Explain the difference between potential energy and kinetic energy.
Sentence Starters
"Potential Energy is. . "
"Kinetic Energy is. . "
"The difference between potential energy and kinetic energy is. . ."
Answers
Answer:
Potential energy is the stored energy in an object or thing, while kinetic energy is the energy which an object contains because of a particular motion.
Explanation:
Potential energy is energy that is stored for example a wind up toy, it has energy but in order for you to get kinetic energy, it has to move. So kinetic energy is moving energy.
The difference is that potential is stored and kinetic is moving energy.
Related Questions
A formula for the relationship between weight and blood pressure in children is given by the formula below where P(x) is measured in millimeters of mercury and x is measured in pounds. Use the formula to answer the questions. P(x)=17.9(9+lnx)10≤x≤100 What is the rate of change of blood pressure with respect to weight at the 60-pound weight level? The rate of change at the 60-pound weight level is approximately mm/ pound. (Do not round until the final answer. Then round to the nearest hundredth as needed.) What is the rate of change of blood pressure with respect to weight at the 70-pound weight level? The rate of change at the 70-pound weight level is approximately mm/ pound. (Do not round until the final answer. Then round to the nearest hundredth as needed.)
Answers
The formula for the relationship between weight and blood pressure in children is:
P(x) = 17.9(9 + ln x) 10 ≤ x ≤ 100To find the rate of change of blood pressure with respect to weight at the 60-pound weight level.We differentiate the above function with respect to x Therefore:
dP/dx = 17.9(1/x)Now substituting x = 60 in the above equation, we get: dP/dx = 17.9(1/60) dP/dx = 0.298The rate of change at the 60-pound weight level is approximately 0.30mm/ pound.Rate of change at the 70-pound weight level:
Similarly, we can find the rate of change at 70-pound weight level, by differentiating the function P(x) with respect to x. Therefore, dP/dx = 17.9(1/x)Now substituting x = 70 in the above equation, we get:dP/dx = 17.9(1/70) dP/dx = 0.2557The rate of change at the 70-pound weight level is approximately 0.26mm/ pound.About BloodBlood is a fluid found in all living things that functions to deliver substances and oxygen needed by body tissues, transports chemical products of metabolism, and also acts as the body's defense against viruses or bacteria. Blood has the function of regulating acid and base balance. ,transports O2, carbohydrates, and metabolites, regulates body temperature by conduction or conduction, carries body heat from heat production centers (liver and muscles).
Learn More About Blood at https://brainly.com/question/920424
#SPJ11
Are the Nobel gases reactive?
Answers
Answer:
The noble gases are relatively nonreactive. In fact, they are the least reactive elements on the periodic table. This is because they have a complete valence shell. They have little tendency to gain or lose electrons.
Explanation:
Explanation:
As you may or may not know, atoms of elements take and give electrons in order to form bonds and, therefore, compounds. However, noble gases have a full valence shell (8 electrons in the valence shell).
Normally, in an atomic reaction, there is an instability in the atoms' valence shells. For example, oxygen has only 6 valence electrons. In order for an atom to be completely stable it needs to have 8 valence electrons. So, two hydrogen atoms with one electron each bond with the oxygen atom, creating a stabilization.
However, in the case of noble gases, their atoms already have a full valence shell, so there is no instability and no need to form bonds with other elements. In fact, noble gases got their name from their inactivity with other elements.
Given the equation
N2(g) +3H2 (g)--> 2NH3 (g)
1 mole of N2 gas is needed to completely react with 3 moles of H2 gas.
How many molecules of H2 gas are needed?

Answers
Answer:
numbers of molecules = 3×6.023×10-23
A 5.25 g sample of metal at 49.5 °C
gives off 10.4 J of energy until it is at
40.5 °C. What is the specific heat
of the metal?
J
c = [?] g ºc
note: q = -10.4 J
Spec. Heat (J/g °C)
Answers
The specific heat of the metal is 0.220106 (J/g °C).
