Enter the electron configuration for I+ using noble gas shorthand notation.
In the first box enter the noble gas (notice the brackets). In the following boxes enter the number that goes in front of the orbital followed by the superscript.
For example, the electron configuration for sulfur is: [Ne]3s2 3p4
so the first box would have Ne in it followed by 3, then 2, then 3 then 4.
If you do not need an orbital, just enter 0 (zero) in the boxes for the coefficient and superscript.
[ ] s f d p
Find the element in the periodic table and count over
to the right the number of negative charges on your anion.
This element has the same electron configuration as your anion.
Which noble gas precedes the element? Knowing the s, p, d, and f blocks
of elements in the periodic table, deduce the the electron configuration
of the element from the preceding noble gas. Remember that the
1p 1d, 2d, 1f, 2f, and 3f orbitals are forbidden energy levels (they do not exist).
Answers
Answer:
[Kr] 4d10 5s2 5p4
Explanation:
The Symbol I represents Iodine. It has atomic number of 53. The full electronic configuration is given as;
1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p5
However the question requested for the configuration of I+.
I+ is a cation and it simply refers to an iodine atom that has lost a single electron. The electronic configuration of I+ is given as;
1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p4
Using Noble gas shorthand representation, we have;
[Kr] 4d10 5s2 5p4
Related Questions
An airplane flew 3043 km from Houston to Seattle in 5.5 hours. What was the average speed, in m/s rounded to the nearest hundredth, of the airplane from Houston to Seattle?
Answers
An airplane flew 3043 km from Houston to Seattle in 5.5 hours. What was the average speed, in m/s rounded to the nearest hundredth, of the airplane from Houston to Seattle?
The answer is 553
Answer:
553
Explanation:i did this question today and it was right!
how many grams of granite at 54.1 c must be added to 467.62 of water at 21 C to make the final temp of both come out to be 29.2 C? Specific heat of granite 0.79 J/g C, specific heat of water 4.184 J/g C
Answers
Answer:
815.59
Explanation:
Assuming no heat lost to the Surrounding
Total heat lost by granite = Total heat gained by water
mc∆T of granite = mc∆T of water
m x 0.79 x (54.1 - 29.2) = 467.62 x 4.184 x (29.2 - 21.0)
m x 19.671 = 16043.48106
m = 16043.48106/19.671
= 815.59J
You measure the following dimensions of a rectangular metal block of metal A: length =13.0; cm; width = 6.4cm; height = 8.2cm It has a mass of 1287 gWhat is the density of metal in g/cm^ 3 ?

Answers
The density of the rectangular block of metal in g/cm³ is 1.87 g/cm³
How to calculate density?Density is a measure of the mass of matter contained by a unit volume. It can be calculated by dividing the mass of the substance by its volume as follows;
Density = mass ÷ volume
According to this question, the dimensions of a rectangular metal block of metal A are as follows: length = 13.0cm; width = 6.4cm; height = 8.2cm. If it has a mass of 1287g, the density can be calculated as follows;
Volume = 13 × 6.4 × 8.2 = 682.24cm³
Density = 1287g ÷ 682.24cm³
Density = 1.87 g/cm³
Learn more about density at: https://brainly.com/question/28858363
#SPJ1
The temperature of 150 mL of water is 25°C. What must happen for the temperature of the
water to increase to 35°C?
A. Thermal energy must be transferred to the water to increase the average kinetic
energy of the water molecules.
B. Thermal energy must be transferred from the water to decrease the average
kinetic energy of the water molecules.
C. Water must be removed to decrease the thermal energy.
D. More water must be added to increase the thermal energy.
Answers
Answer:D
Explanation:
Water is a _____ molecule, which gives it many of its unique properties, including its ability as a universal solvent.
Answers
Answer:
The answer is Polar.
Explanation:
Because polar has many unique properties.
Select the correct terms to complete this statement about charged particles.
Like charges attract | repel, and opposite charges attract repel. According to Coulomb's law, as the distance between two charged particles decreases, the force between the particles decreases I increases. As the magnitude of the charges decreases, the force decreases | increases.
Answers
Like charges repel each other, while opposite charges attract each other. This principle is one of the fundamental aspects of electrostatics. According to Coulomb's law, the force between two charged particles is directly proportional to the product of their charges and inversely proportional to the square of the distance between them.
As the distance between two charged particles decreases, the force between them increases. This is because the closer the particles are, the stronger the electric field they create, leading to a stronger force of interaction.
