Find the pH during the titration of 20.00 mL of 0.1000 M butanoic acid, CH₃CH₂CH₂COOH (Kₐ = 1.54X10⁻⁵), with 0.1000 M NaOH solution after the following additions of titrant:(d) 19.00 mL
Answers
Ph range is 14.35.
What is PH?PH is a proportion of hydrogen particle focus , a proportion of the causticity or alkalinity of an answer. The pH scale for the most part goes from 0 to 14. Fluid arrangements at 25°C with a pH under 7 are acidic, while those with a pH more prominent than 7 are essential or basic. Having a reasonable pH safeguards our bodies from the back to front. Some even say that illnesses and problems can't fill in a body whose pH is in balance. The pH is a logarithmic scale, that is to say, when an answer becomes multiple times more acidic, its pH diminishes by one. In the event that an answer becomes multiple times more acidic, its pH will diminish by two. The pH scale is recognizable to a bunch of standard arrangements whose pH is laid out by peaceful accord. Essential pH standard qualities are resolved utilizing a fixation cell with transaction, by estimating the possible distinction between a hydrogen terminal and a standard cathode like the silver chloride anode.
Learn more about PH, visit
brainly.com/question/21819990
#SPJ4
Related Questions
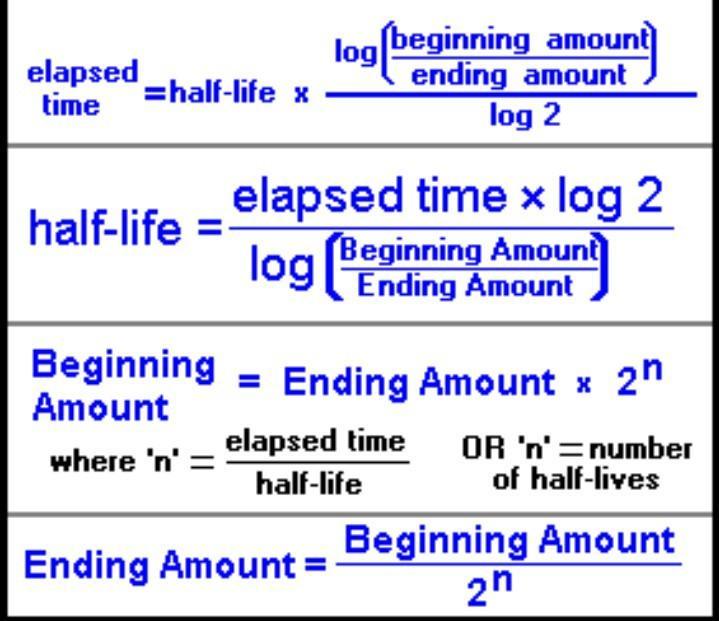
Assuming the half life of element X is 32 hours, and you start with 200.0 g, how much will be left after 96 hours?
Answers
Explanation:
You have to calculate as long as you just look at the image on how to do it
Sorry po
5. What conclusion might scientists draw if they found drastic changes in the types of sediment found in different rock layers?
the area went through major changes in climate over geologic time
the rock layers in that area formed slower than surrounding areas
the rock layers in that area were not exposed to normal weather
the area was likely under water for long periods of time
Answers
Answer:
the area went through major changes in climate over geologic time
Explanation:
Different types of sediment is a good indication of different climates.For example, if an area just experienced the same type of climate, like a desert, we would expect to observe the same types of sediment/rocks in the area
Hope this helps :D
If the temperature of water is observed to decrease when a certain salt is dissolved in it, then: The salt dissolution process is endotheic a for the salt dissolution process is <0 q for the solution is >0 The enthalpy change for the dissolution of the salt is <0.
Answers
If the temperature of the water is observed to decrease when a certain salt is dissolved in it, then the enthalpy change for the dissolution of the salt is <0.
When the temperature of the water is observed to decrease when a certain salt is dissolved in it, then the process of salt dissolution is exothermic. As per the thermodynamics concept, the process of dissolving salts in water may be endothermic or exothermic. It depends on the nature of the salts. If the salts tend to absorb heat from surroundings, it is known as an endothermic reaction and if the salts tend to release heat to the surroundings, it is known as an exothermic reaction.
In this case, as the temperature of the water decreases by dissolving the salt, it means that the reaction is exothermic. Hence, the enthalpy change for the dissolution of the salt is <0.
to know more about temperature here:
brainly.com/question/7510619
#SPJ11
How many moles are in 5NO3?
Answers
Answer:
200
Explanation:
more molecules thats all i know
3 poi
27) Name the neutral element with this Noble Gas shorthand
configuration.

Answers
Calculate the change in internal energy of the gas. Express your answer in joules. An experimenter adds \( 980 \mathrm{~J} \) of heat to \( 1.75 \mathrm{~mol} \) of an ideal gas to heat it from \( 10.
Answers
The change in internal energy of the gas is 10062.6 J.The first step to calculating the change in internal energy of a gas is by using the equation Q = nCΔT.
where Q is the heat added, n is the number of moles of the gas, C is the molar specific heat capacity of the gas, and ΔT is the change in temperature. Here's how to calculate the change in internal energy of the gas:Given;Heat added = 980 J
No of moles (n) = 1.75
molInitial Temperature (T1) = 10.3 °C
Final Temperature (T2) = 23.7 °C
First, calculate the change in temperature:ΔT = T2 - T1ΔT
= 23.7 - 10.3ΔT
= 13.4 °C
Next, convert the temperature change to Kelvin:
ΔT = 13.4 + 273.15ΔT
= 286.55 K
Now, use the equation Q = nCΔT to calculate the change in internal energy:
Q = nCΔTΔU
= QΔU
= nCΔTΔU
= (1.75 mol) (20.8 J/mol K) (286.55 K)ΔU = 10062.6 J
Thus, the change in internal energy of the gas is 10062.6 J.
To know more about internal energy visit:
https://brainly.com/question/11742607
#SPJ11
how to keep chicken water from freezing without electricity
Answers
Answer:
Float a few ping pong balls in your water tub. The slightest breeze will create waves in the water and keep a solid layer of ice from forming for a lot longer.
YOU MUST SHOW ALL STEPS OF THE G.U.E.S.S. METHOD CORRECTLY. NO UNITS=WRONG ANSWER
Q: wave is traveling with a frequency of 2424 Hz and has a wavelength of 0.5 meters. What speed is this wave going?
Answers
Answer:
The equation for wave speed can be used to calculate the speed of a wave when both wavelength and wave frequency are known. Consider an ocean wave with a wavelength of 3 meters and a frequency of 1 hertz. The speed of the wave is: Speed = 3 m x 1 wave/s = 3 m/s.
SO... take your meters and hezert and do tha same
Explanation:
Plz mark me as brainlyist
nitrogen dioxide no2 gas and liquid water h2o react to form aqueous nitric acid hno3 and nitrogen monoxide no gas. suppose you have 5.0 mol of no2 and 7.0 mol of h2o in a reactor. calculate the largest amount of hno3 that could be produced. round your answer to the nearest 0.1 mol.
Answers
The largest amount of HNO₃ that could be produced will be 14 mol
Number of moles of NO₂ = 5.0 mol
Number of moles of H₂O = 7.0 mol
Amount of HNO₃ = ?
Write the balanced chemical equation
3NO₂ + H₂O → 2HNO₃ + NO
As we can see the molar ratio between NO₂ and HNO₃ is 3 : 2
Calculate for 5.0 mol of NO₂
5.0 mol x (2 mol / 3 mol) = 3.33 mol HNO₃
As we can see the molar ratio between H₂O and HNO₃ is 1 : 2
Calculate for 7.0 mol of H₂O
7.0 mol x (2 mol / 1 mol) = 14 mol HNO₃
So H₂O is the excess reactant and to find out the larger amount we use the excess reactant.
So the largest amount of HNO₃ produced is 14 mol
You can also learn about number of moles from the following question:
https://brainly.com/question/12513822
#SPJ4
Give The Products For The Balanced Neutralization Reaction: HNO3(Aq)+LiOH(Aq)→
Answers
The products of the balanced neutralization reaction between HNO3 and LiOH are lithium nitrate and water.