What is the specific heat of the metal? The specific heat of a metal is a measure of how much energy is required to raise the temperature of one gram of the metal by one degree Celsius. It is typically expressed in units of joules per gram-degree Celsius (J/g°C). The specific heat of a metal is determined by its atomic structure and its chemical composition. Different metals have different specific heats, and the range of values can vary greatly. Generally, metals with a higher atomic weight have higher specific heats than those with a lower atomic weight. For example, gold has a specific heat of 0.13 J/g°C, while aluminum has a specific heat of 0.90 J/g°C. Heat capacity, which is a measure of the amount of heat energy required to raise the temperature of a material, is related to specific heat. Generally, the higher the specific heat of a material, the higher its heat capacity.Solution:
This can be calculated using the equation q = mc∆T, where q is the energy given off, m is the mass of the metal (5.25 g), c is the specific heat of the metal, and ∆T is the change in temperature (40.5 °C - 49.5 °C = -9 °C).
Thus, c = -10.4 J / (5.25 g * -9 °C), which can be simplified to 0.220106 (J/g °C).
To learn more about specific heat refer to:
https://brainly.com/question/27991746
#SPJ1
Use linear algebra to balance the chemical equation: C7H₁6 +0₂ → CO₂ + H₂O. 20. Let V be the set of all vectors in ³ whose components sum to zero (e.g. (-5, 2, 3) is in the set V but (0, 0, 1) is not). Is V a subspace of R³2 Give compelling evidence either way. 15. (Determine the quadratic interpolant to the given data set using linear algebraic techniques. (The quadratic interpolant is a quadratic equation that best approximates the data set). {(6.667, 46.307), (4.567, 16.582), (3.333, 4.857)}
Answers
The balanced chemical equation is:
0.5C7H16 + O2 → 0.5CO2 + H2O
For balancing the chemical equation C7H16 + O2 → CO2 + H2O, we can use linear algebraic techniques. We need to determine the coefficients that balance the number of atoms on both sides of the equation.
Let's denote the coefficients for C7H16, O2, CO2, and H2O as a, b, c, and d, respectively.
The balanced chemical equation can be written as:
aC7H16 + bO2 → cCO2 + dH2O
To balance the carbon (C) atoms, we have:
7a = c (Equation 1)
To balance the hydrogen (H) atoms, we have:
16a = 2d (Equation 2)
To balance the oxygen (O) atoms, we have:
2b = 2c + d (Equation 3)
We have three equations (Equations 1, 2, and 3) and four unknowns (a, b, c, d). To solve this system of equations, we can write it in matrix form and find the solution using linear algebraic techniques.
The augmented matrix for the system of equations is:
[ 7 0 -1 0 | 0 ]
[ 0 0 0 -2 | 0 ]
[ 0 -2 2 -1 | 0 ]
By performing row operations to row-reduce the augmented matrix, we can obtain the solution:
[ 1 0 -0.5 0 ]
[ 0 1 -1 -0.5 ]
[ 0 0 0 0 ]
The solution to the system of equations is:
a = 0.5
b = 1
c = 0.5
d = 1
Putting the values of a,b,c, and d we get the balanced chemical equation as:
0.5C7H16 + O2 → 0.5CO2 + H2O
To learn more about balancing, visit:
https://brainly.com/question/31242898
#SPJ11
Two reactant particles collide with proper orientation. The collision will be
effective if the particles have
(A) sufficient potential energy
(B) high activation energy
(C) high ionization energy
(D) sufficient kinetic energy
And explain why
Answers
Answer: D) Sufficient kinetic energy
Explanation:
a. what is the hybridization of the central atom in cse2?
Answers
The orbital hybridization of the central carbon atom in CSe2 is sp.
In chemical bonding, atomic orbitals may be combined to form appropriate hybrid orbitals suitable for bonding. The orbitals that combine during hybridization must be close enough in energy.
In the compound Cse2, carbon is the central atom bonded to two selenium atoms. The carbon atom in CSe2 is sp hybridized.
Learn more about orbital hybridization: https://brainly.com/question/1869903
Which statement is true?
A.
Molar concentration is proportional to the temperature of a reaction.
B
Molar concentration is proportional to the change in the number of moles in a reaction.
c.
Molar concentration is proportional to the partial pressure of a gaseous system.
D
Molar concentration is proportional to the volume of a system.
Answers
Answer:
I'm not really sure. but I think is D
Answer: B, Molar concentration is proportional to the change in the number of moles in a reaction
Explanation: took the test on plato
Order the following phases of matter from the phase with the most space between the
atoms to the least space between the atoms.
Answers
Answer:
Gas>Liquid>Solid
Explanation:In a gas state, molecules are allowed to move freelyand rarely collide into nearby particles. In a liquid state, the molecules are contained within the liquid and occasionally bump into each other. now in a solid state the atoms arrange themselves into a structure that the atoms sit very tightly next to each other, sometimes vibarating
Determine the number of molecules in 3.79 kilograms of the fictional compound Cs7(Cr5O3)4. Include the units, but do not write the chemical formula. Round the answer to 3 significant figures.
Answers
Answer:
1.0555 * 10^24 molecules
Explanation:
Number of molecules = ?
Mass = 3.79 Kg = 3790 g
Molar mass of Cs7(Cr5O3)4 = 2162.25 g/mol
Number of moles = Mass / Molar mass
Number of moles = 3790 g / 2162.25 g/mol
Number of moles = 1.7528 mol
1 mol = 6.022 * 10^23 molecules
1.7528 mol = x
solving for x;
x = 6.022 * 10^23 * 1.7528
x = 1.0555 * 10^24 molecules
What evidence do you need to determine that two rocks have some similar minerals because the minerals from the original rock was eroded then it was later compressed with other minerals to form a different rock.
Answers
Answer:
It comes from same parent material.
Explanation:
The two rocks have some similar minerals which indicates that they are comes from the same parent material but with the passage of time it is mixed with other minerals due to which they are some different in composition from one another so we can conclude that they comes from the same parent material due to the presence of some similar minerals.
PLEASE HELP!! I need to know the answer to this but I'm overly exhausted and the question isn't making and sense...
An unknown element has three naturally occurring isotopes.
Below are their masses (amu) and % abundance
A) Calculate the weighted atomic mass unit for each isotope. Show % conversions and record the AMU for each isotope in the table below
B) What is the average (Total) atomic mass unit of the unknown element.
C) Identify this unknown element from the Periodic Table

Answers
An unknown element has three naturally occurring isotopes total average atomic mass 20.18 amu and element from the periodic table is neon
Isotopes are the members of a family of an element that all have the same number of protons but different numbers of neutrons
There are three neon isotopes and the more abundant ²⁰Ne and ²¹Ne are both essentially all primordial, as there is no significant global production of these isotopes and also ²²N
Neon 20 is made up of ten proton and ten neutron the atomic mass unit of the gas is 19.992 and the abundance of the gas is 90.48% and neon 21 is made up of ten proton and eleven neutron and the atomic mass unit of the gas is 20.994 and the abundance is 0.27% and neon 22 is made up of ten proton and twelve neutron and the atomic mass unit of the gas is 21.991 and the abundance of gas is 9.25%
Unknown element from the periodic table is neon
Know more about isotopes
https://brainly.com/question/13658643
#SPJ1
if the temperature of a radiator is increased from 27ºc to 54ºc, by what factor does the radiating power change?
Answers
The power radiated by the radiator changes by a factor of 14.1573 when the temperature is increased from 27°C to 54°C.
The formula that relates the power radiated by an object with the fourth power of the temperature is known as the Stefan-Boltzmann Law. It is stated as follows: P = σA(T⁴), where P is the power radiated, σ is the Stefan-Boltzmann constant (5.67 × 10⁻⁸ W/m²K⁴), A is the surface area of the radiator, and T is the temperature in Kelvin.
We must first convert the temperature to Kelvin:
TK = T°C + 273.15
TK1 = 27°C + 273.15 = 300.15 K
TK2 = 54°C + 273.15 = 327.15 K
The factor by which the power radiated changes is the ratio of the power at the new temperature to the power at the original temperature. The equation is as follows:
P2/P1 = (T2/T1)⁴
Substituting the given values:
P2/P1 = (327.15/300.15)⁴
P2/P1 = 1.8856⁴
P2/P1 = 14.1573
You can learn more about the temperature at: brainly.com/question/7510619
#SPJ11
Non-combustible (type II) constructed building has a different recurring fire spread problem: fire spreads on the roof deck. A type II building has steel or concrete walls, floors and structural framework; however, the roof covering is combustible, it burns and spreads fire. The roof covering of a type II building can be a layer of asphalt water proofing, with a combustible felt paper covering. Another layer of asphalt may be mopped over the felt paper. A combustible foam insulation may be placed on top of the asphalt, and another layer of asphalt mopped over the foam insulation. When a fire occurs inside a type II building, flames rising to the underside of the steel roof deck may conduct heat through the metal and ignite the combustible roof covering above. Conduction is the transfer of heat through a solid. The asphalt, felt paper and foam insulation may bum and spread fire along the roof covering. After a fire has been extinguished inside a type II building, the officer should go to the roof and examine the roof covering directly above for extension. If necessary, a hose line should be stretched to the roof for extinguishment. Modern type II and type III buildings have combustible membrane roof coverings which are more combustible than the asphalt roof covering. After reading the above information, what are your opinions on Type II construction?
Answers
Type II construction has a recurring fire spread problem related to its combustible roof covering. This can be a significant safety concern for occupants of the building and can cause significant damage to the property.
What is construction?
The transfer of heat through the metal roof deck can ignite the combustible materials above, leading to the spread of fire along the roof covering. It is important for building owners, operators, and firefighters to be aware of this potential hazard and take appropriate measures to prevent or control fires in Type II buildings. This may include upgrading the roofing materials to reduce the risk of fire spread, regular inspections of the roof covering, and prompt response to any signs of fire. It is also important for officers and firefighters to examine the roof covering after a fire has been extinguished to ensure that the fire hasn't spread and to take necessary measures to prevent further damage or reignition.
To know more about construction, visit:
https://brainly.com/question/28996046
#SPJ1
Enter your answer in the provided box. Calculate the wavelength of a
photon of electromagnetic radiation with a frequency of 61.7 MHz. m
Be sure to answer all parts. Calculate the energy of a photon of
electromagnetic radiation with a wavelength of 582.8 nm. * 10 Report
your answer in scientific notation using the provided boxes.
Answers
we find the energy to be approximately \(3.41 * 10^-19\) Joules is the answer.
To calculate the wavelength of a photon with a frequency of 61.7 MHz, we can use the formula: wavelength = speed of light / frequency. The speed of light is approximately\(3 * 10^8\) meters per second.
Converting the frequency to Hz (\(1 MHz = 10^6 Hz\)), we have \(61.7 * 10^6\)Hz.
Plugging these values into the formula, we get: wavelength =\((3 * 10^8 m/s) / (61.7 * 10^6 Hz).\)
Simplifying, we find the wavelength to be approximately 4.862 meters.
Now, to calculate the energy of a photon with a wavelength of 582.8 nm, we can use the equation: energy = Planck's constant × speed of light / wavelength.
Planck's constant is approximately \(6.63 * 10^-34\) Joule-seconds.
Converting the wavelength to meters (\(1 nm = 10^-9 m\)), we have \(582.8 * 10^-9 m.\)
Plugging these values into the equation, we get: energy =\((6.63 * 10^-34J·s) * (3 * 10^8 m/s) / (582.8 * 10^-9 m).\)
Simplifying, we find the energy to be approximately \(3.41 * 10^-19\) Joules.
know more about wavelength
https://brainly.com/question/31143857
#SPJ11
Someone please help will mark as brainliest
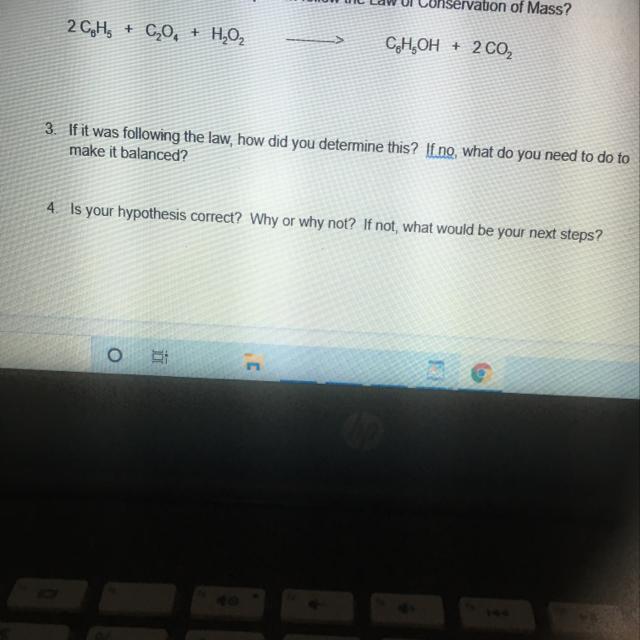
Answers
Answer: no you need a balance skall to make it the correct balance that you want. yes the hypothesis is correct because you change the balance with your balance scale so obviously it's going to make your hypothesis higher.
Explanation: I HOPE THAT HELPED.
Which isotope of sulfur is the most naturally abundant
Answers
Answer:
The Isotope of sulfur.
32S 94.99% stable.
33S 0.75% Stable.
Explanation:
What is the mass percent of a sugar solution having 15 g sugar dissolved in 63 g of solution?
Answers
Answer:
23.81%
Explanation:
The mass percent of a solution, also known as percentage by weight or w/w%, can be calculated using the formula:
mass percent = (mass of solute / mass of solution) x 100%
In this question;
Mass of solute (sugar) = 15g
Mass of solution (sugar + water) = 63g
Hence,
w/w% = 15/63 × 100
w/w% = 0.2381 × 100
w/w% = 23.81%
Therefore, the mass percent of the sugar solution is 23.81%.
Which is a diatomic molecule?
A) Ar
B) CO
C) CO2
D) NaCl
Answers
the first-order decay of radium has a half-life of 1620 years. how many grams of radium decomposes after 555 years if the sample initially weighs 100.0 grams? (hint: [a]t in the integrated rate law is the amount that remains after time t)
Answers
The amount of radium that decomposes after 555 years is:
100.0 g - 74.7 g = 25.3 g.
The integrated rate law for a first-order decay is: ln([A]t/[A]0) = -kt
where [A]t is the amount of substance remaining after time t, [A]0 is the initial amount of substance, k is the rate constant, and t is the time.
To solve for the amount of radium that decomposes after 555 years, we can use the following equation:
ln([A]t/[A]0) = -kt
ln([A]t/100.0 g) = -(0.693/1620 years) * 555 years
ln([A]t/100.0 g) = -0.297
[A]t/100.0 g = e^(-0.297)
[A]t = (e^(-0.297)) * 100.0 g
[A]t = 74.7 g
Therefore, the amount of radium that decomposes after 555 years is:
100.0 g - 74.7 g = 25.3 g.
To know more about decomposes , refer here:
https://brainly.com/question/28896356#
#SPJ11
what is the solubility of cr(oh)₃ at a ph of 11.00? (ksp cr(oh)₃ is 6.70 × 10⁻³¹)
Answers
The solubility of Cr(OH)₃ at a pH of 11.00 is 4.69 × 10⁻¹⁰ mol/L. at a pH of 11.00 is 4.69 × 10⁻¹⁰ mol/L.
Given that the Ksp of Cr(OH)₃ is 6.70 × 10⁻³¹ at a pH of 11.00.To find the solubility of Cr(OH)₃, we use the expression for the solubility product. It is given as:Ksp = [Cr³⁺][OH⁻]³We know that at a pH of 11, the concentration of OH⁻ ions is 1.0 × 10⁻³ M.
The reaction is given as:Cr(OH)₃(s) ⇌ Cr³⁺(aq) + 3OH⁻(aq)The concentration of Cr³⁺ is the same as the solubility of Cr(OH)₃(s).Let the solubility of Cr(OH)₃ be x. Then, the concentration of Cr³⁺ is x.[OH⁻]³ = Ksp/Cr³⁺(1.0 × 10⁻³)³ = 6.70 × 10⁻³¹/xx = (6.70 × 10⁻³¹/(1.0 × 10⁻³)³)¹∕³= 4.69 × 10⁻¹⁰ mol/LTherefore, the solubility of Cr(OH)₃ at a pH of 11.00 is 4.69 × 10⁻¹⁰ mol/L.
To know more about solubility visit:
https://brainly.com/question/31493083
#SPJ11
An example of a molecule that cannot be represented adequately by a single lewis structure is?.
Answers
An example of molecule that cannot be represented by a single Lewis structure is Ozone (O₃).
A Lewis Structure may be defined as the representation of electrons of an atom with the help of dots and symbols. The symbols are used for representing initials of atom and dots are used for electrons. If there is a molecule, which is to be represented as Lewis's structure, then the bonds are shown with the help of straight line. However, there are some atoms which cannot be represented by a single Lewis structure. For those atoms different resonating structures are drawn. Resonating structures are different Lewis structures f similar molecule or atom in which delocalization of electrons takes place. These structures are drawn because a single Lewis structure is unable to explain all the properties of the molecule.
Learn more about Lewis structure at:
brainly.com/question/1407731
#SPJ4

Which examples are possible environmental impacts of flooding? Select the two correct answers. (1 point,
damage to homes
Answers
Reduction in tourism and Groundwater recharge are possible environmental impacts of flooding .
What are floods ?
During floods, people need to quickly move themselves and their most valuable possessions to higher ground. Leaving home in search of a safer place is called evacuation. Floods occur at irregular intervals and vary in magnitude, duration and areas affected.
Water flows naturally from high ground to low ground. This means that low-lying areas can flood quickly before they start reaching higher elevations. In rivers, flooding can occur, especially at bends and meanders in the channel, when flow exceeds the bed's capacity. Floods often damage homes and businesses when they are in the natural floodplains of rivers.
To learn more about the floods , click the link below ;
https://brainly.com/question/12686086
#SPJ9
Why are biopharmaceuticals in high demand?
Answers
Answer:
the market is largely driven by the growing population, increasing burden of chronic disease, and rising inclination toward targeted therapy.
Explanation:
also, the huge demand of biopharmaceutical is facilitated by an accelerating focus in research and related investment.
Do you think elements with large electron affinities will gain or lose electrons? Explain your thinking.
Answers
Answer:
The less valence electrons an atom has, the least likely it will gain electrons. Electron affinity decreases down the groups and from right to left across the periods on the periodic table because the electrons are placed in a higher energy level far
Explanation:
Answer:
Electron affinity increases upward for the groups and from left to right across periods of a periodic table because the electrons added to energy levels become closer to the nucleus, thus a stronger attraction between the nucleus and its electrons. Remember that greater the distance, the less of an attraction; thus, less energy is released when an electron is added to the outside orbital. In addition, the more valence electrons an element has, the more likely it is to gain electrons to form a stable octet. The less valence electrons an atom has, the least likely it will gain electrons.
Electron affinity decreases down the groups and from right to left across the periods on the periodic table because the electrons are placed in a higher energy level far from the nucleus, thus a decrease from its pull. However, one might think that since the number of valence electrons increase going down the group, the element should be more stable and have higher electron affinity. One fails to account for the shielding affect. As one goes down the period, the shielding effect increases, thus repulsion occurs between the electrons. This is why the attraction between the electron and the nucleus decreases as one goes down the group in the periodic table. hope i helped
Explanation:
You are investigating how seasonal temperature changes differ in three zones of a glacial temperate lake throughout the year. You record measurements at each of the three locations in late winter, late spring, late summer, and late autumn. You record temperatures as follows:
Each of the temperature-sampling locations is within the hypolimnion, the thermocline, or the epilimnion. In which zones are sampling locations A, B, and C in this lake?
a. location A, hypolimnion; location B, thermocline; location C, epilimnion
b. location A, thermocline; location B, epilimnion; location C, hypolimnion
c. location A, thermocline; location B, hypolimnion; location C, epilimnion
d. location A, epilimnion; location B, hypolimnion; location C, thermocline
e. location A, hypolimnion; location B, epilimnion; location C, thermocline
Answers
The zones with sampling locations A, B, and C in this lake is (c) location A, thermocline; location B, hypolimnion; location C, epilimnion.
Based on the recorded temperatures, we can determine the location of the sampling sites. Late winter and late autumn are cold months, and therefore the hypolimnion would have the coldest temperatures. In late spring and late summer, the epilimnion would have the warmest temperatures as it is exposed to sunlight and warm air temperatures.
From the given options, the correct answer is (c) location A, thermocline; location B, hypolimnion; location C, epilimnion. This is because the thermocline would have moderate temperatures that change rapidly, which is consistent with the temperature recorded at location A in all seasons. The hypolimnion, which is the coldest layer, is consistent with the temperature recorded at location B in all seasons. Finally, the temperature recorded at location C is consistent with the warmest layer, the epilimnion, in all seasons.
In summary, understanding the temperature variations in different zones of a lake is important in identifying the location of temperature-sampling sites. By recording temperatures at different times of the year, we can observe the changes in temperature that occur in different lake zones and understand how these changes impact aquatic life.
For more such questions on Temperature variations.
https://brainly.com/question/27744030#
#SPJ11
Which type of bond will form between these elements?
O Metallic
O lonic
Covalent

Answers
Answer:
covalent
Explanation:
A solution consists of 42. 00 g of CoSO4 dissolves in 200. 0 mL of water. The molar mass of Cu is 63. 55 g/mol, the molar mass of S is 32. 07 g/mol, and the molar mass of O is 16. 00 g/mol. What is the molarity of the solution?
Answers
Answer:
1.3155M
Explanation:
molar mass of CuSO4
=63.55+32.07+16(4)
=159.62g/mol
n=mass/molar mass
n=42.00/159.62
n=0.2631mol
volume=200mL=0.2L
molarity=mole (n)/volume (v)
molarity=0.2631mol/0.2L
molarity=1.3155M
19 What is the "skin" of the cell?

Answers
Answer:
d cell membrane:)
Explanation:
I need some help with with question:
How could you prepare 100 ml of a 0.40 mol/L of MgSO4 solution from a stock solution of 2.0 mol/L MgSO4. And then how would u prepare it?
Answers
Answer:
C1V1=C2V2
C1 is 2.0mol/l
V1=?
C2=.4mol/L
V2=100ml or for this 0.1L
V1 is 20ml
Best way to prepare this is to measure out 20ml of the 2 molar solution and add 80mL to it to get to 100mL
Explanation: