Explain the relationship between fat and fatty acids.
Answers
Answer:
Fatty acids are the building blocks of the fat in our bodies and in the food we eat. During digestion, the body breaks down fats into fatty acids, which can then be absorbed into the blood. Fatty acid molecules are usually joined together in groups of three, forming a molecule called a triglyceride.
Related Questions
If H 2
and Cl 2
are mixed, how will they interact based on your knowledge of chemical bonds?
Answers
The reaction of the hydrogen and the chlorine atoms would lead to the formation of HCl
Formation of HClIn this reaction, each chlorine molecule (Cl2) breaks apart into two chlorine atoms (Cl), and each hydrogen molecule (H2) breaks apart into two hydrogen atoms (H). These reactive atoms then combine to form hydrogen chloride molecules (HCl).
It's worth noting that the reaction between hydrogen gas and chlorine gas is highly exothermic and releases a significant amount of energy.
Learn more about HCl:https://brainly.com/question/30233723
#SPJ4
A FeCl 3 solution is 0.175 M. How many mL of a 0.175 M FeCl 3 solution are needed to make 650. mL of a solution that is 0.300 M in Cl - ion?
Answers
Based on the molar concentration, the volume of FeCl₃ required is 371 mL
What is the mole ratio of the reaction?The mole ratio of the reaction is determined from the equation of the reaction as follows:
Equation of reaction: FeCl₃ (aq) ----> Fe³⁺ (aq) + 3 Cl⁻ (aq)
From the equation of reaction, the mole ratio of FeCl₃ to Cl⁻ is 1 : 3
The moles of chloride ions in 650 mL of 0.300 M is calculated from the formula below:
Moles = molar concentration * volume in litersMoles of Cl⁻ ions = 0.300 * 650/100
Moles of Cl⁻ ions = 0.195
Moles of FeCl₃ required = 0.195/3
Moles of FeCl₃required = 0.065
volume of FeCl₃ required = 0.065 / 0.175
volume of FeCl₃ required = 0.371 L or 371 mL
Learn more about molar concentration at: https://brainly.com/question/14469428
#SPJ1
The boiling process is considered to be____

Answers
your answer is D. physical change, because a new substance is not formed.
The boiling process is considered to be a physical change as no new substance is formed.
What is a physical change?Physical changes are defined as changes which affect only the form of a substance but not it's chemical composition. They are used to separate mixtures in to chemical components but cannot be used to separate compounds to simpler compounds.
Physical changes are always reversible using physical means and involve a change in the physical properties.Examples of physical changes include melting,boiling , change in texture, size,color,volume and density.Magnetism, crystallization, formation of alloys are all reversible and hence physical changes.
They involve only rearrangement of atoms and are often characterized to be changes which are reversible.
Learn more about physical change,here:
https://brainly.com/question/17931044
#SPJ2
A first order reaction requires 30 minutes for 50% completion. The time required to complete the reaction by 75% will be:
Answers
The time required to complete the reaction by 75% is approximately 51.3 minutes.
The half-life of a first-order reaction is a constant value that is independent of the initial concentration of the reactant. It is given by the equation:
t1/2 = ln(2) / k
where t1/2 is the half-life, ln(2) is the natural logarithm of 2 (approximately 0.693), and k is the rate constant for the reaction.
We can use the given information to determine the rate constant k:
t1/2 = 30 minutes
ln(2) / k = 30 minutes
k = ln(2) / 30 minutes ≈ 0.0231 min^-1
Now we can use the rate constant to determine the time required to complete the reaction by 75%:
ln(Ct / Co) = -kt
where Ct / Co is the fraction of reactant remaining at time t, Ct is the concentration at time t, Co is the initial concentration, and k is the rate constant.
For 50% completion, Ct / Co = 0.5:
ln(0.5) = -0.0231 min^-1 * t
t ≈ 30.1 minutes
For 75% completion, Ct / Co = 0.25:
ln(0.25) = -0.0231 min^-1 * t
t ≈ 51.3 minutes
Therefore, the time required to complete the reaction by 75% is approximately 51.3 minutes.
