Explain how Wegener came up with the Theory of Continental Drift and why it is considered to be a theory.
Answers
Answer:
Wegener thought all the continents were once joined together in an "Urkontinent" before breaking up and drifting to their current positions. For instance, Wegener thought the continents might have plowed through the ocean crust like icebreakers smashing through ice.
Related Questions
If you triple the force on an object , the acceleration will
a
reduced by 1/3
b
double
c
tripled
d
halve
Answers
Answer:
C triple
Explanation:
please crown me
A food company claims that 1 out of 5 boxes of its breakfast cereal contain a prize. Trevor created a spinner with five
equal-size sections to simulate this situation. One section was labeled "Prize," and the remaining sections were labeled
"No Prize. " To conduct the simulation, Trevor spun the spinner and recorded the outcome.
Which outcome simulates the claim made by the food company?
A The spinner landed on "Prize" 30 times and on "No Prize" 120 times.
B The spinner landed on "Prize" 75 times and on "No Prize" 15 times.
© The spinner landed on "Prize" 30 times and on "No Prize" 150 times.
0 The spinner landed on "Prize" 40 times and on "No Prize" 10 times.
Answers
Outcome B, where the spinner landed on "Prize" 75 times and on "No Prize" 15 times, simulates the claim made by the food company.
The claim made by the food company states that 1 out of 5 boxes of its breakfast cereal contains a prize. In other words, the probability of getting a prize is 1/5 or 0.2. To simulate this claim, we need the spinner to land on the "Prize" section approximately 1 out of 5 times. In Outcome B, the spinner landed on "Prize" 75 times and on "No Prize" 15 times. To determine the probability of landing on "Prize," we divide the number of times the spinner landed on "Prize" by the total number of spins:
Probability of landing on "Prize" = 75 / (75 + 15) = 75 / 90 ≈ 0.833
The probability of landing on "Prize" in Outcome B is approximately 0.833, which is greater than the desired probability of 0.2. Therefore, Outcome B does not accurately simulate the claim made by the food company. None of the given outcomes accurately simulate the claim made by the food company.
learn more about probability here:
https://brainly.com/question/32117953
#SPJ11
please help me i really am struggling

Answers
Answer:
It is visible light
Explanation:
Which proportionality applies to Avogadro's law?
O V 1/P
O V T
O P T
O V n
Answers
Avogadro’s law is written as V1/n1=V2/n2 or Vn=k.
Hope this helped!
V n is the proportionality that applies to Avogadro's law. Therefore, the correct option is option D.
What is Avogadro's law?According to Avogadro's law, equal volumes of various gases each contain an equal amount of molecules at the same temperature and pressure. On the presumption of an ideal (perfect) gas, this experimental relation can be deduced first from kinetic theory of gases. For actual gases, the law is roughly applicable when temperatures and pressures are both low enough.
Avogadro's number, or even the Avogadro constant, is the precise quantity of molecules inside one gram-mole of such a material, defined as that of the molecular weight metric grams, which is equal to 6.022×10²³.
Avogadro’s law
V1/n1=V2/n2 or Vn=k.
Therefore, the correct option is option D.
To know more about Avogadro's law, here:
https://brainly.com/question/4133756
#SPJ5
For the following word equations, write it as a chemical equation, then balance it.
a) potassium + oxygen gas ------ potassium oxide
Answers
4K+O2-----------2K2O

How many lone pairs are there in the Lewis structure CO2?
Answers
This implies that the CO2 molecule does not include any lone pairs.
What is Lewis structure of CO2?According to the Lewis structure of CO2, which stands for carbon dioxide, there are two double bonds between each oxygen atom and the carbon atom. The arrangement of the atoms and the distribution of electrons within a molecule are represented by the Lewis structure of the molecule.
Why does CO2 molecules not include lone pairs?The core carbon atom in the Lewis structure of CO2 is surrounded by two oxygen atoms.
Each link in the Lewis structure is represented by a pair of dots, and the quantity of valence electrons on each atom is specified. Each oxygen atom in CO2 possesses six valence electrons, compared to the four valence electrons on the carbon atom.
Each link in the Lewis structure is represented by a pair of dots, and the quantity of valence electrons on each atom is specified. Each oxygen atom in CO2 possesses six valence electrons, compared to the four valence electrons on the carbon atom.
There are no additional electrons that may be referred to as "lone pairs" in a double bond because two pairs of electrons are shared between two atoms.
To know more about lone pairs, visit:
https://brainly.com/question/30194336
#SPJ4
.
Where are molecular compounds usually found?
Answers
Answer:A molecule is composed of two elements. In general, the elements that combine to form binary molecular compounds are both nonmetals.
This contrasts with ionic compounds, which usually involve bonds between metal ions and nonmetal ions.
Explanation:
Calculate the mass of hydrogen produced when 72 g of magnesium
reacts with sulfuric acid.
Answers
Since this is a single replacement reaction, the equation for the reaction is:
\(\text{Mg}+\text{H}_{2}\text{SO}_{4} \longrightarrow \text{MgSO}_{4}+\text{H}_{2}\)
From this, we know that for every mole of magnesium consumed, 1 mole of hydrogen is produced.
The atomic mass of magnesium is 24.305 g/mol, so 72 grams of magnesium is 72/24.305 = 2.9623534252211 moles.
This means we need to find the mass of 2.9623534252211 moles of hydrogen.
Hydrogen has an atomic mass of 1.00794 g/mol, so doubling this to get the formula mass of of \(\text{H}_{2}\), we get 2.01588 g/mol, which his a mass of:
(2.01588)(2.9623534252211). which is about 5.97 g
What are the current and or future uses of genetically modified strawberries
Answers
In the future, genetically modified strawberries may become more widely available if they pass regulatory approvals and are deemed safe for consumption. They could potentially provide benefits such as reduced pesticide use, longer shelf life, and improved nutrition. However, there are also concerns about the environmental impact and potential health risks associated with genetically modified crops, which will need to be addressed before they can be widely adopted.
which of the following require oxygen to grow? group of answer choices facultative anaerobes aerobes anaerobes all of the above
Answers
Aerobes are organisms that require oxygen to grow and survive. The correct answer is: aerobes.
They have metabolic pathways that depend on the presence of oxygen for efficient energy production through aerobic respiration. Without oxygen, aerobes cannot carry out their metabolic processes effectively.
Facultative anaerobes, on the other hand, can grow and survive in the presence or absence of oxygen. They have the ability to switch between aerobic and anaerobic metabolic pathways depending on the availability of oxygen. In the presence of oxygen, facultative anaerobes can utilize aerobic respiration, and in the absence of oxygen, they can switch to anaerobic fermentation.
Anaerobes are organisms that do not require oxygen for growth and can even be inhibited or killed by its presence. They have metabolic pathways that allow them to carry out fermentation or other anaerobic processes for energy production.
Therefore, the correct answer is aerobes, as they specifically require oxygen to grow.
Learn more about Aerobes from the link given below.
https://brainly.com/question/29615844
#SPJ4
According to Valence Shell Electron Pair Repulsion (VSEPR) theory, which statement is correct? If a molecule has four pairs of electrons, it will always have a tetrahedral structure. If a molecule is made up of three atoms, it will always have a linear structure. If a molecule is bent, it will always have unpaired electrons around the central atom. If a molecule has three atoms around a central atom, it will always be trigonal planar.
Answers
Answer: Option 1
Explanation: hopes it helps
Valence Shell Electron Pair Repulsion (VSEPR) theory is employed in prophesying the geometrical organisation of the specific molecule with the aid of electron sets found encompassing the atom.
The correct answer is:
Option A. If a molecule has four pairs of electrons, it will always have a tetrahedral structure.
This can be explained as:
To reduce the electron repellence atom having four pairs of electrons will always accommodate to a tetrahedral structure.An atom comprising four pairs of electrons will have no lone pairs and hence, tetrahedral geometry.An example of tetrahedral geometry is methane (CH4).Therefore atom containing four pairs of electrons will have a tetrahedral shape.
To learn more about VSEPR theory follow the link:
https://brainly.com/question/12775505
Charlie is frying an egg in a pan located over a gas burner. He develops a model to determine the energy produced by the flame in the gas
burner by calculating the energy absorbed by the egg. Which assumption will Charlie need to make in order for his model to be considered a
closed system?
A. Heat flows from the egg to the surroundings.
O
B. Heat flows from the pan to the surroundings.
O
C. No heat is lost from the flame or the egg to the surroundings.
OD. The egg receives heat from the flame and the surroundings.
Answers
A closed system is one in which there is no exchange of materials or energy with the surroundings hence the correct assumption is that "No heat is lost from the flame or the egg to the surroundings."
Another name for a closed system is an isolated system. In such system, there is exchange of material or energy between the system and the surroundings.
In this case, the system comprises of the pan and the eggs. If the system is to be a closed system then " No heat is lost from the flame or the egg to the surroundings."
Learn more; https://brainly.com/question/8987993
need help please answer

Answers
Answer:
True
Explanation:
The answer is True if it's wrong forgive me if its right heart it.
How many milligrams are found in 0.1% w/v copper
sulfate?
Answers
Therefore, in 100 milliliters of the solution, there are 100 milligrams of copper sulfate.
In a 0.1% w/v copper sulfate solution, the amount of copper sulfate present can be calculated by considering that 0.1% represents 0.1 grams per 100 milliliters (w/v). To convert this to milligrams, we multiply the grams by 1000. Therefore, in 100 milliliters of the solution, there are 100 milligrams of copper sulfate.
To calculate the amount of copper sulfate in a different volume of the solution, you can use this proportion: 100 milligrams of copper sulfate is to 100 milliliters of solution as X milligrams of copper sulfate is to Y milliliters of solution. Cross-multiplying and solving for X will give you the amount of copper sulfate in the desired volume.
Remember to check the concentration unit and adjust the calculations accordingly if the concentration is given in a different form (e.g., w/w, v/v, etc.).
To learn more about copper sulfate click here: brainly.com/question/33043457
#SPJ11
Which one(s) of the following statements about the buddy system for allocating memory is/are TRUE? A. The buddy system is ideal for quickly allocating pages on demand for user-space applications B. The buddy system ensures allocation that is free of internal fragmentation C. With the buddy system, memory segments can be either divided or coalesced, to satisfy an allocation request D. The buddy system allocates memory that is physically contiguous None of the mentioned
Answers
The correct statement about the buddy system for allocating memory is:
C. With the buddy system, memory segments can be either divided or coalesced to satisfy an allocation request.
The buddy system is a memory allocation technique used in operating systems. It divides memory into fixed-size blocks or segments, typically in powers of two. When a memory allocation request is made, the system looks for a free block of suitable size. If the block is larger than required, it can be divided or split into smaller blocks to satisfy the request. Conversely, if adjacent blocks are free, they can be coalesced or merged to form a larger block for allocation.
A. The buddy system is not specifically designed for quickly allocating pages on demand for user-space applications. It is a general memory allocation strategy used in various contexts.
B. The buddy system may still result in internal fragmentation. Internal fragmentation occurs when allocated memory blocks are slightly larger than the requested size, leaving unused space within the block.
D. The buddy system does not guarantee physically contiguous memory allocation. It can allocate memory from different available blocks, which may or may not be physically contiguous.
Therefore, the only true statement is C. With the buddy system, memory segments can be either divided or coalesced to satisfy an allocation request.
To know more about system visit:
https://brainly.com/question/13453484
#SPJ11
8.) If 396 g of Carbon Dioxide (CO2) are produced, what mass of Oxygen
(02) reacted? *
1 C3H3 + 5 O2 → 3 CO2 + 4 H20

Answers
Answer:
480 g of oxygen.
Explanation:
C3H8 + 5O2 ---> 3CO2 + 4H2O
Using the molar masses:
3*12 + 6*16 g of CO2 were formed from 10*16 g O2
132g g CO2 from 160 g O2
1g CO2 from (160/132) g O2
396 g from (160/132) * 396
= 480 g of oxygen.
How many electrons are in the valence shell?
a. three
b. two
c. five to eight
d. four
e. one

Answers
When fluorine gas is put into contact with calcium metal at high temperatures, calcium fluoride powder is created in an exothermic reaction
Answers
Answer:
True,When fluorine gas is put into contact with calcium metal at high temperatures, calcium fluoride powder is created in an exothermic reaction.
please help asap in 10 mins
What are the conditions necessary for electro-chemical corrosion to occur?
Answers
Answer:
Presence of an Electrolyte
Metal Surface
Oxygen or Other Oxidizing Agent
Difference in Potential
Electrochemical Pathway
Explanation:
How many mL of 0.45 M CaCl2 have 14.15 g of CaCl2 in them?
Answers
In a liter of 0.15 M \(CaCl\)₂, there are 0.15 moles of \(CaCl\)₂. Calculating it in grams.
1 mole \(CaCl\)₂ = 110.98 g
16.65 g \(CaCl\)₂ in 1 liter from 0.15 moles \(CaCl\)₂ x 110.98 g/mole
So, it needs \(CaCl\)₂—54 g—in liters (or milliliters)
3.24 L or 3240 ml is equal to 16.65 g/L or 54 g/L.
Alternatively put:
You get 54 g of \(CaCl\)₂ converted to moles.
54 g x 1 mole/110.98 g = 0.487 moles of \(CaCl\)₂ in 3.24 L, or 3240 ml of solution.
According to the chemical formula for \(CaCl\)₂, there are two moles of \(Cl_{1} -\)and one mole each of the total ions \(Ca_{2}+\) and \(Cl_{1} -\) in every mole of the molecule.
The amount of solute that dissolves in one liter of solution is measured by a substance's molarity (M). Divide the number of moles of solute by the liters of solution volume to determine the molarity of a solution: Molarity (M) = moles of solute/liters of solution = molL.
To know more about molarity of \(CaCl\)₂,click on the link below:
https://brainly.com/question/16601268
How do you identify the components necessary for a combustion reaction?
Answers
The ways to identify the components necessary for a combustion reaction include:
Combustible substance.Ignition temperature. A gas which supports it.What is Combustion?This is referred to as burning and involves high-temperature exothermic redox chemical reaction.
This type of reaction involves the use of a gas such as oxygen which supports it and other flammable substances.
Read more about Combustion here https://brainly.com/question/23992512
#SPJ1
determine the hydronium ion concentration of an aqueous solution that has a pH of 4.0
Answers
Answer:
Imagewww.chem.purdue.edu › Equilibrium
Calculating the Hydroxide Ion Concentration from pOH
To calculate the pH of an aqueous solution you need to know the concentration of the hydronium ion in moles per liter ...
Calculating pH
Calculating hydronium ion ...
Calculating pOH
Calculating hydroxide ion ...
Calculating pKa
People also ask
How do you find hydronium ion concentration when given pH?
The hydronium ion concentration can be found from the pH by the reverse of the mathematical operation employed to find the pH. [H3O+] = 10-pH or [H3O+] = antilog (- pH) Example: What is the hydronium ion concentration in a solution that has a pH of 8.34? On a calculator, calculate 10-8.34, or "inverse" log ( - 8.34).
The burning of fossil fuels contributes to the addition of greenhouse gases to the atmosphere. These gases trap thermal energy in the Earth’s atmosphere. Which of the following would be the most reasonable changes that could result from an increase in the burning of fossil fuels?
Answers
Explanation:
The burning of fossil fuels contributes to the addition of greenhouse gases to the atmosphere. These gases trap thermal energy in the Earth's atmosphere.
The materials which contain hydrocarbons which are generated from the remains of decay of plants and animals that are buried underground for many years are called fossil fuels.
What causes by burning fossil fuels?The burning of fossil fuels can affect the environment, climatic conditions and human health. The burning of fossil fuels produces large amount of green house gases like CO₂, CH₄, etc. The increase in the amount of green house gases increases the heat and temperature of the earth's surface which leads to global warming.
The increase of temperature due to global warming can cause rise in the sea level, the glaciers of the earth will melt and coastal areas will be submerged under water due to this.
The burning of fossil fuels also releases toxic gases like SO₂, NO₂ which can cause acid rain and the emission these gases causes air pollution.
Thus the burning of fossil fuels has adverse effects.
To know more about fossil fuels, visit;
https://brainly.com/question/29770775
#SPJ6
Why does the pressure inside a container of gas increase if more gas is added to the container?
Why does the pressure inside a container of gas increase if more gas is added to the container?
There are greater differences in the distances between the molecules.
There is a corresponding increase in the number of molecules striking the walls of the container per unit time.
There is a increase in the force of the collisions between the molecules and the walls of the container.
Answers
The pressure inside a container of gas increases if more gas is added to the container due to the increase in the number of molecules striking the walls of the container per unit time and the increase in the force of the collisions between the molecules and the walls of the container.
Pressure is defined as force per unit area and is usually measured in atmospheres (atm), millimeters of mercury (mmHg), or kilopascals (kPa).The molecules of gas in a container are in constant motion and collide with the walls of the container. When more gas is added to the container, the molecules have less space to move around and collide with the walls more frequently.
This leads to an increase in the number of collisions per unit time and therefore an increase in the force per unit area exerted on the walls of the container. This increase in force leads to an increase in pressure inside the container.In summary, the pressure inside a container of gas increases if more gas is added to the container due to an increase in the number of collisions and the force of the collisions between the molecules and the walls of the container.
Know more about pressure here:
https://brainly.com/question/24719118
#SPJ8
If only 0.500 mol of NO2(g) is placed in a 1.0 L container and the reaction is allowed to come to equilibrium, 0.186 mol of N2O4(g) is formed. Find the value of Keq.
Solve using ICE table.
Answers
The equilibrium constant of the system is 11.3.
What is the value of Keq?We know that the equilibrium constant shows the extent to which reactants are converted into products.
Now;
2NO2 ⇄ N2O4
[NO2] = 0.500 mol /1 L = 0.500 M
[N2O4] = 0.186 mol /1 L = 0.186 M
The ICE table is;
2NO2 ⇄ N2O4
I 0.5 0
C -2x +x
E 0.5 - 2x 0.186
The concentration of NO2 at equilibrium = 0.5 - 2(0.186) = 0.128
Keq = 0.186/( 0.128)^2
Keq = 11.3
Learn more about equilibrium constant:https://brainly.com/question/10038290
#SPJ1
Write the balanced nuclear equation for electron capture by 110 in 49. Include both the mass numbers and the atomic numbers with each nuclear symbol. Use the sup-subscript button in the answer palette to enter these numbers correctly. Greek letters can be accessed in the drop-down menu that says main keypad.
Answers
The balanced nuclear equation for electron capture by 110 in 49 can be written as \(^49110In + e⁻ → ^49109Sn\), where \(^49110In\) represents the initial nucleus, e⁻ represents the captured electron, and ^49109Sn is the resulting nucleus.
The balanced nuclear equation for electron capture by 110 in 49 (indium-110) can be represented as: \(^49110In + e⁻ → ^49109Sn\)
In this equation,\(^49110In\) is the initial nucleus of indium-110, and e⁻ represents the captured electron. During electron capture, an inner orbital electron is absorbed by the nucleus. The resulting nucleus is represented as \(^49109Sn\), which is tin-109.In the process of electron capture, the atomic number decreases by 1, as an electron combines with a proton to form a neutron. The mass number remains the same, indicating the conservation of mass during the reaction. This balanced nuclear equation represents the electron capture process for indium-110.
Learn more about balanced nuclear equation here:
https://brainly.com/question/30852800
#SPJ11
Which one is a single replacement reaction? (Whoever gets it correct first I’ll mark)

Answers
The equation that represents a single replacement reaction given the various options is 2Al(s) + 6HCl -> 2AlCl₃(aq) + 3H₂O(g) (option 2)
What is a single replacement reaction?A single replacement reaction, also known as single displacement reaction is a reaction in which elements higher in the electro-chemical series displace or replace elements lower in the electro-chemical series displace from a solution.
The following example illustrates single replacement reaction:
A + BC -> AC + B
From the above reaction, we can see that A has replace/displace B to from AC.
With the above information, we can determine the equation that represents single replacement reaction. Details below:
Equation from the questions:
2Al + 3Cl₂ -> 2AlCl₃2Al(s) + 6HCl -> 2AlCl₃(aq) + 3H₂O(g)2AlCl₃(aq) -> 2Al + 3Cl₂ AlCl₃ + 3KOH -> Al(OH)₃ + 3KClFrom the above, we can see that only 2Al(s) + 6HCl -> 2AlCl₃(aq) + 3H₂O(g) conform to single replacement reaction.
Thus, the correct answer to the question is: 2Al(s) + 6HCl -> 2AlCl₃(aq) + 3H₂O(g) (option 2)
Learn more about single replacement reaction:
https://brainly.com/question/29662825
#SPJ1
Phosphoric acid (H3PO4) in water solution at 25 °C with a concentration of 0. 21 kg mol/m³ is passing through a porous ceramic filter. The thickness of the filter is 4. 2 mm, and its tortuosity numerical value is 13 times the numerical value of its void fraction. The mass transfer rate was estimated to be 1. 4 x 10-9 kg mol H3PO4/ s. M².
a) Predict the diffusion coefficient of Phosphoric acid in water using the Wilke-Chang method.
[CO1, PO1,C4]
b) Calculate the Phosphoric acid concentration at the other side of the ceramic filter.
[CO1, PO1, C4]
Answers
The Wilke-Chang method is used to predict the diffusion coefficient of Phosphoric acid in water. The Wilke-Chang method is a widely used empirical equation to estimate the diffusion coefficient of a solute in a solvent.
Fick's Law of diffusion states that the mass transfer rate of a solute across a porous membrane is proportional to the concentration gradient and the diffusion coefficient.
a. It takes into account the molecular weight, viscosity, and density of the solute and solvent. By plugging in the relevant values for Phosphoric acid and water, we can calculate the diffusion coefficient.
To calculate the diffusion coefficient of Phosphoric acid in water using the Wilke-Chang method, we need to know the molecular weight of Phosphoric acid (H3PO4) and water. The molecular weight of H3PO4 is 98 g/mol, and the molecular weight of water is 18 g/mol.
The Wilke-Chang equation is given by:
D = (1 / Φ) * [(1/M1 + 1/M2) / (√(1/μ1) + √(1/μ2))] * (T / P)
where D is the diffusion coefficient, Φ is the void fraction of the ceramic filter, M1 and M2 are the molecular weights of the solute and solvent, μ1 and μ2 are the viscosities of the solute and solvent, and T is the temperature in Kelvin.
b. We have the mass transfer rate and the thickness of the ceramic filter, so by rearranging Fick's Law equation, we can calculate the concentration at the other side of the filter.
The equation is given by:
J = -D * ∆C/∆x
where J is the mass transfer rate, D is the diffusion coefficient, ∆C is the change in concentration, and ∆x is the thickness of the ceramic filter.
We have the mass transfer rate (1.4 x 10^(-9) kg mol H3PO4/s.m²) and the thickness of the ceramic filter (4.2 mm = 0.0042 m), so by rearranging the equation, we can calculate the change in concentration (∆C) and then use it to find the concentration at the other side of the ceramic filter.
To know more about diffusion coefficient refer to this:
https://brainly.com/question/33711482
#SPJ11
0.92 lbm of water fills a container whose volume is 1.92 ft3. the pressure in the container is 100 psia. calculate the total internal energy and enthalpy in the container. use data from the steam tables. the total internal energy in the container is btu. the enthalpy in the container is btu.
Answers
The total internal energy in the container is 329.77 Btu and the enthalpy in the container is 385.14 Btu.
Using the steam tables, we can determine the specific volume of water at the given pressure and temperature. The specific volume of water is 0.01658 \(ft^3/lbm\).
The mass of water in the container is 0.92 lbm, so the total volume of the water is:
V = m/v = 0.92 lbm / 0.01658 \(ft^3/lbm\) = \(55.539 ft^3\)
Assuming the water is at saturation, we can find the total internal energy and enthalpy by using the values in the steam tables for saturated water at 100 psia.
From the steam tables, the total internal energy of saturated water at 100 psia is 358.05 Btu/lbm, so the total internal energy in the container is:
U = m * u = 0.92 lbm * 358.05 Btu/lbm = 329.77 Btu
From the steam tables, the enthalpy of saturated water at 100 psia is 419.02 Btu/lbm, so the enthalpy in the container is:
H = m * h = 0.92 lbm * 419.02 Btu/lbm = 385.14 Btu
Therefore, the total internal energy in the container is 329.77 Btu and the enthalpy in the container is 385.14 Btu.
Learn more about enthalpy :
https://brainly.com/question/13996238
#SPJ4
Which of the following molecules has a trigonal planar shape? A P E X
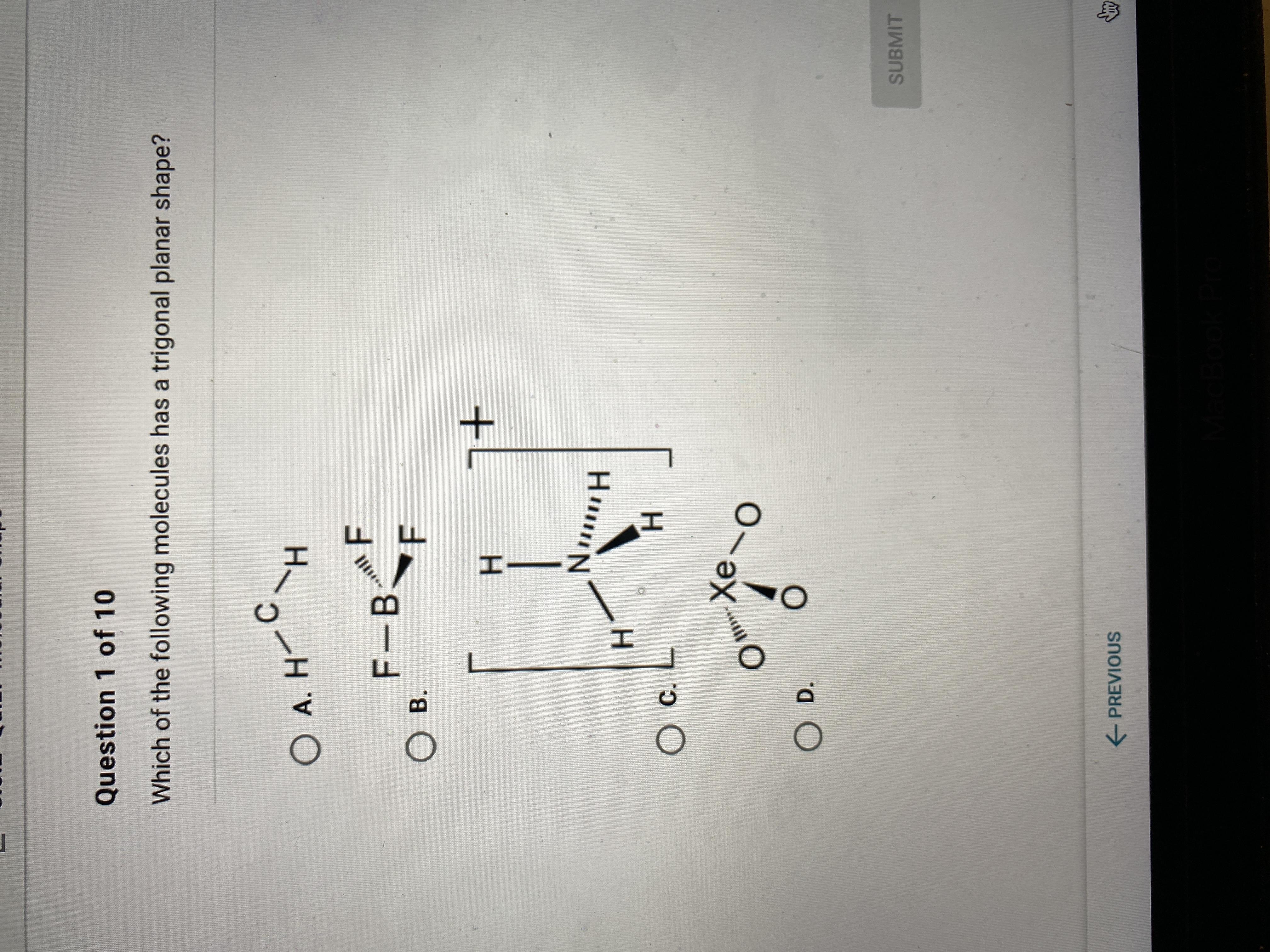
Answers
Answer:
B. BF3
Explanation:
I did summoning jutsu on it and got it correct