
What is hydrogen?Hydrogen is a chemical element with the symbol H and atomic number 1. It is the most abundant element in the universe, making up about 75% of the universe's elemental mass. Hydrogen is a colorless, odorless, and tasteless gas, which is the most basic and simplest of all elements. It is a highly flammable, light, and combustible gas and burns with a pale blue flame. Hydrogen is a component of water, which is composed of two atoms of hydrogen and one atom of oxygen. In addition, it is found in many organic compounds and is used in the production of ammonia, methanol, and hydrochloric acid
This means that there are not enough moles of water to completely react with the CH4. Therefore, the limiting reactant is CH4.
The number of liters of hydrogen produced from the reaction of 80.0 g of CH4 and 16.3 g of water is 16.3 L. This is because the number of moles of hydrogen produced is 8.88mol, and at STP the volume of 1mol of a gas is 22.4L. Therefore, the volume of 8.88mol of hydrogen is 8.88 x 22.4L = 197.3L. Since the total volume of gas produced is 197.3L and 16.3g of water was used in the reaction, the amount of hydrogen produced is 16.3L.
To learn more about hydrogen
https://brainly.com/question/24433860
#SPJ9
pOH of the 0.001M NaOH solution is
Answers
The pOH of the 0.001 M NaOH solution is approximately 3.
To determine the pOH of a solution, we need to know the concentration of hydroxide ions (OH-) in the solution.
In the case of a 0.001 M NaOH solution, we can assume that all of the NaOH dissociates completely in water to form Na+ and OH- ions. Therefore, the concentration of hydroxide ions in the solution is also 0.001 M.
The pOH is calculated using the equation:
pOH = -log[OH-]
Substituting the concentration of hydroxide ions, we have:
pOH = -log(0.001)
Using a calculator, we can evaluate the logarithm:
pOH ≈ 3
Therefore, the pOH of the 0.001 M NaOH solution is approximately 3.
Know more about hydroxide ions here:
https://brainly.com/question/28464162
#SPJ8
(7.12F) Which of the following statement(s) is part of the cell theory?*
O A A cell is the smallest unit of life in living organisms
O B All living things are made of cells
O C All cells come from pre-existing cells
O D All of the above
Answers
Which statements are TRUE of the picture below? Choose 3 statements.
a
KCl dissolves in water because it is an ionic compound and water is a polar solvent.
b
KCl dissolves in water because it is an ionic compound and water is a nonpolar solvent.
c
The negative chloride ions (Cl-) of KCl are pulled into solution by the slightly negative Hydrogen atoms on the water molecule.
d
The negative chloride ions (Cl-) of KCl are pulled into solution by the slightly positive Hydrogen atoms on the water molecule.
e
The positive potassium (K+) ions of KCl are pulled into solution by the slightly negative Oxygen atom on the water molecule.
f
The positive potassium (K+) ions of KCl are pulled into solution by the slightly positive Oxygen atom on the water molecule.
Answers
Answer:
e
The positive potassium (K+) ions of KCl are pulled into solution by the slightly negative Oxygen atom on the water molecule.
Explanation:
I need help with this

Answers
The theoretical yield of water formed from the reaction of 46.8g of octane and 287g of oxygen gas is 58.58 g.
What is a balanced chemical equation?A balanced chemical equation is a representation of a chemical reaction using chemical formulas and symbols. It shows the reactants and products of the reaction, and the coefficients in front of the formulas ensure that the number of atoms of each element is the same on both sides of the equation.
The balanced chemical equation for the reaction between octane and oxygen gas is:
2 C8H18 + 25 O2 → 16 CO2 + 18 H2O
According to the stoichiometry of the balanced equation, 2 moles of octane react with 25 moles of oxygen gas to produce 18 moles of water. Therefore, the molar ratio of octane to water is 2:18 or 1:9.
First, we need to determine which reactant is limiting, i.e., which reactant will be completely consumed in the reaction. To do this, we can calculate the amount of water that would be produced if each reactant were to react completely and then compare the results.
The molar mass of octane (C8H18) is 114.23 g/mol, so 46.8 g of octane is equal to:
46.8 g / 114.23 g/mol = 0.41 mol
The molar mass of oxygen gas (O2) is 32.00 g/mol, so 287 g of oxygen gas is equal to:
287 g / 32.00 g/mol = 8.97 mol
Now, we can calculate the theoretical yield of water based on the amount of each reactant:
Octane: 0.41 mol octane × 9 mol H2O/1 mol octane = 3.69 mol H2O
Oxygen gas: 8.97 mol oxygen gas × 9 mol H2O/25 mol O2 = 3.25 mol H2O
Since the stoichiometry of the reaction indicates that the amount of water produced should be proportional to the amount of octane used, and the calculation shows that less water would be produced if we used all of the oxygen gas, we conclude that oxygen is the limiting reactant.