To know more about first order reaction refer here:
https://brainly.com/question/31661139?#
#SPJ11
What volume of air at room conditions (20c , 760 torr) would be required to combust a full lighter of butane
Answers
The amount of air required to combust a full lighter of butane can be calculated using the stoichiometry of the combustion reaction. The complete combustion of butane (C4H10) can be represented by the following equation:
C4H10 + 6O2 -> 4CO2 + 5H2O
From this equation, we can see that for every mole of butane combusted, 6 moles of oxygen are required. To determine the volume of air required, we need to know the concentration of oxygen in air.
Air is composed of approximately 21% oxygen and 79% nitrogen, and since the pressure of air is 760 torr, the partial pressure of oxygen is:
0.21 * 760 torr = 160 torr
learn more about butane here:
https://brainly.com/question/14818671
#SPJ4
What is stoichiometry?
Answers
Answer:
hope it help u !!!!
Explanation:
Stoichiometry is the relationship between the relative quantities of substances taking part in a reaction or forming a compound, typically a ratio of whole integers
compare the size of ions to the size of atoms from which they form
Answers
Cations are always smaller than the atoms from which they form. Anions are always larger than the atoms from which they form. Ions are usually bigger than the atoms from which they are formed.
When an atom receives or loses electrons, the atom's electron configuration changes, resulting in a net positive or negative charge.
This net charge expands the electron cloud surrounding the nucleus, making the ion bigger in size than the neutral atoms from which it arose. When a metal atom loses one or more electrons to create a cation, it shrinks in size because the positive charge of the nucleus pulls the remaining electrons more strongly.
When a nonmetal atom obtains one or more electrons to create an anion, it normally expands in size.Because of the increasing amount of electrons, the electron cloud surrounding the nucleus grows. It should be noted that this comparison is not absolute and is dependent on the individual factors involved. Some ions are smaller than their neutral atom counterparts, while others are similar in size.
learn more about atoms here:
https://brainly.com/question/29695801
#SPJ4
The complete question is:
Compare the size of ions to the size of atoms from which they form.
$0.500 mL of 0.300 mol / L potassium chromate is added to 30.0 mL of 0.0105 mol/L lead (II) nitrate. Find liming reagent
Answers
0.500 mL of 0.300 mol / L potassium chromate is added to 30.0 mL of 0.0105 mol/L lead (II) nitrate here Lead (II) nitrate is the limiting reagent in this reaction.
To determine the limiting reagent in the reaction between potassium chromate (K2CrO4) and lead (II) nitrate (Pb(NO3)2), we need to compare the amount of each reactant present and identify which one is completely consumed.
Firstly, we need to balance the chemical equation for the given reaction:
2 K2CrO4 + Pb(NO3)2 → PbCrO4 + 4 KNO3
Now, let's calculate the number of moles for each reactant:
Concentration of potassium chromate solution = 0.300 mol/L
Volume of potassium chromate solution = 0.500 mL = 0.500 L
Number of moles of potassium chromate = volume × concentration = 0.500 L × 0.300 mol/L = 0.150 mol
Concentration of lead (II) nitrate solution = 0.0105 mol/L
Volume of lead (II) nitrate solution = 30.0 mL = 0.0300 L
Number of moles of lead (II) nitrate = volume × concentration = 0.0300 L × 0.0105 mol/L = 0.000315 mol
The balanced chemical equation states that the stoichiometric ratio of lead (II) nitrate to potassium chromate is 2:1. This means that 2 moles of potassium chromate react with 1 mole of lead (II) nitrate.
By comparing the moles of each reactant, we can see that potassium chromate is abundant (0.150 mol), whilst lead (II) nitrate is scarce (0.000315 mol).
Lead (II) nitrate is the limiting reagent in this instance because the reactant with the less moles is the limiting reagent.
Learn more about limiting reagent:
https://brainly.com/question/26905271
Two atoms that differ only in the number of neutrons they contain are known as:_________
a. isotopes.
b. ions.
c. anions.
d. isomers.
Answers
The correct answer is (a) isotopes.
Isotopes are two or more atoms of the same element that have the same number of protons but differ in the number of neutrons they contain. Since the number of protons determines the element's identity, isotopes of an element have identical chemical properties. However, due to the difference in the number of neutrons, isotopes may have slightly different atomic masses.
Let's break down the answer choice and explain each option:
(a) Isotopes: This is the correct answer. Isotopes are atoms of the same element that have different numbers of neutrons but the same number of protons.
(b) Ions: Ions are atoms or molecules that have gained or lost electrons, resulting in a net positive or negative charge. This is not the correct term for atoms differing in neutron number.
(c) Anions: Anions are negatively charged ions that have gained electrons. This is not the correct term for atoms differing in neutron number.
(d) Isomers: Isomers are compounds that have the same molecular formula but different structural arrangements. This is not the correct term for atoms differing in neutron number.
Therefore, the correct term for two atoms that differ only in the number of neutrons they contain is (a) isotopes.
Learn more about isotopes from the given link: https://brainly.com/question/27475737
#SPJ11
Question 1
Three factors affect surface currents : (Select 3)
A: Water density
B: Global winds
C: Coriolis Effect
D: Continental deflection
Answers
Answer:
c,a,d
Explanation:
Answer:
i belive it is
B: Global winds
C: Coriolis Effect
D: Continental deflection
Explanation:
on edge
What intermolecular forces are in solid NaCl salt?
Answers
The intermolecular forces are in the solid NaCl salt is ion - ion forces or the dipole - dipole forces of the interaction.
The Na is the metal and is capable of the donating the electrons and the Cl is the non metal and have capability of accepting the electrons. This makes the Na to form the cation and the Cl to make the anion. The dipole - dipole forces are present in the molecule which contains the oppositely charged ions one is positively charged ion and the other is the negatively charged ion.
Thus , the NaCl solid has the ion - ion intermolecular forces of the interaction .
To learn more about intermolecular force here
https://brainly.com/question/30542287
#SPJ4
___+___=__KOH+H2 It Is chemistry question
Answers
Answer:
It's from the quation:
\( \boxed{ \mathsf{ \underline{2K} +\underline{2 H_2O} \rightarrow \:\underline{2}KOH + H_2 }}\)
(How did I know?)
I learnt it while studying the elements of group 1.
- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - -
Group 1 elements:K, here, is Potassium. It's position in the periodic table is Group 1, Period 4.
Group 1 elements are also called Alkali metals. Group 1 elements have 1 electron in their valence shell(outermost shell), the electron that takes part in chemical reactions.Valance electrons of elements of this group contribute to the valency of the group's elements I.e.,
valency of Alkali metals is 1!
- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - -
Reaction with water:Alkali metals react vigorously with cold water to form their hydroxides alongwith the evolution of Hydrogen gas.What are hydroxides?
Hydroxides of an element comprises one atom each of oxygen and Hydrogen bonded together, acting as an anion, the hydorxide anion — OH-.
Example:
KOH (hydroxide of Potassium) NaOH (Hydroxide of Sodium)- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - -
Back to the question:In the question, The reactants are missing but the products are known, that are, Potassium Hydroxide and water.
Keeping the above mentioned property in mind, the reactants come clear as Potassium(K) and Cold water(H2O).
that makes the equation:
\( \mathsf{K + H_2O \rightarrow \: KOH + H_2 }\)
- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - -
Balancing the equation:For an equation to be balanced, the number of atoms of each elements on both side of the equation must be equal.
Number of atoms of each element on the LHS:
K = 1 H = 2O = 1(subscript in front of an atom represents its number of atoms in the compund)
Number of atoms of each element on the LHS:
K = 1 H = 1 + 2 = 3O = 1The number of Hydrogen atoms on boths sides is NOT balanced!
Hit and trial method:
If we add a "2" in front of K, H2O and KOH
\( \mathsf{ \underline{2K} +\underline{2 H_2O} \rightarrow \:\underline{2}KOH + H_2 }\)
Number of atoms on both the sides become equal.