The balanced neutralization reaction between HNO3 (nitric acid) and LiOH (lithium hydroxide) can be represented as follows:
HNO3 (aq) + LiOH (aq) → LiNO3 (aq) + H2O (l)
In this reaction, nitric acid reacts with lithium hydroxide to form lithium nitrate and water. The products of the reaction are LiNO3 (lithium nitrate) and H2O (water).
Lithium nitrate is a white crystalline solid that is commonly used in the manufacturing of fireworks, fertilizers, and various other industrial applications. It is also used in the treatment of bipolar disorder and depression. Water, on the other hand, is a colorless and odorless liquid that is essential for the survival of all living organisms.
To know something about the neutralization reaction, click below.
https://brainly.com/question/11039778
#SPJ11
The alkali metals are
Answers
Answer:
They are lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr).
Hope this helps :)
Answer:
Groups one of the periodic table.
Lithium Li
Sodium Na
Potassium K
Rubidium Rb
Cesium Cs
Francium Fr
A(g) + B(g) = C(g) + D(g)
18. For which of the following equilibrium constants will reactions of the general type shown above
give the greatest yield of products C+D when equilibrium is attained ?
(A) K= 1 x 10^-15
(B) K= 1 x 10-²
(C) K= 1 x 10²
(D) K= 1 x 10^50
Answers
Comparing the given equilibrium constants, we find that K values increase from (A) to (D): 1 x 10^-15 < 1 x 10^-2 < 1 x 10^2 < 1 x 10^50.
Since a higher K value signifies a greater yield of products, the greatest yield of products C and D will be obtained when the equilibrium constant is the highest. the answer is (D) K = 1 x 10^50.
option d
The equilibrium constant (K) is a measure of the extent of a chemical reaction at equilibrium. It is defined as the ratio of the concentrations (or partial pressures) of the products to the concentrations (or partial pressures) of the reactants, each raised to the power of their respective stoichiometric coefficients.
In the given reaction A(g) + B(g) = C(g) + D(g), a higher equilibrium constant (K) indicates a greater yield of products C and D at equilibrium. This means that the reaction is more favorable in the forward direction, leading to a higher concentration of C and D compared to A and B.
A high equilibrium constant indicates that the reaction strongly favors the formation of products C and D and will shift towards the products' side at equilibrium. In this case, the forward reaction is highly favored, resulting in a significant yield of products C and D.
option d
for more such questions on equilibrium
https://brainly.com/question/19340344
#SPJ8
when did democritus contribute to the atomic theory
Answers
Answer:
Around 400 B.C.E., the Greek philosopher Democritus introduced the idea of the atom as the basic building block matter.
Explanation:
Hope this helps.
calculate the average kinetic energy, in j/mol, for a mole of kr at 273.0 k. assume ideal gas behavior.
Answers
The average kinetic energy of a mole of Kr at 273.0 K, assuming ideal gas behavior, is 3411.33 J/mol.
To calculate the average kinetic energy of a mole of Kr at 273.0 K, we need to use the following equation:
K.E. = (3/2) * R * T
where K.E. is the kinetic energy per mole, R is the gas constant, T is the temperature in Kelvin, and (3/2) is the average kinetic energy of a molecule in an ideal gas.
The value of R is 8.314 J/mol*K, and the temperature is 273.0 K. Substituting these values into the equation, we get:
K.E. = (3/2) * 8.314 J/mol*K * 273.0 K
K.E. = 3/2 * 2274.22 J/mol
K.E. = 3411.33 J/mol
Therefore, the average kinetic energy of a mole of Kr at 273.0 K, assuming ideal gas behavior, is 3411.33 J/mol.
To know more about average kinetic energy, refer
https://brainly.com/question/26080657
#SPJ11
convert 33.6 degree Celsius to degree Fahrenheit
Answers
Answer:
Your answer is 92.48 degrees Fahrenheit, or just 92 degrees.
Explanation:
The formula for finding degrees Celsius and turning them into Fahrenheit is: (C x 9/5) + 32. So,
33.6 x 9/5 = 60.48
60.48+ 32= 92.48.
Checks out!
Please like and mark brainliest!
Hope it helps!
21) Technetium-99 is a nuclear isomer that is used in tens of millions of medical diagnostic procedures annually and has a half-life of six hours. Suppose you have a 100mg sample of Technetium-99. a) Write a function that models the sample. b) Approximate how much of the sample will be remaining after one day. 4
Answers
After one day, approximately 8.67mg of the sample will be remaininga) The function that models the sample of Technetium-99 is given by
f(t) = P₀e^(-kt)
Where,P₀ = initial quantity = 100mgk = decay constantt = timef(t) = remaining quantity after t time.
A half-life of 6 hours is given. The decay constant can be found using the half-life formula:
T½ = (ln 2)/k6
= (ln 2)/kk
= (ln 2)/6f(t)
= P₀e^(-kt)f(t)
= 100e^(-0.1155t)mg
b) After one day, 24 hours = 4 half-lives Remaining amount,
f(t) = P₀e^(-kt)f(24)
= 100e^(-0.1155 × 24)
= 100e^(-2.772)
≈ 8.67mg
After one day, approximately 8.67mg of the sample will be remaining. The function that models the sample is
f(t) = 100e^(-0.1155t), where t is time in hours and f(t) is the remaining quantity in milligrams.
To know more about Technetium-99 visit:-
https://brainly.com/question/29970596
#SPJ11
After one day, approximately 8.67mg of the sample will be remaininga) The function that models the sample of Technetium-99 is given by
f(t) = P₀e^(-kt)
Where,P₀ = initial quantity = 100mgk = decay constantt = timef(t) = remaining quantity after t time.
A half-life of 6 hours is given. The decay constant can be found using the half-life formula:
T½ = (ln 2)/k6
= (ln 2)/kk
= (ln 2)/6f(t)
= P₀e^(-kt)f(t)
= 100e^(-0.1155t)mg
b) After one day, 24 hours = 4 half-lives Remaining amount,
f(t) = P₀e^(-kt)f(24)
= 100e^(-0.1155 × 24)
= 100e^(-2.772)
≈ 8.67mg
After one day, approximately 8.67mg of the sample will be remaining. The function that models the sample is
f(t) = 100e^(-0.1155t), where t is time in hours and f(t) is the remaining quantity in milligrams.
To know more about Technetium-99 visit:-
https://brainly.com/question/29970596
#SPJ11
Realice una historieta que resuma su comprensión acerca de la teoría atómica y los diferentes modelos atomicos que se
han propuesto a lo largo de la historia.
Answers
Respuesta:
Los modelo atómicos han permitido representar el modo de funcionamiento de los átomos. A lo largo de la historia han surgido un numero de modelos atómicos diferentes incluyendo los modelos de Bohr, Thomson, Rutherford, Sommerfeld, Dalton y Schrödinger.
Explicación:
El modelo atómico propuesto por John Dalton (1808) demostró que las sustancias químicas reaccionan en proporciones fijas y cómo mediante su combinación se producen elementos diferentes. Dalton fue el primero en postular la existencia de elementos indivisibles llamados átomos. A continuación, Thomson (1904) desarrolló un modelo en el cual el átomo estaba compuesto por protones con carga positiva y electrones con carga negativa los cuales se incrustaban uniformemente dentro de este átomo, asemejándose a las pasas de uva de un budín. En 1911, Ernest Rutherford desarrolló un nuevo modelo donde la masa principal del átomo tenía carga positiva y se localizaban en el núcleo, mientras que los electrones con carga negativa se posicionaban en la región externa del átomo. Subsecuentemente, Niels Bohr (1913) represento el funcionamiento del átomo de hidrógeno mediante un protón inmóvil en el núcleo atómico y un electrón girando a su alrededor. El modelo atómico de Sommerfeld permitió generalizar el diagrama de Bohr a otros tipos de átomos mas allá del Hidrógeno, incluyendo diferentes niveles energéticos para cada átomo particular. El modelo de Schrödinger (1926) permitió corregir aquellas discordancias surgidas del modelo atómico de Bohr. Schrödinger incluyó diferentes niveles y subniveles de energía a los electrones e incorporó órbitas elípticas a su movimiento, con lo cual permitiendo predecir los efectos relativos de los campos magnético y eléctrico sobre el movimiento de los electrones.
express250,000ml in liters
Answers
Answer:
250,000 ML is equal to 250 Liters.
Explanation:
The term 'Mili' multiplies the term 'Liter' by 1,000. Per every liter is 1000 milliliters.
What is the basic mechanism that naturally creates freshwater within the hydrologic cycle? precipitation reverse osmosis evaporation runoff infiltration
Answers
The basic mechanism that naturally creates freshwater within the hydrologic cycle is through the processes of evaporation, precipitation, infiltration, and runoff.
1. Evaporation: Water from the Earth's surface (e.g., oceans, lakes, and rivers) is heated by the sun and turns into water vapor, rising into the atmosphere.
2. Condensation: The water vapor cools as it rises, condensing into clouds.
3. Precipitation: When the clouds become heavy enough, the water droplets combine and fall back to the Earth's surface as precipitation (e.g., rain, snow, or hail).
4. Infiltration: Precipitation that reaches the ground can infiltrate the soil, becoming part of the groundwater system.
5. Runoff: Precipitation that does not infiltrate the soil will flow over the land surface as runoff, eventually entering rivers, lakes, and oceans.
This continuous movement of water through the various stages is known as the hydrologic cycle. Note that reverse osmosis is not part of this natural process; it is a human-engineered method used for water purification.
More on hydrologic cycle: https://brainly.com/question/13334963
#SPJ11
1. Shankar and Sameer performed an experiment to differentiate primary, secondary and tertiary amines in a laboratory.Shankar correctly identified those 3 compounds but Sameer could only identify primary amine. a) What may be the reagent used by Sameer 1) Ammonical Silvernitrate solution 2) Chloroform & Caustic potash 3) Aqueous Copper Sulphate solution
Answers
Answer: 2) Chloroform & Caustic potash
Explanation:
The carbylamine reaction is a kind of chemical test which is done to detect primary amines in an unknown solution. It cannot detect secondary and tertiary amines.
The reaction involves the heating with up of the unknown solution with alcoholic potassium hydroxide or caustic potash and the chloroform.
In the presence of primary amine, the production of isocyanide results.
clearance tests used to determine the glomerular filtration rate must measure substances that are:
Answers
Clearance tests used to determine the glomerular filtration rate must measure substances that are freely filtered at the glomerulus and not reabsorbed or secreted by the renal tubules.
This ensures that the substance is solely cleared by glomerular filtration and can provide an accurate estimation of the filtration rate. Substances commonly used in clearance tests include inulin, creatinine, and certain radioactive tracers.
These substances are not metabolized or significantly reabsorbed by the renal tubules, allowing their concentration in the urine to reflect the glomerular filtration rate.
By measuring the clearance of these substances, the efficiency of the kidneys in filtering blood can be assessed.
To know more about the Clearance tests refer here :
https://brainly.com/question/32339523#
#SPJ11
A large rift valley can be found along the east coast of Africa. It has been slowly widening over time, and it is now wide enough to contain many large lakes.
Which of these best explains the slow widening of this rift valley over time?
Group of answer choices
Earth's rotation
wind and water erosion
the Moon's gravitational pull
lithospheric plate movement
Answers
Answer:
wind and water erosion
Answer:
C is the answer
When a fuel is burned, any carbon that does not undergo combustion can be released as particles. What do we call this product?

Answers
Answer:
"soot" or "smoke" or "particulates" or "ash" or "carbon particulates"
Explanation:
The carbon that does not undergo combustion is released as particles. This particles are called soot or dust. Incomplete combustion also leads to the production carbon monoxide.
What is combustion ?Combustion is an exothermic process in which a gas reacts with the atmospheric oxygen producing carbon dioxide and water. This reaction involves heat to the surroundings.
Sometimes the combustion does not occurs completely. When there is insufficient oxygen for the fuel to completely react with the air to form carbon dioxide and water leads to incomplete combustion. It also occurs when a heat sink, such a solid surface or flame trap, extinguishes the combustion.
One of the byproducts of incomplete combustion is carbon monoxide. The typical incomplete combustion process releases carbon, which results in the formation of soot and dust.
Find more on combustion:
https://brainly.com/question/13153771
#SPJ2
What conclusion did Rutherford draw from his gold-foil experiment? A. Almost all the mass of an atom is concentrated in the nucleus. B. Atoms contain three different subatomic particles. C. Electrons are tiny particles that carry a negative charge. D. The mass of a proton is nearly equal to the mass of a neutron.
Answers
Answer:
A. Almost all of the mass is concentrated in the nucleus.
Explanation:
Because when he shot the alpha particles towards the atoms, most passed through (which meant atom is mostly empty space), but some bounced back and such (which meant mass is concentrated in the nucleus.)
The reason they bounced is because alpha particles are postive and nucleus is positive as well, and we know positives don't attract, rather they repel thus they bounced.
Sort the characteristics based on whether they describe a dog, a computer, or both.
has internal
organization
has living
cells
uses energy
produces
offspring
lacks genetic
material
Answers
Answer:
both have internal organization dog only has living cells both use energy dogs only produce offspring computers only lack genetic material
what are 6 uses of filtration?
Answers
Answer:
Coffee Filter.
Tea-bags.
Water Filters.
Sand Filtration.
HEPA Air Filters.
Automotive Filters.
Belt Filters.
Dialysis.
Explanation:
ASAPPPP EASYYYY!!!
Total number of single, double, and triple bonds for C2H6(g).
_ _ _
Answers
Answer:
2 single, 0 double, and 1 triple
Explanation:
H-C≡C-H
A Rottweiler dog is born with a long tail and when it is a few days old its tail is cut to be shorter. Several years later, the dog has eight puppies. Based on this information, how many puppies would you expect to have short tails like their mother's? A. 4 B. 8 C. 0 D. 2
Answers
Answer:
I think it's A or D
Explanation:
apologies if I get this wrong..
Answer:c.0
Explanation: hopefully this isn’t wrong, but wouldn’t it be 0 since it was something the mother did later on so it really wasn’t a trait of her? And also the mother didn’t born with a short tail?
3. When 2. 750 g of Pb3O4 is heated to a high temperature, it decomposes to produce 0. 0640 g of
oxygen gas and 2. 686 g of some new lead oxide compound. Use this data to determine the
empirical formula of the new lead oxide compou
Answers
The empirical formula of the new lead oxide compound is PbO₇.
We can use the given data to determine the moles of Pb₃O₄, oxygen gas, and the new lead oxide compound produced,
moles of O₂ = 0.0640 g / 32.00 g/mol = 0.00200 mol
moles of new lead oxide compound = (2.686 g - 2.750 g) / 223.2 g/mol = -0.000287 mol
Now we can use the moles of oxygen gas and the new lead oxide compound to determine the empirical formula of the new lead oxide compound. From the balanced chemical equation for the decomposition of Pb₃O₄,
Pb₃O₄ → 3PbO + 0.5O₂
we see that 0.5 mol of O₂ is produced for every 3 mol of PbO produced. Therefore, the mole ratio of O₂ to the new lead oxide compound is,
0.00200 mol O₂ / -0.000287 mol new lead oxide compound ≈ -6.96
To get integer mole ratios, we can multiply both sides of the ratio by a common factor to obtain whole numbers. In this case, multiplying by 7 gives.
0.0140 mol O₂ / -0.00201 mol new lead oxide compound ≈ -7
This means that for every 7 moles of oxygen gas produced, there are approximately 1 mole of the new lead oxide compound produced.
The empirical formula of the new lead oxide compound can be expressed as PbOₓ. Since we know that the ratio of Pb to O in the compound is 1 : x, and we have determined that the ratio of O to the new lead oxide compound is approximately 7:1,
PbO₇ ≈ PbOₓ
To know more about empirical formula, here
brainly.com/question/14044066
#SPJ4
--The complete question is, When 2.750 g of Pb3O4 is heated to a high temperature, it decomposes to produce 0. 0640 g of oxygen gas and 2. 686 g of some new lead oxide compound. Use this data to determine the empirical formula of the new lead oxide compound.--
does an oxygen atom form two covalent bonds?
Answers
Answer:
yes
Explanation:
Two covalent bonds form between the two oxygen atoms because oxygen requires two shared electrons to fill its outermost shell.