Therefore, the theoretical yield of water formed is 3.25 mol H2O × 18.02 g/mol = 58.58 g H2O.
To know more about balanced chemical equation, visit:
https://brainly.com/question/28294176
#SPJ1
Calculate the mass in 488 mol of calcium carbonate, CaCO3
Answers
Calcium carbonate is the active ingredient in agricultural lime.
Calcium carbonate is the calcium salt with formula CCaO3. It has a role as an antacid, a food coloring, a food firming agent and a fertilizer and much more. It is a calcium salt, a carbonate salt, a one-carbon compound and an inorganic calcium salt.
Calcium carbonate is used therapeutically as a phosphate buffer.
488 moles of calcium carbonate (CaCO3) can be calculated by using its molecular weight. Calcium carbonate has a molecular weight of 100.0869 g/mol. To calculate the mass of 488 moles, we can multiply the molecular weight by the number of moles:
mass = molecular weight x number of moles
mass = 100.0869 g/mol x 488 mol
mass = 48,842.4072 g
So, the mass of 488 moles of calcium carbonate is approx. 48,842.4072 grams.
To learn more about calcium click here,
https://brainly.com/question/27798376
How many chromosomes do we not understand?
Answers
Answer:
we don't understand why humans have only 46 chromosomes
Answer:
46 chromosomes is what we don't understand
How many grams of solid Na2CO3 are required to neutralize exactly 2 liters of an HCI solution of pH 2.0?
Answers
Answer:
The answer is 1.06g.
Explanation:
Analysis of question:
1. Identify the information in the question given.
volume of HCl is 2 dm3pH of HCl is 2.02. What the question want?
mass of Na2CO3 is ?(unknown)3. Do calculation.1st-Write a balanced chemical equation:Na2CO3 + 2HCl (arrow) 2NaCl + H20 + CO2
2nd-Determine the molarity of HCl with the value of 2.0.pH= -log[H+]
2.0= -log[H+]
log[H+]= -2.0
[H+]= 10 to the power of negative 2(10-2)
=0.01 mol dm-3
molarity of HCl is 0.01 mol dm-3
3rd-Find the number of moles of HCln=MV
=0.01 mol dm-3 × 2 dm3
=0.02 mol of HCl
4th-Find the second mol of it.Based on the chemical equation,
2.0 mol of HCl reacts with 1.0 mol of Na2CO3
0.02 mol of HCl reacts with 0.01 mol of Na2CO3
Na2CO3>a=1 mol
2HCl>b=2 mol
5th-Find the mass of it.mass= number of mole × molar mass
g=0.01 × [2(23)+ 12+ 3(16)]
g=0.01 × 106
# =1.06 g.
moles of each product that would form as a result of the decomposition of aspirin
Answers
The decomposition of aspirin (acetylsalicylic acid,\(C_{9} H_{8} O_{4}\)) can occur through the hydrolysis reaction, resulting in the formation of acetic acid (\(CH_{3} COOH\)) and salicylic acid (\(C_{7} H_{6}O_{3}\)).
The decomposition of aspirin (acetylsalicylic acid, \(C_{9} H_{8} O_{4}\)) can occur through the hydrolysis reaction, resulting in the formation of acetic acid (\(CH_{3} COOH\)) and salicylic acid (\(C_{7} H_{6}O_{3}\)). To determine the moles of each product formed, we need to consider the balanced chemical equation for the reaction:
\(C_{9} H_{8} O_{4} = > C_{7} H_{6}O_{3} +CH_{3} COOH\)
From the equation, we can see that for every 1 mole of aspirin, 1 mole of salicylic acid and 1 mole of acetic acid are produced.
Therefore, the moles of salicylic acid and acetic acid formed will be equal to the number of moles of aspirin that decomposes. If we know the amount of aspirin in moles, we can directly calculate the moles of each product based on stoichiometry.
For more question on aspirin
https://brainly.com/question/25794846
#SPJ8
Which of the following is true for polar jet streams?
They bring cool air.
They bring warm air.
They blow at the equator.
They blow from north to south.
Answers
Answer:
A: They bring cool air.
Explanation:
Took the test and got it correct. :)
Jet streams are fast flowing meandering air currents. Polar jet streams bring cool air through the atmosphere. Polar jets meet subtropical jets at some regions.
What are polar jet streams ?The polar jet stream, which travels through the lower layers of the atmosphere in the Northern Hemisphere, is a swiftly moving band of westerly winds.
The confluence of warm air ascending from the tropics and cold air masses descending from the arctic produces the jet. The denser cold air lowers and deflects the warmer air areas north, creating deep troughs and steep ridges that give the jet stream its wavy appearance.
As pockets of cold air intermittently sneak down from the Arctic, this pattern spreads across the mid-latitudes of North America, Europe, and Asia, producing opposing waves and flows that speed eastward due to the earth's rotation.
Find more on jet streams:
https://brainly.com/question/885071
#SPJ2
Whats the answer 50 points and brainliest if its right

Answers
Answer:
D
Explanation:
When salt water is heated leaving behind solid salt, this is separating a mixture by what method?
A)
chromatography
B)
evaporation
Eliminate
filtration
D)
sifting
Answers
Answer:
option B is correct answer of this question
it is. Evaporation
Answer:
It's filtration!
Explanation:
Filtration is a physical chemical operation that separates solid matter and fluid from a mixture!
succinic acid has a simple formula of C2H3O2 and a molecular weight of 118 gram/mol. what is the molecular formula of succinic acid
A) C4H6O4
B) C2H3O2
C) C8H12O8
D) C3H6O3
Answers
Find Empirical mass.
\(\\ \bullet\sf\longmapsto C_2H_3O_2\)
\(\\ \bullet\sf\longmapsto 2(12u)+3(1u)+2(16u)\)
\(\\ \bullet\sf\longmapsto 24u+3u+32u\)
\(\\ \bullet\sf\longmapsto 59g/mol\)
Now Divide it by Molecular Mass..
\(\\ \bullet\sf\longmapsto \dfrac{118}{59}\)
\(\\ \bullet\sf\longmapsto 2\)
Now molecular formula
\(\\ \bullet\sf\longmapsto 2(C_2H_3O_2)\)
\(\\ \bullet\sf\longmapsto C_4H_6O_4\)
What did scientists infer from Robert Boyle’s Law in terms of kinetic theory?
Answers
Based on the kinetic theory of gases, scientists could infer from Robert Boyle’s Law that an inverse relationship exists between the pressure of gases and their volume if the temperature is kept constant because the decrease in the volume of the vessel containing the gases will result in less room for movement of the gases and hence, the frequency of collisions increases and subsequently the pressure of the gases will increase.
What is the kinetic theory of gases?According to the kinetic theory of gases, a gas is made up of numerous submicroscopic particles (atoms or molecules), all of which are moving randomly and continuously. The container's walls and the quickly moving particles frequently collide in this situation resulting in the gas pressure.
Learn more about the kinetic theory of gases at: https://brainly.com/question/134712
#SPJ1
What would happen if HF were added to water?It would not ionize at allIt would completely ionizeIt would partially ionizeIt would dissolve
Answers
Hydrofluoric acid is a weak acid, which means that it is partially ionized when it is in presence of water.
This means that the correct answer is It would partially ionize.
A 500 mL gas sample is collected over water at a pressure of 740mmHg and 25°C. What is the volume of the dry gas at STP? (STP = 1 atm and 0°C) Vapor pressure at 25° of H2O equals 24mmHg.
Answers
1) List the known and unknown quantities.
Sample: gas.
Volume: 500 mL.
Pressure: 740 mmHg
Temperature: 25 ºC.
Vapor pressure at 25 ºC: 24 mmHg.
2) Pressure of the gas.
\(P_{gas}=P_{atm}-P_{water\text{ }vapor}\)\(P_{gas}=740\text{ }mmHg-24\text{ }mmHg\)\(P_{gas}=716\text{ }mmHg\)The pressure of the gas is 716 mmHg
3) Moles of gas
3.1- List the known quantities.
Volume: 500 mL.
Temperature: 25 ºC.
Pressure: 716 mmHg.
Ideal gas constant: 0.082057 L * atm * K^(-1) * mol^(-1).
3.2- Set the equation.
\(PV=nRT\)3.3- Convert the units of the volume, the temperature, and the pressure.
Volume.
1 L = 1000 mL
\(L=500\text{ }mL*\frac{1\text{ }L}{1000\text{ }mL}=0.500\text{ }L\)Temperature.
\(K=25\text{ }ºC+273.15\text{ }K\)\(K=298.15\text{ }K\)Pressure
1 atm = 760 mmHg
\(atm=716\text{ }mmHg*\frac{1\text{ }atm}{760\text{ }mmHg}=0.942\text{ }atm\)3.4- Plug in the know quantities in the ideal gas equation.
\((0.942\text{ }atm)(0.500\text{ }L)=n*(0.082057\text{ }L*atm*K^{-1}*mol^{-1})(298.15\text{ }K)\)3.5- Solve for n (moles).
Divide both sides by (0.082057 L * atm * K^(-1) * mol^(-1)) * (298.15 K)
\(\frac{(0.942atm)(0.500L)}{(0.082057\text{ }L*atm*K^{-1}mol^{-1})(298.15K)}=\frac{n(0.082057\text{ }L*atm*K^{-1}mol^{-1})(298.15K)}{(0.082057\text{ }L*atm*K^{-1}mol^{-1})(298.15K)}\)\(n=\frac{(0.942atm)(0.500L)}{(0.082057L*atm*K^{-1}*mol^{-1})(298.15K)}=\)\(n=0.0193\text{ }mol\)
4) Dry volume at STP
STP conditions are
Temperature: 273 K
Pressure: 1 atm.
At STP conditions 1 mol of a gas occuppies 22.4 L. We can use this as a conversion factor.
1 mol gas = 22.4 L
\(V=0.0193\text{ }mol\text{ }gas*\frac{22.4\text{ }L}{1\text{ }mol\text{ }gas}=0.432\text{ }L\)The volume of the dry gas at STP is 0.432 L.
.
Answer:
1) List the known and unknown quantities.
Sample: gas.
Volume: 500 mL.
Pressure: 740 mmHg
Temperature: 25 ºC.
Vapor pressure at 25 ºC: 24 mmHg.
2) Pressure of the gas.
The pressure of the gas is 716 mmHg
3) Moles of gas
3.1- List the known quantities.
Volume: 500 mL.
Temperature: 25 ºC.
Pressure: 716 mmHg.
Ideal gas constant: 0.082057 L * atm * K^(-1) * mol^(-1).
3.2- Set the equation.
3.3- Convert the units of the volume, the temperature, and the pressure.
Volume.
1 L = 1000 mL
Temperature.
Pressure
1 atm = 760 mmHg
3.4- Plug in the know quantities in the ideal gas equation.
3.5- Solve for n (moles).
Divide both sides by (0.082057 L * atm * K^(-1) * mol^(-1)) * (298.15 K)
4) Dry volume at STP
STP conditions are
Temperature: 273 K
Pressure: 1 atm.
At STP conditions 1 mol of a gas occuppies 22.4 L. We can use this as a conversion factor.
1 mol gas = 22.4 L
The volume of the dry gas at STP is 0.432 L.
Explanation:
A 57.07 g sample of a substance is initially at 24.3°C. After absorbing of 2911 J of heat, the temperature of the substance is 116.9 CWhat is the specific heat (SH) of the substance?
Answers
Answer:
Approximately \(0.551\; \rm J\cdot kg^{-1} \cdot \left(^\circ\! C \right)^{-1}\).
Explanation:
The specific heat of a material is the amount of energy required to increase unit mass (one gram) of this material by unit temperature (one degree Celsius.)
Calculate the increase in the temperature of this sample:
\(\Delta T = (116.9 - 24.3)\; \rm ^\circ\! C= 92.6\; \rm ^\circ\! C\).
The energy that this sample absorbed should be proportional the increase in its temperature (assuming that no phase change is involved.)
It took \(2911\; \rm J\) of energy to raise the temperature of this sample by \(\Delta T = 92.6\; \rm ^\circ\! C\). Therefore, raising the temperature of this sample by \(1\; \rm ^\circ\! C\) (unit temperature) would take only \(\displaystyle \frac{1}{92.6}\) as much energy. That corresponds to approximately \(31.436\; \rm J\) of energy.
On the other hand, the energy required to raise the temperature of this material by \(1\; \rm ^\circ\! C\) is proportional to the mass of the sample (also assuming no phase change.)
It took approximately \(31.436\; \rm J\) of energy to raise the temperature of \(57.07\; \rm g\) of this material by \(1\; \rm ^\circ C\). Therefore, it would take only \(\displaystyle \frac{1}{57.07}\) as much energy to raise the temperature of \(1\; \rm g\) (unit mass) of this material by \(1\; \rm ^\circ \! C\!\). That corresponds to approximately \(0.551\; \rm J\) of energy.
In other words, it takes approximately \(0.551\; \rm J\) to raise \(1\; \rm g\) (unit mass) of this material by \(1\; \rm ^\circ \! C\). Therefore, by definition, the specific heat of this material would be approximately \(0.551\; \rm J\cdot kg^{-1} \cdot \left(^\circ\! C \right)^{-1}\).
What are all of the mole ratios of MgCl2 -> Mg+ Cl2
Answers
Answer:
2 moles
In words, 1 mole of Mg reacts with 2 moles of HCl to form 1 mole of MgCl₂ and 1 mole of H₂. The molar ratio of HCl to MgCl₂ is 2:1.