(TIP: This comes thru practice:/. but main point is even out the oxygen and Hydrogen atoms first. like if it's 3 multiply it by 2 and you get an even number, I.e., 6)
Number of atoms of each element on the LHS:
K = 2H = 4O = 2Number of atoms of each element on the LHS:
K = 2H = 2 + 2 = 4 O = 2- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - -
Answer:Hence, the balanced form of the equation is:
\( \boxed{ \mathsf{ \underline{2K} +\underline{2 H_2O} \rightarrow \:\underline{2}KOH + H_2 }}\)
In his experiment Alejandro sees that his numbers don't make very much sense
compared to his neighbors. He didn't make any mistakes in his experiment so this
must be a random error.
True
False
Answers
False. Both random and systematic errors can have an impact on experimental data.
Random mistakes are generated by unpredictability in measuring settings and are typical of minor magnitude. In contrast, systematic mistakes are generated by systematic biases in the experimental technique and result in a constant divergence from the correct value.
If Alejandro's results differ greatly from those of his neighbors, it could be due to chance, but it could also be due to systemic flaws in his experiment. Without more examination, Alejandro cannot assume that the variance is purely due to random mistakes. He should thoroughly examine his experimental approach for potential sources of systematic error. It may also be beneficial to repeat the experiment to establish whether the discrepancy is due to random error or if it is consistent.
To Learn More About magnitude click
https://brainly.com/question/15681399
#SPJ4
The specific type of bond that results from unequal sharing of electrons in the bond is
Answers
Answer:
It's called A polar covalent bond
true or false? in apocalyptic literature, the source of suffering for god’s people is their faithfulness.
Answers
The given statement " Apocalyptic literature, the source of suffering for god’s people is their faithfulness" is true. Because, it includes texts found in religious or biblical contexts, the suffering experienced by God's people is often attributed to their faithfulness or lack thereof.
These texts typically depict a period of intense tribulation, persecution, or catastrophic events that are believed to occur as part of a divine plan or judgment.
According to the narrative structure of apocalyptic literature, the suffering endured by the faithful is seen as a test of their devotion and commitment to their beliefs. It is believed that their faithfulness will ultimately be rewarded, either through deliverance or entrance into a new divine realm.
This theme can be found in various apocalyptic texts, such as the Book of Revelation in the New Testament of the Bible, where the faithful are depicted as enduring suffering and persecution due to their unwavering allegiance to God.
To know more about Apocalyptic literature here
https://brainly.com/question/17601404
#SPJ4
The statement “in apocalyptic literature, the source of suffering for God’s people is their faithfulness” is generally true.What is apocalyptic literature?Apocalyptic literature is a kind of literature that is heavily symbolic and depicts catastrophic events.
It was created during times of hardship and political strife, such as during the reign of the Roman Empire, which made it impossible for people to freely practice their religion and made them feel as though the end of the world was near.Suffering for God’s people in apocalyptic literature:In apocalyptic literature, the faithful are expected to suffer. As a result, the source of suffering for God’s people is their faithfulness.
Know more about apocalyptic literature here:
https://brainly.com/question/32340527
#SPJ11
Temperature and humidity levels need to be monitored and recorded
(A) weekly, preferably daily.
(B) for each shift.
(C) at least daily.
(D) at least monthly.
Answers
To maintain accurate and consistent data on temperature and humidity levels, they should be monitored and recorded (C) at least daily.
Monitoring temperature and humidity levels is crucial for various applications, such as ensuring optimal working conditions, preserving perishable goods, and maintaining proper indoor air quality. By recording these levels daily, you can quickly identify any fluctuations or issues that may arise and take appropriate action. While weekly or monthly checks may provide some information, daily monitoring is the most effective way to stay on top of environmental conditions.
To achieve the best results and maintain an optimal environment, temperature and humidity levels should be monitored and recorded at least daily.
To know more about humidity visit:
brainly.com/question/22069910
#SPJ11
the enthalpy of neutralization for the reaction of a strong acid with a strong base is -56 kj/mol of water produced. how much energy will be released when 195.0 ml of 0.400 m hcl is mixed with 148.0 ml of 0.500 m naoh?
Answers
57.1 kJ energy will be released.
What is energy?Scientists refer energy as the ability to do work. Modern civilization is possible because humans have learned how to transform energy from one form to another and use it to do work. Energy do exist in many forms. Examples include light energy, thermal energy, mechanical energy, gravitational energy, electrical energy, acoustic energy, chemical energy, nuclear or atomic energy. Each form can be transformed or changed into other forms. Energy is made up of nothingness. Energy is a term used to describe the properties of matter and non-material fields. For example, if an object has velocity, we can say that it has kinetic energy. Potential energy also has different forms.To learn more about energy from the given link :
https://brainly.com/question/1932868
#SPJ4
Calculate the osmotic pressure associated with 50. 0 g of an enzyme of molecular weight 98,000 g/mol dissolved in water to give 2600 ml of solution at 30. 0 oc.
Answers
An enzyme with a molecular weight of 98,000 g/mol dissolved in water has an associated osmotic pressure of = 4,882 atm.
What is the body's osmotic pressure?
The pressure that a water solution of salts exerts against a permeability barrier in either direction is known as osmotic pressure. This pressure is brought on by variations in the quantities of salt content in the sea and the body, respectively.
How is osmotic pressure determined?
Concentrations and temperature both have an impact on osmotic pressure. Temperature as well as solute density both have an impact on how much pressure is generated when water moves through a membrane. Osmotic pressure rises with temperature and concentration.
Briefing:
2600 mL x 1 L / 1000 mL = 2.6 L
Temperature ⇒ Go from ° C to K
T (K) = t (° C) + 273.15 = 30.0 ° C + 273.15 = 303.15 K
Number of moles of solute ⇒ It can be calculated since we have the mass of the enzyme and its molecular mass:
98.0 g of enzyme ____ 1 mol
50.0 g of enzyme _____ X = 0.510 moles
Calculation: 50.0 g x 1 mol / 98.0 g = 0.510 moles
Now, you can replace the values in the Van´t Hoff equation and you will get the result:
π = (n x R x T) / V
π = (0.510 mol x 0.08206 L.atm / mol.K x 303.15 K) / 2.6 L = 4.882 atm
To know more about osmotic pressure visit:
https://brainly.com/question/15501096
#SPJ4
What is a polar molecule
Answers
Answer:
Here, I hope this helps
Explanation:

Answer:
┃
V
Explanation:
A polar molecule is a chemical species in which the distribution of electrons between the covalently bonded atoms is not even. Each atom has a certain electro-negativity. When bonded to another atom, the atom with the higher electro-negativity will tend to attract more electrons.
Does the half life of oxygen-18 impacts its use ( explain)
Answers
Answer:
O-18 is used as a tracer in many hydrologic studies. It is most often used as a component in a mixing-model and hydrograph separation, as O-18 acts conservatively and is applied naturally and uniformly over broad areas. O-18 can be used when different sources (old water/new water) have different isotopic values.
a chemical equation is a statement using chemical that expresses both the identities and the relative of the reactants and products involved in a chemical or physical change. multiple choice question. numbers; masses formulas; quantities formulas; masses names; quantities
Answers
A chemical equation is a statement using chemical formulas expresses both the identities and the relative quantities of the reactants and products involved in a chemical or physical change. The correct answer is B.
An expression of the identities and relative quantities of the reactants and products involved in a chemical or physical transformation is called a chemical equation. Chemical equations are statements made using chemical formulas.
What exactly is a chemical equation?In chemical equations, variables like the direction of a reaction and the physical states of the reacting parties are represented by symbols. In 1615, the French chemist Jean Beguin created the first chemical equation.
Chemical equations, such as the one below, can be used to depict chemical reactions on paper (for the reaction between hydrogen gas and oxygen gas to form water).
To know more about chemical equations, click the link given below:
brainly.com/question/19626681
#SPJ4
identify the conditions for a standard electrochemical cell. select one or more: pressure of 1 atm temperature of 298 k solution concentrations of 1 m pressure of 5 atm solute masses of 1 g temperature of 273 k
Answers
The conditions for a standard electrochemical cell. select one or more : pressure of 1 atm temperature of 298 k solution concentrations of 1 M.
The electrochemical cell is the cell that is capable of generating the electrical energy from the chemical reactions or by the use of the electrical energy to cause the chemical reaction. The conditions for a standard electrochemical cell. select one or more : pressure of 1 atm temperature of 298 k solution concentrations of 1 M.
There are the two types of the electrochemical cells is as follows : the galvanic called the electrolytic cells. the galvanic cell is also called as the voltaic cell.
To learn more about temperature here
https://brainly.com/question/14995282
#SPJ4
If you start with 16 atoms of a parent radioisotope, after how many half-lives will only one atom of the parent remain, on average?
Answers
The half-life of a radioactive isotope is the amount of time it takes for half of the original atoms to decay. Therefore, after one half-life, there will be half as many parent atoms remaining.
After two half-lives, there will be only one-fourth of the original parent atoms remaining. After three half-lives, there will be only one-eighth of the original parent atoms remaining, and so on.
Using this information, we can determine that it takes four half-lives for 16 parent atoms to decay to only one parent atom remaining. This is because after the first half-life, there are eight parent atoms remaining. After the second half-life, there are only four parent atoms remaining.
After the third half-life, there are only two parent atoms remaining. And after the fourth half-life, there is only one parent atom remaining.
To learn more about; Radioactive
https://brainly.com/question/1236735
#SPJ11
Which of these is a mineral?
1) sugar
2) oxygen
3) water
4) halite
Answers
Halite is a common evaporite mineral.
Sugar, Oxygen, and water are NOT minerals.
So therefore the answer is 4. Halite
Answer:
D
Explanation:
Oxygen is an element.
sugar is a hydrocarbon derivative.
water is a compound made of hydrogen and oxygen. There are no metals in it at all.
So you are left with Halite. The common name for halite is salt. The chemical formula is NaCl
Answer: D
write a balanced equation for the reaction of thiosulfate (s2o32-) and permanganate (mno4-) ions in acidic solution to form sulfate and manganese(ii) ions.
Answers
When potassium permanganate is reacted with thiosulfate, then there is formation of potassium sulfate and magnese ion.
What is potassium permanganate?The inorganic substance potassium permanganate has the formula KMnO₄. It is a crystalline salt that is purple-black and dissolves in water into a solution of K+ and MnO₄.
As a potent oxidizing agent, dermatitis medicine, wound cleaner, and general disinfectant, potassium permanganate is widely employed in the chemical industry and labs.
A balanced equation for the reaction of thiosulfate permanganate ions in acidic solution to form sulfate and manganese (ii) ions is
KMno₄ + Na₂S₂o₃ + H₂O ⇒ K₂So₄ + KOH + MnO₂ + Na₂So₄
Thus, the equation is KMno₄ + Na₂S₂o₃ + H₂O ⇒ K₂So₄ + KOH + MnO₂ + Na₂So₄.
To learn more about potassium permanganate, follow the link;
https://brainly.com/question/20630418
#SPJ1
the fermentation of glucose c6h12o6 produces ethanol, c2h5oh and co2.the equation for the reaction is as follows: c6h12o6 --> 2c2h5oh 2co2. how many moles of co2 are produced when 0.400 moles of c6h12o6 are used?
Answers
0.800 moles of CO2 are produced when 0.400 moles of glucose are used in the fermentation reaction.
The number of moles of CO2 produced when 0.400 moles of glucose (C6H12O6) are used can be calculated from the balanced chemical equation for the reaction. According to the equation, for every 1 mole of glucose that reacts, 2 moles of CO2 are produced:
C6H12O6 -> 2C2H5OH + 2CO2
Therefore, when 0.400 moles of glucose are used, the number of moles of CO2 produced can be calculated as follows:
0.400 moles of glucose * 2 moles of CO2 per 1 mole of glucose = 0.800 moles of CO2
So, 0.800 moles of CO2 are produced when 0.400 moles of glucose are used in the fermentation reaction.
Learn more about fermentation here:
https://brainly.com/question/13050729
#SPJ4
The salts of carboxylic acids, such as sodium benzoate, are often used in foods as
A) flavor enhancers.
B) colorings.
C) sweeteners.
D) preservatives.
Answers
The salts of carboxylic acids, like sodium benzoate, are typically used as preservatives in foods. This is because carboxylic acids have antimicrobial properties that help prevent the growth of bacteria, yeast, and fungi in food products.
Sodium benzoate is commonly used in soft drinks, fruit juices, and other acidic foods to extend their shelf life. While carboxylic acids can have a tart flavor, they are not generally used as flavor enhancers or sweeteners in foods. Similarly, carboxylic acids are not typically used as colorings, as they do not provide any pigmentation to food products.
Hi! The salts of carboxylic acids, such as sodium benzoate, are often used in foods as D) preservatives. These compounds help maintain food quality by inhibiting the growth of microorganisms, such as bacteria and mold, thus prolonging the shelf life of the products. While they do not function as flavor enhancers, colorings, or sweeteners, their primary role in the food industry is to ensure safety and freshness for consumers.
learn more about the acid here
https://brainly.com/question/29796621
#SPJ11
What characteristic do all parts of the electromagnetic spectrum share?
They have the same wavelength.
They have the same frequency.
They interact in the same way with any matter.
They travel at the same speed through empty space.
Answers
Answer:
I agree with D
Explanation:
Took the test on Edge
The characteristic do all parts of the electromagnetic spectrum share is that they travel at the same speed through empty space. The speed of these waves are the speed of light in vacuum.
What is electromagnetic spectrum?Electromagnetic spectrum is the arrangement of different types of radiations in the order of their increasing frequency or decreasing wavelength.
The order of the waves in the increasing order of frequency is radio waves, microwaves, infrared rays, visible light, ultraviolet rays, x-rays and gamma rays.
The frequency and wavelength of all these waves are different. However, they travel at the same speed in empty space equal to the speed we say for the light.
Find more on electromagnetic spectrum:
https://brainly.com/question/15576247
#SPJ6
the solubility of o2 in water is 5.85 x 10-4 m at 25 oc and 0.45 atm of o2 pressure . what will the solubility be when the partial pressure of o2 is one-fourth of the original pressure? group of answer choices 0.0023 m 0.0052 m 0.0013 m 0.00026 m 0.00015 m
Answers
The solubility of gas (O2) in water will be 1.49 x 10^-4 M when the partial pressure of O2 is one-fourth of the original pressure i.e. option E : 0.00015 M
The solubility of a gas in water is directly proportional to the partial pressure of the gas above the solution. This relationship is described by Henry's law, which states that the solubility of a gas in a solvent is proportional to the pressure of the gas above the solvent.
Therefore, if the partial pressure of O2 is one-fourth of the original pressure, the solubility of gas (O2) in water will be proportional to the new pressure, which is 0.45 atm / 4 = 0.1125 atm.
So, the new solubility of gas (O2) in water will be:
New solubility = original solubility * (new pressure / original pressure)
New solubility = 5.85 x 10^-4 M * (0.1125 atm / 0.45 atm)
New solubility = 1.49 x 10^-4 M
Therefore, the solubility of gas (O2) in water will be 1.49 x 10^-4 M when the partial pressure of O2 is one-fourth of the original pressure.
To know more about the solubility of gas, click here : https://brainly.com/question/23204201
#SPJ4
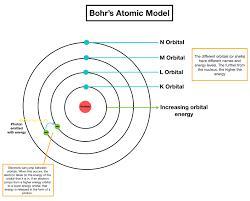
Why is electron filling similar to going up the stairs?.
Answers
If we look into the Bohr's atomic model, it is like a staircase.
Where every next orbit is like a stair.
As we can not rest in air, we either have to stay on level 1 or can go up with enough energy to climb next level.
Same way, electron can either stay on one orbit or can go to higher orbits with enough energy to jump from lower energy level to the higher energy level, can say orbit.
According to Bohr, atom is a system consisting of a dense nucleus surrounded by orbiting electrons.
He compared his atomic structure with the structure of the Solar System.
To know more about Bohr's atomic model:
brainly.com/question/3964366
#SPJ4