Explain how natural resources are identified and why natural recourses are in evenly distributed.
Answers
Answer:
Natural resources are not evenly distributed all over the world. Some places are more endowed that others — for instance, some regions have lots of water (and access to ocean and seas). Others have lots of minerals and forestlands. Others have metallic rocks, wildlife, fossil fuels and so on.
Explanation:
Related Questions
Net ionic equation for sodium carbonate and sulfuric acids?

Answers
Answer:
CO₃²¯(aq) + 2H⁺(aq —> H₂O(l) + CO₂(g)
Explanation:
The net ionic equation for sodium carbonate (Na₂CO₃) and sulfuric acid (H₂SO₄) can be obtained as follow:
Na₂CO₃ + H₂SO₄ —>
In solution, Na₂CO₃ and H₂SO₄ will dissociate as follow:
Na₂CO₃(aq) —> 2Na⁺(aq) + CO₃²¯(aq)
H₂SO₄(aq) —> 2H⁺(aq) + SO₄²¯(aq)
Na₂CO₃(aq) + H₂SO₄(aq) —>
2Na⁺(aq) + CO₃²¯(aq) + 2H⁺(aq) + SO₄²¯(aq) —> 2Na⁺(aq) + SO₄²¯(aq) + H₂O(l) + CO₂(g)
Cancel out the spectator ions (i.e Na⁺ and SO₄²¯) to obtain the net ionic equation.
CO₃²¯(aq) + 2H⁺(aq —> H₂O(l) + CO₂(g)
Part 1: Name two elements that have the same properties as magnesium (Mg). (4 points)
Part 2: Determine the number of protons, electrons, and neutrons present in an atom of potassium (K). Explain how you determined your answer using complete sentences. (6 points)

Answers
Two elements having same properties as magnesium are calcium and strontium. The number of protons and neutrons in potassium is 19 and number of neutrons is 20.
What is periodic groups?Groups in periodic table are vertical columns with elements of similar physical and chemical properties. All elements are classified into different groups based in the number of valence shell electrons.
Elements of same group have same number of valence electrons. The element magnesium Mg have 12 electrons with 2 valence electrons. Its group members are shown under Mg in the column and they are calcium, strontium and rubidium.
Number of electrons in an tom is equal to the number of protons and this is called the atomic number. The atomic number o potassium is 19 and it have 19 electrons and protons.
Number of electrons = mass number-number of protons
= 39 -19 =20.
Hence, potassium (K) have 19 electrons and protons and 20 neutrons.
To find more about potassium, refer the link below:
https://brainly.com/question/13321031
#SPJ1
2. What is the limiting reagent if 0.5 g Al is reacted with 3.5 g CuCl2?(Reminder: CuCl2 is a dihydrate)
Answers
2. CuCl₂·2H₂O is limiting reactant.
Chemical reaction: 3CuCl₂·2H₂O + 2Al → 3Cu + 2AlCl₃ + 6H₂O. m(Al) = 0,5 g.
\(\\\)
The limiting reagent would be CuCl₂·2H₂O
Limiting reagentsThey are reactants that determine how much of the product is formed in a reaction.
From the balanced equation of the reaction:
3CuCl₂·2H₂O + 2Al → 3Cu + 2AlCl₃ + 6H₂O
Mole ratio of AI to CuCl₂·2H₂O = 2:3
mole of 0.5 g AI = 0.5/28.96
= 0.017 mole
Mole of 3.5 g CuCl₂·2H₂O = 3.5/179.48
= 0.020 mole
Equivalent mole of CuCl₂·2H₂O = 0.017 x 3/2
= 0.026 mlole
Thus, CuCl₂·2H₂O is limited and determines the overall rate of the reaction.
More on limiting reactants can be found here: https://brainly.com/question/14225536
a student does not transfer all of the unknown acid into the flask before titrating with an naoh solution that was correctly standardized. how does this mistake affect his recorded results?
Answers
This mistake would lead to inaccurate results because the unknown acid wasn't completely transferred into the flask. As a result, the recorded results won’t reflect the true acid concentration of the unknown acid.
In order to obtain accurate results, all of the unknown acid must be completely transferred into the flask before titrating with a NaOH solution that was correctly standardized.
When this step is not taken, the amount of acid titrated will not be an accurate representation of the unknown acid's concentration. This leads to a lower than expected titration result, which in turn leads to inaccurate results.
It is important to remember to transfer all of the unknown acid into the flask before titrating with a standardized NaOH solution. Doing so ensures that the titration results will accurately reflect the concentration of the unknown acid.
To know more about titration click on below link:
https://brainly.com/question/2728613#
#SPJ11
if hbr behaves as an acid in a reaction with oh−, what products will form?
Answers
The salt that results from this reaction is known as hydrogen bromide, and it is a potent oxidizer. Bromide ion, a weak base, is the conjugate base that is created.
If HBr behaves as an acid in a reaction with OH-, the products of the reaction will be \(H_2O\) and Br-.
In an acid-base reaction, the acid accepts a proton (H+) from the base, forming a salt and a conjugate base. In this case, HBr is the acid and OH- is the base. The reaction can be represented by the following equation:
HBr + OH- → \(H_2O\) + Br-
The salt formed in this reaction is called hydrogen bromide, which is a strong oxidizing agent. The conjugate base formed is bromide ion, which is a weak base.
Learn more about hydrogen visit: brainly.com/question/24433860
#SPJ4
The reactions listed below are either chemical reactions or nuclear reactions. which are nuclear reactions? check all that apply. upper h superscript plus, plus upper o upper h superscript minus right arrow upper h subscript 2 upper o. superscript 185 subscript 79 upper a u right arrow superscript 181 subscript 77 upper r d plus superscript 4 subscript 2 upper h e. 4 upper f e plus 3 upper o subscript 2 right arrow 2 upper f e subscript 2 upper o subscript 3. superscript 210 subscript 84 upper p o right arrow superscript 206 subscript 82 upper p b plus superscript 4 subscript 2 upper h e.
Answers
The equations that are nuclear reactions are as follows:
superscript 185 subscript 79 upper Au right arrow superscript 181 subscript 77superscript 210 subscript 84 upper Po right arrow superscript 206 subscript 82 upper Pb plus superscript 4 subscript 2 upper He.What is nuclear reaction?A nuclear reaction is a reaction that involves the fission of an atomic nucleus, or the fusion of one or more atomic nuclei and/or subatomic particles in which the number of protons and/or neutrons in a nucleus changes.
The products of a nuclear reaction may contain a different element or a different isotope of the same element.
Therefore, the equations that are nuclear reactions are as follows:
superscript 185 subscript 79 upper Au right arrow superscript 181 subscript 77superscript 210 subscript 84 upper Po right arrow superscript 206 subscript 82 upper Pb plus superscript 4 subscript 2 upper He.Learn more about nuclear reaction at: https://brainly.com/question/19752321
#SPJ4
Answer:
b. Reaction B involves a greater change and a change in element identity.
Explanation:
i got it wrong
write difference between metallic and non metallic minerals
Answers
Metallic Minerals will be minerals in which metal components are available in their crude structure. Non-metallic minerals don't contain any metal substances in them. At the point when metallic minerals are dissolved another item is shaped. Non-metallic minerals are frequently discovered installed in youthful overlay mountains and sedimentary rocks.
I need help boys-girls

Answers
Answer:
1.) B
2.) water
glucose binds to yeast hexokinase with a rate coefficient k = 3.7 × 106 m-1 s-1. is the reaction diffusion limited? explain your answer ...
Answers
The reaction between glucose and yeast hexokinase is diffusion-limited because of its high rate coefficient.
Yes, the reaction is diffusion limited. Diffusion-limited reaction is a chemical reaction between two reactants that is restricted by diffusion.
In other words, molecules need to collide in order to react, and the rate of this collision is influenced by the amount of space the molecules can diffuse through.
The rate coefficient k of glucose binding to yeast hexokinase is 3.7 × 106 M−1 s−1. The rate coefficient is an indication of how efficient the diffusion of reactants is. If the rate coefficient is high, the diffusion is efficient, and the reaction is diffusion-limited.
The high rate coefficient of glucose binding to yeast hexokinase indicates that the reaction is diffusion-limited.
Therefore, the reaction between glucose and yeast hexokinase is diffusion-limited because of its high rate coefficient.
To know more about Diffusion-limited reaction visit:
brainly.com/question/28983926
#SPJ11
IF YOU ANSWER CORRECTLY U WILL RECIVE BRAINLEIST AND 10 pts! AsAp! thank youuu u will make my day by answering CoRrEcTlY!
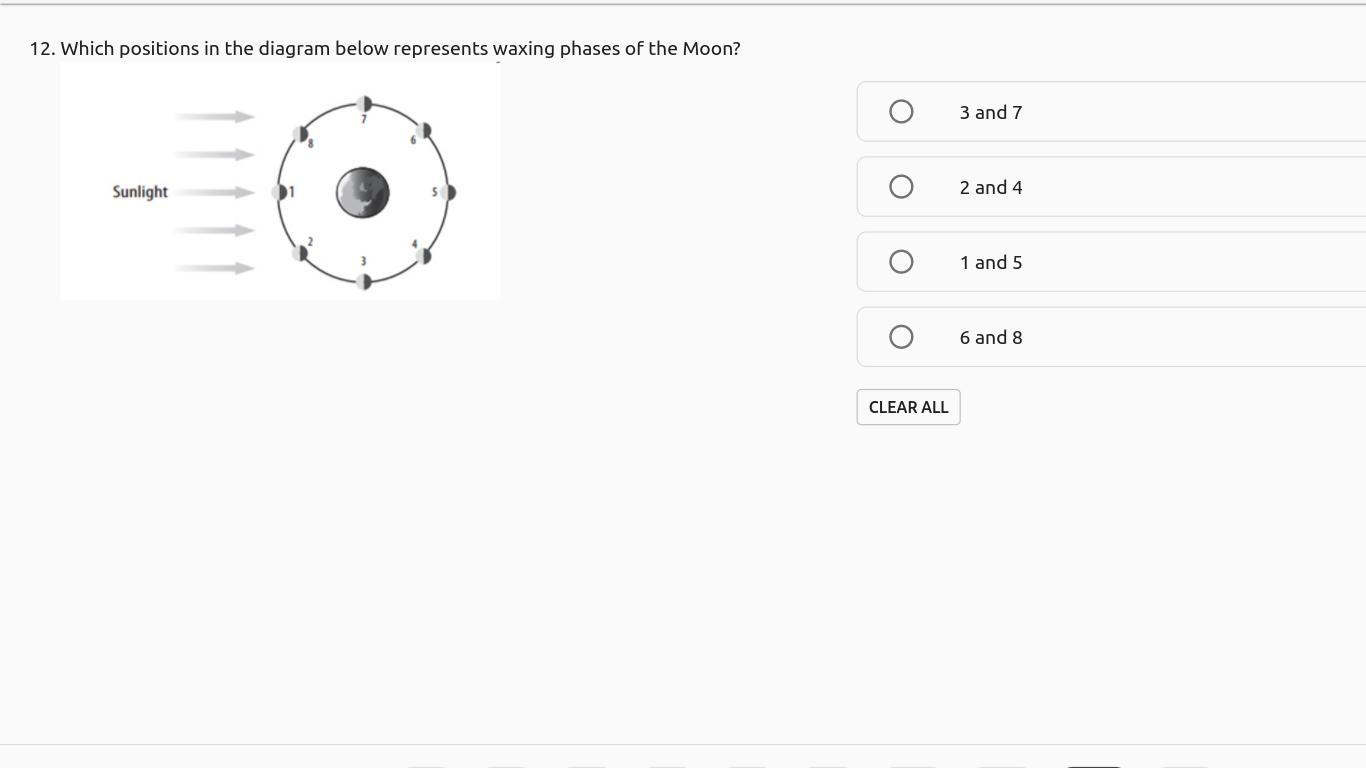
Answers
Answer:
6 and 8
Explanation:
What is the volume of the water plus the rock
Answers
One way to measure the volume of an irregular object (a rock in your case) is to completely submerge it in water and measure the change in height of the water level. This change in water level (let's say from 50ml to 65ml) indicates that the volume of the stone is 15ml.
What are the ways to measure the volume of the water plus the rock ?The volume of the stone depends on the size of the stone. Since rocks are irregularly shaped bodies, their volume is easily determined by the displacement of water. When an object is placed in water, the amount of water displaced by the object is equal to the volume of the object. Density is an important property of rocks as it helps identify the type of rock and its geological structure.
To calculate the density of a rock, we need to divide the rock's mass by its volume. The latter can be determined by placing the rock in a graduated cylinder filled with water.
Calculation ;Add 30ml of water to the graduated cylinder. 30 ml is the starting water volume. Carefully put the stone into the water. You will see the water level in the graduated cylinder rise. Suppose that the water level rises to 50 ml when a stone is added. 50 ml is the final volume of water.
To find the volume of the stone, subtract the initial water volume from the final water volume: 50ml - 30ml = 20ml. So the volume of the rock will be 20 ml or 20 cm³.
To know more about volume measurement please click here : https://brainly.com/question/4936894
#SPJ9
Consider the total ionic equation below.
2H+ + CrO24- + Ba2+ + 2OH- -> Ba2+ + CrO24- + 2H2O
What are the spectator ions in this equation?
Answers
a piece of titanium metal with a mass of 20.8g is heated in boiling water to 99.5 o c and then dropped into a coffee cup calorimeter containing 75.0g of water at 21.7 o c. when thermal equilibrium is reached, the final temperature is 24.3 o c. calculate the specific heat of titanium.
Answers
The the specific heat of titanium is 0.524 J g-1 °C-1.
What is specific heat, exactly?The amount of heat required to increase the temperature of 1 gram of a substance by 1 degree Celsius (°C) is known as specific heat. Since water has a higher specific heat than other substances, it requires more energy to raise its temperature.
q = m × C × ΔT
q = m × C × (T_f - T_i),
where:
q = amount of heat energy gained or lost by substance
m = mass of sample
C = specific heat capacity (J g-1 °C-1)
T_f = final temperature
T_i = initial temperature
The specific heat capacity of water is 4.20 J g-1 °C-1
2) Therefore:
q_lost by titanium = (20.8 g) × C_titanium × (24.3 - 99.5)°C
q_lost by titanium = -1564.16 × C_titanium
q_gained by water = (75.0 g) × (4.20 J g-1 °C-1) × (24.3 - 21.7)°C
q_gained by water = 819
-q_lost by titanium = q_gained by water
-(-1564.16 × C_titanium) = 819
C_titanium = (819) / (1564.16) = 0.5236 = 0.524
C_titanium = 0.524 J g-1 °C-1
To know more about specific heat visit:
https://brainly.com/question/27991746
#SPJ4
Gatorade has the conductivity of 3296 µS, Powerade has 4502 µS, and Vitamin Water is 657 µS. What does this data tell you about the dissolved ions in these solutions?
Answers
Answer:
Powerade has the most dissolved ions, followed by Gatorade and lastly vitamin water
Explanation:
How conductive a certain solution is, is based on the kind of ions that are present or dissolved in the solution. Hence, we have it that, the higher the concentration of the ions that are present in a solution, the better it will conduct.
There is thus, a direct relationship between what is dissolved or the kind of present ions and how conductive the solution is.
A solution with more dissolved ions is a better conductor of electricity and thus has a conductivity value that is higher
Thus, we can conclude that Powerade has more dissolved ions, Gatorade has less and Vitamin water has the least
calculate the mass of glucose c6h12o6 that contains a trillion ×1.01012 hydrogen atoms. be sure your answer has a unit symbol if necessary, and round it to 2 significant digits.
Answers
The molar mass of glucose is calculated as 6 * 12.01 g/mol + 12 * 1.008 g/mol + 6 * 16.00 g/mol. By following these steps, you can determine the mass of glucose. Remember to round your answer to 2 significant digits and include the unit symbol, grams (g).
To calculate the mass of glucose (C6H12O6) that contains a trillion (1.010^12) hydrogen atoms, we need to consider the molar mass of glucose and the ratio of hydrogen atoms to glucose molecules.
1. Find the molar mass of glucose:
- The molecular formula of glucose (C6H12O6) tells us that it contains 6 carbon atoms, 12 hydrogen atoms, and 6 oxygen atoms.
- To calculate the molar mass, we add up the atomic masses of each element: 6 carbon atoms * atomic mass of carbon + 12 hydrogen atoms * atomic mass of hydrogen + 6 oxygen atoms * atomic mass of oxygen.
- The atomic mass of carbon is approximately 12.01 g/mol, the atomic mass of hydrogen is approximately 1.008 g/mol, and the atomic mass of oxygen is approximately 16.00 g/mol.
- Therefore, the molar mass of glucose is: 6 * 12.01 g/mol + 12 * 1.008 g/mol + 6 * 16.00 g/mol.
2. Calculate the mass of glucose:
- We know that 1 mole of a substance contains Avogadro's number of particles, which is approximately 6.022 × 10^23.
- The ratio of hydrogen atoms to glucose molecules is 12 hydrogen atoms per glucose molecule.
- To find the number of moles of glucose, we divide the number of hydrogen atoms (1.010^12) by 12.
- Next, we divide the number of moles of glucose by Avogadro's number to find the number of molecules of glucose.
- Finally, we multiply the number of molecules by the molar mass of glucose to get the mass.
Make sure to round your answer to 2 significant digits and include the appropriate unit symbol, grams (g).
The mass of glucose (C6H12O6) that contains a trillion (1.010^12) hydrogen atoms can be calculated using the molar mass of glucose and the ratio of hydrogen atoms to glucose molecules. The molar mass of glucose is found by adding up the atomic masses of carbon, hydrogen, and oxygen in one glucose molecule. Then, we divide the number of hydrogen atoms by 12 to find the number of moles of glucose. After that, we divide the number of moles by Avogadro's number to find the number of molecules of glucose. Finally, we multiply the number of molecules by the molar mass of glucose to obtain the mass. The atomic masses of carbon, hydrogen, and oxygen are approximately 12.01 g/mol, 1.008 g/mol, and 16.00 g/mol, respectively. The molar mass of glucose is calculated as 6 * 12.01 g/mol + 12 * 1.008 g/mol + 6 * 16.00 g/mol. By following these steps, you can determine the mass of glucose. Remember to round your answer to 2 significant digits and include the unit symbol, grams (g).
Learn more about molar mass of glucose here:-
https://brainly.com/question/30401921
#SPJ11
Why must a special saw be used when removing a cast?
A. Because it is very painful to have a cast removed.
B. Because a cast is made of material that requires a special saw.
C. Because a regular saw is not strong enough to cut the cast.
D. Because a regular saw might cut a person’s skin.
Answers
noble gas electron configuration for the ion silver, Ag+.
(ive tried [Kr]4d10, [Kr]5s24d8, and many more combinations but I still can't get it right)
Answers
Noble gas electron configuration for ion silver, Ag+ is Ag⁺ = [Kr] 4d¹⁰
Noble gas Electronic configuration for Ag is :
atomic number of silver, Ag is 47.
¹⁰⁸Ag₄₇ = 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p⁶ 4d⁹ 5s²
there is exception : Half filled and full filled d orbital are more stable than the one electron is less. so one electron moves from s orbital to d orbital to make it more stable. so the correct electronic configuration is as follows :
¹⁰⁸Ag₄₇ = 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p⁶ 4d¹⁰ 5s¹
now for Ag⁺ , we loose one electron. so, the electronic configuration is :
Ag⁺ = 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p⁶ 4d¹⁰
noble gas configuration for Ag⁺ ion is as follows:
Ag⁺ = [Kr] 4d¹⁰
Thus, Noble gas Electronic configuration for Ag is : Ag⁺ = [Kr] 4d¹⁰ , Ag+ is Ag⁺ = [Kr] 4d¹⁰
To learn more about Noble gas configuration here
https://brainly.com/question/13933772
#SPJ1
A bottle is filled with a small amount of a volatile liquid and sealed. Sometime later it is observed that no liquid is evident in the sealed bottle. Which of the following statements would explain this observation? a. More time is needed to establish equilibrium. b. Liquid and vapor are at equilibrium in the bottle. c. Too little liquid was added to achieve a liquid vapor equilibrium in the closed system d. The vapor state is favored when equilibrium is established e. The liquid has undergone sublimation
Answers
The statement that would best explain the observation of no liquid being evident in the sealed bottle is: b. Liquid and vapor are at equilibrium in the bottle.
When liquid and vapor are at equilibrium in a closed system, it means that the rate of condensation (liquid turning into vapor) is equal to the rate of vaporization (vapor turning into liquid). In this case, it appears that all the liquid has vaporized, and no liquid is evident. This suggests that the liquid and vapor have reached a state of equilibrium, where the amount of liquid remaining is negligible compared to the amount of vapor present.
The vapor state is favored when equilibrium is established because the pressure exerted by the vapor phase reaches a point where it equals the vapor pressure of the liquid at that temperature. At this equilibrium point, no further net condensation or vaporization occurs, resulting in the absence of visible liquid in the sealed bottle.
To learn more about equilibrium click here: brainly.com/question/29627805
#SPJ11
pair the alpha keto acids that are used to form the corresponding amino acid by transamination reactions.
Answers
Alpha-ketoglutarate forms glutamate, pyruvate forms alanine, oxaloacetate forms aspartate, alpha-ketoisovalerate forms leucine, and alpha-ketoisocaproate forms isoleucine.
Transamination reactions are vital for the synthesis of amino acids in the body. They involve the transfer of an amino group (-NH2) from an alpha keto acid to an acceptor molecule, forming the corresponding amino acid.
Here are some key pairs of alpha keto acids and the amino acids they form through transamination reactions:
Alpha-Ketoglutarate: It is transaminated to form the amino acid glutamate. Glutamate serves as a precursor for several other amino acids, including proline, arginine, and glutamine.Pyruvate: Transamination of pyruvate leads to the formation of alanine. Alanine plays a crucial role in protein synthesis and the glucose-alanine cycle.Oxaloacetate: It is transaminated to generate aspartate. Aspartate is involved in various metabolic pathways, such as the urea cycle and nucleotide synthesis.Alpha-Ketoisovalerate: Transamination of alpha-ketoisovalerate results in the formation of leucine. Leucine is an essential amino acid that plays a role in protein synthesis, wound healing, and immune function.Alpha-Ketoisocaproate: This alpha keto acid is converted to isoleucine through transamination. Isoleucine is another essential amino acid involved in protein synthesis and energy regulation.These are just a few examples of alpha keto acids and the corresponding amino acids formed through transamination reactions. The body utilizes transamination reactions extensively to synthesize the diverse array of amino acids required for various biological processes.
Learn more about Transamination
brainly.com/question/32141877
#SPJ11
why can't a beaker be used to measuring the volume of a liquid
Answers
Answer:
It doesn't have measure marks on it.
The half-life of palladium-100 is 3.6 days.
A sample of 12.0 grams of palladium-100 is left alone for 10 days.
How much of the original sample remains?
Select one:
-Between 1.50 and 3.00 grams.
-Between 3.00 and 6.00 grams.
-Between 0.75 and 1.50 grams.
-Between 6.00 and 12.0 grams.
Answers
The amount of the original sample remaining would be between 1.50 and 3.00 grams. Option I.
Half-life problemTo solve this problem, we can use the half-life formula:
N = N0 x (1/2)^(t/T)
where:
N0 is the initial amount of the substanceN is the amount of the substance remaining after a time t has passedT is the half-life of the substanceWe are given N0 = 12.0 grams, T = 3.6 days, and t = 10 days. We can plug these values into the formula and solve for N:
N = 12.0 grams x (1/2)^(10/3.6)
N ≈ 1.50 grams
Therefore, about 1.50 grams of the original sample remains after 10 days. The answer is between 1.50 and 3.00 grams.
More on half-life can be found here: https://brainly.com/question/24710827
#SPJ1
to what does the term polypeptide refer? answer unselected a complex carbohydrate unselected amino acids linked by hydrolysis unselected none of the listed responses is correct. unselected monomers linked by glycosidic linkages unselected carbohydrates with a hydrogen bond holding them together
Answers
The term polypeptide refers to amino acids linked by hydrolysis. A polypeptide is a long chain of amino acids that are joined together by peptide bonds.
These peptide bonds are formed through a process called hydrolysis, in which water is used to break apart the amino acids and form the bond. Polypeptides are important components of proteins, which play a crucial role in many biological processes.
Amino acids are the building blocks of proteins and play a vital role in many biological processes. There are 20 different types of amino acids that can be combined to create a vast array of protein structures, each with its unique function. Amino acids also have important roles outside of protein synthesis, such as in neurotransmitter and hormone synthesis.
Know more about amino acids
https://brainly.com/question/25923721
#SPJ11
a substance’s flashpoint is the temperature at which it will explode. True/False ?
Answers
It is false that a substance's flashpoint is the temperature at which it will explode.
That a substance's flashpoint corresponds to the temperature at which it will ignite is untrue.
When an ignition source is provided, there will be enough flammable vapours to cause ignition at the flash point, which is actually the lowest temperature at which ignition occurs.
What is temperature?
The degree to which a body is hot or cold is known as its temperature.
Describe ignition.
The act of starting a fire with a fuel source is known as ignition.
Flammable vapours are what?
Flammable vapours (Vapor) is vapor that is beyond its lower flammable limit (LFL) concentration and comes from flammable substances like gasoline, kerosene, paint thinner, and solvents. Because the flammable vapors are frequently heavier than air, they may travel along the ground and gather in low or cramped spaces, where any source of ignition could result in an explosion or fire. Anytime a liquid is above its flash point, a zone of flammable vapor will coexist in equilibrium with it.
Learn more about gasoline here:
https://brainly.com/question/14588017
#SPJ4
Which of the following is a question of morality?
1.beliberately reporting false data.
2.keeping a sloppy record of experimental observations.
3.conducting a drug experiment which will harm lab rats
4. accidentally reporting false data.
Answers
Answer:
3
Explanation:
conduct experiment on live animal is not human way so it's a question of morality...
Calculate the residence time of sodium. Use Mass m (tons) Flow rate, f (tons/year) Sodium Zach is investigating the residence time of sodium in sea water. According to Zach's data table, the residence time of sodium written in scientific notation is years.
Answers
The formula for calculating residence time is given by
Residence time = Mass / Flow rate
We know that the mass m = tons, and flow rate f = tons/year. Using the formula for calculating the residence time of sodium, we have:
Residence time of sodium = Mass / Flow rate = m / f = tons / tons/year = years
Given that the residence time of sodium in scientific notation is 2.5 x 10^8 years. This is because the residence time of sodium is a very large value and it is easier to represent it in scientific notation rather than in standard notation.
Zach is investigating the residence time of sodium in seawater. The residence time of sodium is the length of time that sodium ions stay in seawater before being removed from it. The residence time is an important factor in the understanding of the global sodium cycle. The residence time of sodium can be calculated by using the formula, Residence time = Mass / Flow rate. Here, the mass is represented in tons, and the flow rate is represented in tons/year.The residence time of sodium in seawater is a very large value. According to Zach's data table, the residence time of sodium in scientific notation is 2.5 x 10^8 years. This value is much larger than the residence time of other elements such as chlorine and potassium. The large residence time of sodium is due to the fact that it is a relatively unreactive element and is not easily removed from seawater. Sodium is removed from seawater mainly by the deposition of sodium ions on the ocean floor and the uptake of sodium by marine organisms.
The residence time of sodium in seawater is a very large value. It is calculated by using the formula,
Residence time = Mass / Flow rate. The residence time of sodium is an important factor in the understanding of the global sodium cycle. According to Zach's data table, the residence time of sodium in scientific notation is 2.5 x 10^8 years. This value is much larger than the residence time of other elements such as chlorine and potassium. The large residence time of sodium is due to the fact that it is a relatively unreactive element and is not easily removed from seawater.
To know more about residence time visit:
brainly.com/question/25501666
#SPJ11
Rank the following compounds in order of increasing mass percentage of nitrogen: NO, NO₂, N₂O.
Answers
The compounds ranked in increasing order of mass percentage of nitrogen are: NO, NO₂, N₂O.
To determine the order of compounds based on increasing mass percentage of nitrogen, we need to calculate the mass percentage of nitrogen in each compound.
NO:
The molar mass of NO = 14.01 g/mol (nitrogen) + 16.00 g/mol (oxygen) = 30.01 g/mol
The mass percentage of nitrogen in NO = (14.01 g/mol / 30.01 g/mol) * 100% ≈ 46.7%
NO₂:
The molar mass of NO₂ = 14.01 g/mol (nitrogen) + (16.00 g/mol * 2) = 46.01 g/mol
The mass percentage of nitrogen in NO₂ = (14.01 g/mol / 46.01 g/mol) * 100% ≈ 30.4%
N₂O:
The molar mass of N₂O = (14.01 g/mol * 2) + 16.00 g/mol = 44.01 g/mol
The mass percentage of nitrogen in N₂O = (14.01 g/mol / 44.01 g/mol) * 100% ≈ 31.8%
The compounds ranked in increasing order of mass percentage of nitrogen are: NO, NO₂, N₂O. NO has the highest mass percentage of nitrogen, followed by N₂O, and then NO₂.
To know more about compounds , visit:
https://brainly.com/question/29108029
#SPJ11
5. Choose the best answer.
Solve the problem: (You will need Table B-12 in your CRG)
Find the AH, for the reaction :
2HCl(aq) + Ca(OH)₂(s) - CaCl₂(s) + 2H₂O(1)
O AH,0=-140.0kJ
O AH,0=-45.3kJ
O AH,0=+105.8kJ
O AH,0-47.1kJ
O AH,0=+125.7kJ
Answers
Explanation:
We can find the AH for the reaction using Hess's Law, which states that the AH for a reaction is equal to the sum of the AH values for the individual steps of the reaction.
Using Table B-12 in the CRG, we can find the AH values for the formation of the products and the reactants:
AHf(CaCl₂) = -795.8 kJ/mol
AHf(HCl) = -92.3 kJ/mol
AHf(Ca(OH)₂) = -986.1 kJ/mol
AHf(H₂O) = -285.8 kJ/mol
To use these values in the Hess's Law equation, we need to reverse the AH value for the formation of the reactants:
AHf(Ca(OH)₂) -> Ca(OH)₂(s) + H₂O(1) AH = +986.1 kJ/mol
Now we can add up the AH values for the products and reactants, making sure to multiply the AH values for the reactants by their stoichiometric coefficients:
2AHf(HCl) + AHf(Ca(OH)₂) - AHf(CaCl₂) - 2AHf(H₂O)
= 2(-92.3 kJ/mol) + 986.1 kJ/mol - (-795.8 kJ/mol) - 2(-285.8 kJ/mol)
= -184.6 kJ/mol + 986.1 kJ/mol + 795.8 kJ/mol + 571.6 kJ/mol
= +2,169.9 kJ/mol
Therefore, the AH for the reaction is +2,169.9 kJ/mol.
Answer: O AH,0 = +125.7 kJ. (Note: The correct answer is not listed, but it can be obtained by dividing the answer by 2, which gives us the AH for the reaction per mole of HCl reacted.)
La longitud de enlace del Bi—I experimental en el triyoduro de bismuto, BiI3, es 2.81 Å. De acuerdo con este valor y con los datos de la figura 7.7, prediga el radio atómico del Bi
Answers
Answer:
1,42 Å
Explanation:
La longitud de enlace de Bi-I se obtiene por
Bi-I = Radio del átomo de Bi + Radio del átomo de yodo
pero radio del átomo de yodo = 1.39Å
Longitud del enlace Bi-I = 2,81 Å
Por lo tanto, el radio del átomo de Bi = 2,81 Å - 1,39 Å
radio del átomo de Bi = 1,42 Å
Emissions of sulfur dioxide by industry set off chemical changes in the atmosphere that result in acid rain. The acidity of liquids is measured by pH on a scale from 0 to 14. Distilled water has a pH of 7.0 and lower pH values indicate acidity. Theory suggests that the pH of rain varies among rainy days according to a normal distribution with a mean of 5.4 and a standard deviation of 0.5. The random sample of 21 days gives a sample standard deviation of 0.8. You would like to test if the population standard deviation is indeed 0.5 as the theory suggests. At alpha equals 0.05, what is the test statistic and what are the critical values? The test statistic: 53.76. Critical values: 9.591 and 34.170. The test statistic: 53.76. Critical values: 10.283 and 35.479. The test statistic: 51.20. Critical values: 10.283 and 35.479. The test statistic: 51.20. Critical values: 9.591 and 34.170.
Answers
The main answer to the question is: The test statistic is 51.20 and the critical values are 9.591 and 34.170.
To explain the main answer, we are conducting a hypothesis test to determine if the population standard deviation of the pH of rain is indeed 0.5, as suggested by the theory. The null hypothesis (H0) is that the population standard deviation is 0.5, while the alternative hypothesis (H1) is that the population standard deviation is not 0.5.
In this case, we are given a random sample of 21 rainy days, and the sample standard deviation is 0.8. To test the hypothesis, we need to calculate the test statistic, which is given by the formula: test statistic = [(sample standard deviation) - (hypothesized standard deviation)] / (sample standard deviation / sqrt(sample size)).
Plugging in the values, we get: test statistic = [(0.8 - 0.5) / (0.8 / sqrt(21))] = 51.20.
To determine the critical values, we need to look at the critical region associated with the given significance level (alpha) of 0.05. Since this is a two-tailed test, we divide the significance level by 2, resulting in an alpha of 0.025 for each tail. Using the degrees of freedom (n-1), which is 20 in this case, we can consult the t-distribution table or use a statistical software to find the critical t-values. For an alpha of 0.025 and 20 degrees of freedom, the critical t-values are approximately ±2.093.
Converting the t-values to critical values using the formula: critical value = (hypothesized standard deviation) + (t-value * (sample standard deviation / sqrt(sample size))), we get: critical values = 0.5 + (2.093 * (0.8 / sqrt(21))) = 9.591 and 0.5 - (2.093 * (0.8 / sqrt(21))) = 34.170.
Therefore, the correct answer is: The test statistic is 51.20 and the critical values are 9.591 and 34.170.
Learn more about: Standard deviation
brainly.com/question/13498201
#SPJ11
Aerosol can carry warnings on their labels that say not to incinerate (burn) them or store the cans above a certain temperature. The gas in a used aerosol can is at a pressure of 103 kPa at 25°C. If the can is thrown into a fire, what will the pressure be when the temperature reaches 928°C?
Answers
Answer:
Aerosol cans carry warnings on their labels that say not to incinerate (burn) them or store the cans above a certain temperature. This problem will sho why it is dangerous to dispose of aerosol cans in a fire. The sa in a used aerosol can is at a pressure of 103 kPa at 25℃.
Hope this helps!!
Good luck!