On the other hand, as the magnitude of the charges decreases, the force between the particles also decreases. This is because the force is directly proportional to the product of the charges. If one or both of the charges are smaller, the force they exert on each other will be weaker.
In summary, according to Coulomb's law, decreasing the distance between charged particles increases the force between them, while decreasing the magnitude of the charges decreases the force. This understanding of the relationship between charge, distance, and force is crucial in explaining the behavior of charged particles and the interactions between them.
Know more about Coulomb's law here:
https://brainly.com/question/26892767
#SPJ8
Explain two positive aspects of using methane recapture systems.
Answers
Answer:
Two positive aspects of using methane recapture systems are able to generate significant electricity. Another benefit is that the process of anaerobic digestion creates heat that can be used to warm buildings where animals are kept
Answer: The correct answer is;
Two positive aspects of using methane recapture systems include lowering the impact on greenhouse gasses and the production of energy. Methane is a very potent greenhouse gas that is contributing to global warming. As a result, the recapturing process reduces the methane impacts of global warming by reclaiming and reusing the gas for other purposes. Recaptured methane can be stored and used to generate electricity or used as fuel to power updated vehicles and other engines on the farm. The overall benefits from this combination are reducing impacts causing global warming and lower the cost of electricity or fuel on the farm.
Explanation: This answer has been confirmed correct.
What is the median reaction of second end point in HCL and NaOH titration
Answers
The median reaction at the second end point in the HCl and NaOH titration is: HCl + NaOH → NaCl + H2O
In a titration between hydrochloric acid (HCl) and sodium hydroxide (NaOH), the reaction involved is the neutralization reaction between an acid and a base. The balanced equation for this reaction is:
HCl + NaOH → NaCl + H2O
In this reaction, one mole of HCl reacts with one mole of NaOH to form one mole of NaCl (sodium chloride) and one mole of water.
During the titration process, the reaction occurs gradually as the base is added to the acid solution.
The first end point of the titration is reached when the moles of HCl and NaOH are stoichiometrically equivalent, meaning they react in a 1:1 ratio. At this point, all the HCl has been neutralized by the NaOH, and no excess of either reagent remains.
However, if the titration is continued beyond the first end point, the reaction between HCl and NaOH can still occur, albeit in a different ratio.
The second end point refers to the point where the moles of NaOH added exceed the stoichiometrically required amount to neutralize the HCl completely. As a result, any excess NaOH added after the second end point reacts with the excess HCl in a 1:1 ratio.
Therefore, the median reaction at the second end point in the HCl and NaOH titration is:
HCl + NaOH → NaCl + H2O
For more such question on median reaction visit:
https://brainly.com/question/14189499
#SPJ8
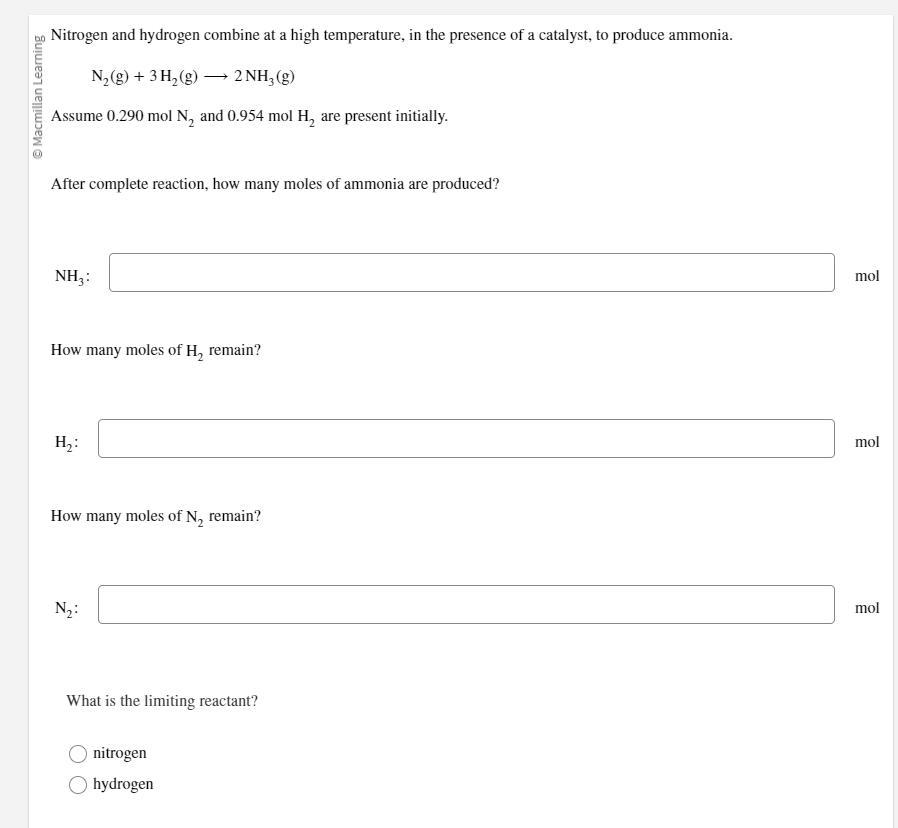
Nitrogen and hydrogen combine at a high temperature, in the presence of a catalyst, to produce ammonia.
N2(g)+3H2(g)⟶2NH3(g)
Assume 0.290 mol N2 and 0.954 mol H2 are present initially.
After complete reaction, how many moles of ammonia are produced?
Answers
Answer:
17 reactions
Explanation:
The measure of the length of events and the duration of intervals between events
Answers
The measure of the length of events and the duration of intervals between events is time.
What is time?The duration of events or the gaps between them can be measured, compared, or even ordered using time. The lengthy period of time that the Earth's geologic history takes up is known as geologic time. Starting at the beginning of the Archean Eon formal geologic time runs until the present. Geology is defined as the "Science of the Earth."
Geology is the fundamental Earth science that examines how the earth created, its structure and composition, and the various forces acting on it. It is sometimes known as geoscience or earth science.
Learn more about time at;
https://brainly.com/question/479532
#SPJ1
Calculate the isomer number for 3-chlrobutan-2-ol and 4-chloropentane -2, 3-diol??
Answers
There are 288 possible isomers for 3-chlorobutan-2-ol.
There are 7200 possible isomers for 4-chloropentane-2,3-diol.
3-chlorobutan-2-ol:
Total number of isomers = number of possible arrangements of carbon atoms x number of possible arrangements of other atoms
= 24 x 12
= 288
Therefore, there are 288 possible isomers for 3-chlorobutan-2-ol.
4-chloropentane-2,3-diol:
Total number of isomers = number of possible arrangements of carbon atoms x number of possible arrangements of other atoms
= 120 x 60
= 7200
Therefore, there are 7200 possible isomers for 4-chloropentane-2,3-diol.
Isomers are molecules with the same molecular formula (the same amount and types of atoms) but different atomic arrangements in space. This means that despite having the same molecular formula, isomers have distinct physical and chemical properties.
learn more about isomer here
https://brainly.com/question/26298707
#SPJ9
glucose is a six carbon sugar. Albumin is a protein with 607 amino acids. the average molecular weight of a single amino acid is 135 g/mol. there is no reason to run these solutes at the 20 MWCO because
Answers
There is no reason to run these solutes at the 20 MWCO because they are both much smaller than the MWCO of the membrane.
The MWCO (molecular weight cut off) is the molecular weight of a solute at which it will be retained by a membrane during a process such as ultrafiltration or dialysis. If a solute has a molecular weight higher than the MWCO of a membrane, it will be retained and not pass through the membrane. If the molecular weight of a solute is lower than the MWCO, it will pass through the membrane.
In this case, glucose has a molecular weight of 180 g/mol (6 carbons x 12 g/mol per carbon + 6 oxygens x 16 g/mol per oxygen) and albumin has a molecular weight of approximately 81,942 g/mol (607 amino acids x 135 g/mol per amino acid). Both of these solutes have molecular weights that are much lower than 20,000 g/mol, which is a typical MWCO for ultrafiltration or dialysis membranes.
They would both easily pass through the membrane and be lost during the process. Instead, a membrane with a much lower MWCO would be needed if we wanted to retain these solutes during a process such as ultrafiltration or dialysis.
Learn more about glucose here:
https://brainly.com/question/2396657
#SPJ1
The elevated ridges of the brain are
called the
are patches of gray matter that
regulate skeletal muscle movement.
is the gray matter is located in the
outermost region of the brain
involved with integration.
--
The are large fiber tracts that
allow the two hemispheres to
communicate with each other.
--
[Choose]
[Choose]
[Choose]
[Choose]
<
>
Answers
The elevated ridges of the brain are called gyri. Motor cortex are patches of gray matter that regulate skeletal muscle movement.
Cerebral cortex is the gray matter is located in the outermost region of the brain involved with integration.
Corpus callosum are large fiber tracts that allow the two hemispheres to communicate with each other.
How to explain the informationThe elevated ridges of the brain are called the gyri. Motor cortex are patches of gray matter that regulate skeletal muscle movement.
Cerebral cortex is the gray matter is located in the outermost region of the brain involved with integration. Corpus callosum are large fiber tracts that allow the two hemispheres to communicate with each other.
Learn more about brain on
https://brainly.com/question/1247675
#SPJ1
Please help
Calcium carbonate can undergo a chemical reaction to produc calcium oxide (CaO) and carbon dioxide (CO₂). Which possible masses of calcium oxide and carbon dioxide can form when 400g of calcium carbonate undergoes this chemical reaction?
*
1 point
400g CaO and 0g CO₂
224g CaO and 176g CO₂
40g CaO and 10g CO₂
The atomic mass of the element neon (Ne) is 20.180 amu. Which measurement is correct?
1 point
1 gram Ne = 20.180 moles = 1.215×1025 atoms
1 gram Ne = 20.180 moles = 6.022×1023 atoms
1 mole Ne = 20.180 grams = 1.215×1025 atoms
1 mole Ne = 20.180 grams = 6.022×1023 atoms
When reactants of a reaction are known, which fact can always be deduced about the products?
*
1 point
the state of the products
the number of atoms in the products
the number of products
the mass of each product
The atomic mass of carbon (C) is 12.01amu and oxygen (O) is 16.00amu. Which molar mass is correct for carbon dioxide (CO₂)?
*
1 point
28.01 g/mol
44.01 g/mol
192.16 g/mol
268.01 g/mol
How many moles is in 43g of CO₂?
*
1 point
1204 mol
1.54 mol
2.59 x 10²⁵
According to the law of conservation of matter, which statement best describes a balanced chemical equation?
1 point
The number of moles of atoms of each element must be the same in the reactants and in the products.
The total number of moles of atoms must be the same in the reactants and in the products.
The total number of molecules must be the same in the reactants and in the products.
The number of moles of each molecule must be the same in the reactants and in the products.
Nitrogen gas combines with hydrogen gas to produce ammonia (N₂+3H₂⟶Ammonia). Based on the law of conservation of mass, which could be the product of the reaction?
*
1 point
NH₂
NH₃
2NH₃
3NH₂
How do you calculate the number of moles from mass?
*
1 point
it is the product of moles and molar mass
it is the quotient of mass and molar mass
it is the product of mass and molar mass
it is the quotient of avogadro's number and molar mass
Sugar reacts with oxygen gas to produce carbon dioxide and water (C₆H₁₂O₆ + 6O₂⟶6CO₂+6H₂O). Which ratio of components is correct?
*
1 point
For every 12 moles of CO₂ produced, 2 moles of C₆H₁₂O₆ is needed.
For each mole of O₂, 1 mole of C₆H₁₂O₆ is needed.
For every 5 moles of water produced, 6 moles of oxygen is needed.
What is the molar mass of NH₄Cl?
*
1 point
53.5 g/mol
50.5 g/mol
497.0 g/mol
Answers
The possible masses of calcium oxide and carbon dioxide that can form when 400g of calcium carbonate undergoes decomposition is
224g CaO and 176g CO₂; option CThe correct measurement of the element neon (Ne):
1 mole Ne = 20.180 grams = 6.022 × 10²³ atomsWhen reactants of a reaction are known, the fact that can always be deduced about the products is:
the number of products; option CThe correct molar mass correct for carbon dioxide (CO₂) is 44.01 g/mol; option B
The number of moles in 43 g of CO₂ is 0,977 moles.
According to the law of conservation of matter, the statement that best describes a balanced chemical equation:
The number of moles of atoms of each element must be the same in the reactants and in the products.
Based on the law of conservation of mass, the product of the reaction of nitrogen gas and hydrogen is 2 NH₃.
To calculate the number of moles from mass, take the quotient of mass and molar mass; option B
The ratio of components in the reaction of sugar with oxygen gas to produce carbon dioxide and water is:
For every 12 moles of CO₂ produced, 2 moles of C₆H₁₂O₆ is needed; option A
The molar mass of NH₄Cl is 53.5 g/mol; option A.
What is the molar mass of a substance?The molar mass of a substance is the mass of one mole of the substance.
The molar mass of carbon dioxide (CO₂) is calculated below:
molar mass of carbon dioxide, CO₂ = 12 + 16 * 2
molar mass of carbon dioxide, CO₂ = 44.0 g/mol
The moles of CO₂ in 43g of CO₂ is calculated as follows:
moles = mass / molar mass
Moles of CO₂ = 43 / 44 = 0.977 moles
The mass of CaO and CO₂ from the decomposition of 400 g of calcium carbonate is given below:
1 moles of CaCO₃ produces 1 mole of CaO and CO₂
4 moles of CaCO₃ produces 4 moles of CaO and CO₂
Mass of 4 moles of CaO = 4 * 56 = 224 g
Mass of 4 moles of CO₂ = 4 * 44 = 176 g
Learn more about molar mass at: https://brainly.com/question/837939
#SPJ1
which is the odd one out out of these individual questions?
Nitrous oxide
Carbon dioxide
Air
Oxygen
Carbon monoxide
Helium
Nitrous oxide
Carbon dioxide
Air
Salt (Sodium chloride)
Sodium metal
Sodium bicarbonate (baking soda)
Answers
1. Air
Others are chemical compounds, air is a mixture.
2. Carbon monoxide
Others are elements, carbon monoxide is compound.
3. Air
Others are compounds, air is not.
4. Sodium metal
Sodium metal is element, others are compounds.
ch3cl would you expect the following compound to have a dipole moment? if the molecule has a dipole moment, specify its direction. select the single best answer. ch3cl the dipole moment is oriented from the h atoms towards the cl atom. the dipole moment is oriented from the cl atom towards the h atoms. one specific direction of the dipole moment does not exist. the molecule has no dipole moment. ch3cl
Answers
CH3Cl would have a dipole moment, oriented from the H atoms towards the Cl atom.
This is because the Cl atom is more electronegative than the H atoms, so the electrons in the covalent bond between them will be pulled more towards the Cl atom. This creates an uneven distribution of charge and a net dipole moment.
CH3Cl, also known as chloromethane or methyl chloride, is a colorless, flammable gas with a sweet odor. It is a hydrocarbon, meaning it consists of hydrogen and carbon atoms, and is classified as a haloalkane due to the presence of a chlorine atom. CH3Cl is used in a variety of industrial processes, including as a refrigerant and a propellant. It is also used to produce other compounds, such as acetic acid and methylene chloride.
Learn more about CH3Cl:
https://brainly.com/question/30265607
#SPJ4
Bailey swam 0.86 km, biked
for 22.4 km, and ran 4.25 km.
How many meters did she
travel total?
A: 2,751
B: 7,350
C: 21,428
D: 27,510
Answers
Answer:
2,751 Meters.
Explanation:
0.86 + 22.4 + 4.25 = 27.51 km.
Convert that to meters which gives you 2,751 meters.
1) In the nuclear equation below, what does the letter X represent? Show your work.

Answers
how do kinetic energy, particle motion, and particle attraction help explain the differences in the forms of bromine?
Answers
Answer: The particles can move apart only if they have enough kinetic energy to overcome this force of attraction. If particles do not have enough kinetic energy to overcome the force of attraction between them, matter exists as a solid. What is particle kinetic energy? Kinetic energy is energy that an object has because of its motion. The Kinetic Molecular Theory explains the forces between molecules and the energy that they possess.
How do Earth’s plates able to move?
Answers
Answer:
Plates at our planet's surface move because of the intense heat in the Earth's core
Explanation:
Hopefully this helps, I believe in you! ^^
Answer/Explanation:
movement can be cause by the intense heat in the earth's core, which causes molten rock in the mantle layer to move. it moves in a pattern called 'convection cell' which can form when the warm material rises, then cools, and eventually sink down. then the process repeats (cooled, warms up and rises.. etc..)
I hope this was what you were kinda looking for:) - T
PS. HAVE A GOOD DAY! I LIKEYOUR PROFILE HAFHAS
How many moles of hydrogen
are in 3.06 x 10^-3 g of glycine C2H5NO2
Answers
Answer:
2.04x10⁻⁴ mol
Explanation:
First we convert 3.06x10⁻³ grams of glycine into moles of glycine, using its molar mass:
3.06x10⁻³ g ÷ 75 g/mol = 4.08x10⁻⁵ mol C₂H₅NO₂In order to calculate the number of hydrogen moles, we multiply the number of glycine moles by 5, as there are 5 hydrogen moles per glycine mol:
4.08x10⁻⁵ mol C₂H₅NO₂ * 5 = 2.04x10⁻⁴ mol HPlease help me!!!!!!

Answers
Answer:
17.8ml-15.6ml is 2.2 ml then convert to cm so 2.2cm^3
combustion always result in to formation of water. what other type of reactions may result into formation of water? examples of these reactions
Answers
As combustion always result into the formation of water, the other type of reactions that may result into formation of water are Acid-Base Neutralization Reactions and Hydrogen and Oxygen Reaction.
Acid-Base Neutralization Reactions:
A neutralisation reaction is a chemical process in which an acid and a base combine to produce salt and water as the end products.
H⁺ ions and OH⁻ ions combine to generate water during a neutralisation reaction. Acid-base neutralisation is the most common type of neutralisation reaction.
Example: Formation of Sodium Chloride (Common Salt):
HCl + NaOH → NaCl + H₂O
Hydrogen and Oxygen Reaction:
Water vapour is created when hydrogen gas (H₂) and oxygen gas (O₂) are combined directly. This reaction produces a lot of heat and releases a lot of energy.
Example: 2 H₂ + O₂ → 2 H₂O
Learn more about reactions:
https://brainly.com/question/25769000
Adding energy to solid water will turn it into ?
Answers
Answer:
Energy added to solid water will turn it into liquid water; add energy into liquid water and it will be turned into water vapor.
Explanation:
Adding energy is basically adding heat; the more heat, the more excited the molecules of H2O gets. In solid water, the molecules aren't really moving because they don't have a lot of energy, so it is solid. In liquid water (which is water in room temperature), it has a medium amount of energy; the molecules aren't stuck together but it isn't completely dispersed, so it is in liquid form. However, in water vapor, the energy becomes very high and the molecules are excited. The hydrogen bonds holding the molecules together break and the water is released as a vapor.
Pls help me with this it’s so difficult

Answers
pH level is measured on different scales and they range from 0 to 14.
A pH level of 7 is neutral as it is considered to be neither acidic or basic. However, a pH value of less than 7 means it is more acidic, and a pH value of more than 7 means it is more basic.
What is pH?This refers to the level of acid/base that can be found in a solution, usually water.
Therefore, for example, the higher the concentration of OH- in a solution, the more basic the solution is and there is a reversible reaction for H+ and OH-
Hence, we can see that your question is incomplete so a general overview was given to help you better understand the concept.
Read more about pH levels here:
https://brainly.com/question/940314
#SPJ1
2. Which state of matter is characterized by particles that are close to each other but are not arranged in a definite pattern?
A)liquid
B)plasma
C)solid
D)gas
Answers
Answer:
Solid
Explanation:
Cus its solid, take a brick for example. It's hard and has no space unlike liquid or gas.
Select True or False: The entropy of a perfectly ordered crystalline substance at 0 K is 0 J/mol. Bloom's Level: Understand
Answers
True, the entropy of a perfectly ordered crystalline substance at 0 K is 0 J/mol.
The third law of thermodynamics states that, the entropy of a system approaches a constant value as the temperature of the system approaches absolute zero.
The entropy of any perfectly ordered, crystalline substance at absolute zero temperature is zero. That is, the entropy of a pure, perfect crystalline substance at 0 K is 0 J/K.
Thus, we can conclude that the given statement is true. The entropy of a perfectly ordered crystalline substance at 0 K is 0 J/mol.
Learn more about third law of thermodynamics here: https://brainly.com/question/1156831
Bromine gas in a container is heated over a flame. What happens to the average kinetic energy of the bromine
particles?
Olt decreases rapidly.
It increases quickly.
O It remains the same.
O It decreases slowly.
Answers
Answer:
The answer is b
Explanation:
I got my answer off of quizlet
Answer:
B) It increases quickly.
Explanation:
Edg 2020
Which statement describes the law of conservation of energy? A. All systems will exchange matter and energy with their surroundings. B. All systems can exchange energy, but not matter, with their surroundings. C. Energy cannot be created nor destroyed, but it changes from one form to another. D. Energy is destroyed in most chemical reactions when new products are formed.
\\\\\
Answers
Answer:
C, energy cannot be created nor destroyed.
Explanation:
Many scientists think that this single event caused the extinction of most the dinosaurs which led to the rise of mammals which geologic principle is this an example of
A. Geographism
B. Gradualism
C. Catastrophism
D. Uniformitarianism
Answers
Answer:
C. Catastrophism
Explanation: