Answers
Answer:
See figure 1
Explanation:
For this question, we have to remember that definition of an "isomer" in an isomer we have the same condensed formula (in this case \(C_6H_14\)) and different structures. The first structure is a linear structure of 6 carbons (hexane). Then we can have a 5 carbon linear structure in which we have to add a methyl group. This methyl group can be attached to carbon 2 or carbon 3 (2-methylpentane and 3-methylpentane). Finally, we can have a 4 carbon linear structure in which we have to add 2 methyl groups. We can do this addition in carbon 2 (2,2-dimethylbutane) or we can do this addition in carbon 2 and carbon 3 (2,3-dimethylbutane).
See figure 1
I hope it helps!
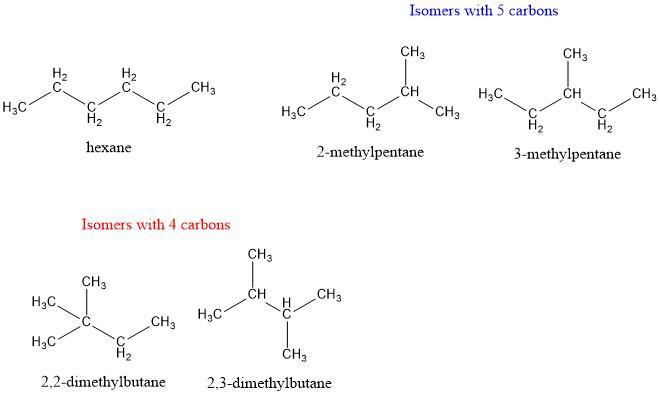
The first component is a six-carbon linear structure. Then we'll need to add a methyl group to a five-carbon linear structure.
Constitutional isomers:This methyl group can be linked to either carbon 2 or carbon 3 in the carbon chain. Finally, we can make a four-carbon linear structure by adding two methyl groups. This addition can be done with carbon 2 alone or with carbon 2 and carbon 3 together.
The constitutional isomers have the same chemical formula, but their atom connections are different. Chain isomers, position isomers, and functional group isomers are the three forms of constitutional isomers.
Find out more information about 'Constitutional isomers'.
https://brainly.com/question/9972986?referrer=searchResults
Related Questions
2NH3+2O2- N2O+3H2O
If 80.0 grams of O2 reacted in above reaction ,how many grams of N2O will be produced?
Answers
Answer:
55.0125 grams NO2
Explanation:
So we have 80 grams of O2, first convert to moles
80 g O2 * 1 mol/32 g O2 = 2.5 mol O2
Next use the mole ratio of O2 to NO2
2.5 mol O2* 1 mol NO2/2 mol NO2=1.25 mol NO2
Since the question is asking how many grams, convert NO2 to grams
1.25 mol NO2 * 44.01 NO2/1 mol NO2= 55.0125 grams
whats the difference between a covalent molecular and covalent network structure
Answers
Answer:
Covalent molecular structures are compounds containing molecules with covalent bonds. Covalent network structures are compounds composed of a network structure with covalent bonds between atoms throughout the material.
Explanation:
Answer:
Covalent molecular structures are compounds containing molecules with covalent bonds. Covalent network structures are compounds composed of a network structure with covalent bonds between atoms throughout the material.
2.
Which mixture could be a useful buffer in a solution?
acetic acid (CH3CO2H) and hydrochloric acid (HCl)
sodium hydroxide (NaOH) and elemental sodium (Na)
ammonia (NH3) and ammonium chloride (NH4Cl)
acetic acid (CH3CO2H) and ammonia (NH3)
Pls answer quickly
Answers
Ammonia (\(NH_3\)) and ammonium chloride (\(NH_4Cl\)) mixture could be a useful buffer in a solution. Option C
A buffer is a solution that can resist changes in pH when small amounts of acid or base are added. It consists of a weak acid and its conjugate base or a weak base and its conjugate acid. The buffer system works by the principle of Le Chatelier's principle, where the equilibrium is shifted to counteract the changes caused by the addition of an acid or a base.
In option A, acetic acid (\(CH_3CO_2H\)) is a weak acid, but hydrochloric acid (HCl) is a strong acid. This combination does not form a buffer because HCl is completely dissociated in water and cannot provide a significant concentration of its conjugate base.
Option B consists of sodium hydroxide (NaOH), which is a strong base, and elemental sodium (Na), which is a metal. This combination does not form a buffer as there is no weak acid-base pair involved.
Option D contains acetic acid (\(CH_3CO_2H\)), a weak acid, and ammonia (\(NH_3\)), a weak base. Although they are weak acid and base, they do not form a buffer system together as they are both weak acids or bases and lack the required conjugate acid-base pair.
Option C, ammonia (\(NH_3\)), is a weak base, and ammonium chloride (\(NH_4Cl\)) is its conjugate acid. This combination can form a buffer system. When ammonia reacts with water, it forms ammonium ions (NH4+) and hydroxide ions (OH-).
The ammonium ions act as the weak acid, while the ammonia acts as the weak base. The addition of a small amount of acid will be counteracted by the ammonium ions, and the addition of a small amount of base will be counteracted by the ammonia, thus maintaining the pH of the solution relatively stable.
Therefore, option C, consisting of ammonia (\(NH_3\)) and ammonium chloride (\(NH_4Cl\)), is the suitable mixture that could be a useful buffer in a solution.
For more such question on buffer visit:
https://brainly.com/question/13076037
#SPJ8
A group of students is investigating how a force applied to an object affects the motion of that object. The diagram shows the setup for the investigation. To create a force, the students add a weight to the weight holder and observe whether the cart moves. The students repeat this step five times, using a different amount of weight each time. Identify the independent and dependent variables of this investigation.
Answers
Answer:
87 is the wight of the hight
Explanation:
Identify the Lewis acid and the Lewis base in each the following reactions. (Omit states of matter.) a. B(OH)2(aq) + H2O(l) + B(OH)4 - (aq) + H+ (aq) Acid: Base: b. H2O(1) + CN- (aq) + HCN(aq) + OH- (aq) Acid: Base: C. HgI,(s) +21+ (aq) → Hg1,2(aq) Acid: Base:
Answers
Base: Water, b. \(HCN\) Acid Base \(OH-\) , c. Base: I- Acid:\(HgI2\) . Chemical substances known as acids have the ability to donate a proton \((H+)\) to a base or another molecule.
Chemical substances known as acids have the ability to donate a proton \((H+)\) to a base or another molecule. They have a sour flavour, have the power to dissolve metals, and can make litmus paper turn red. On the pH scale, where 7 is neutral and lower numbers indicate higher acidity, acids have a pH below 7. Hydrochloric acid, sulfuric acid, and acetic acid are a few typical examples of acids. Acids are essential for many chemical processes, such as digestion, the creation of energy, and the synthesis of numerous significant chemicals. Also, they are employed in a number of sectors, such as industry, food production, and agriculture.
Learn more about Acid Base here:
https://brainly.com/question/15717190
#SPJ4
The same mass of 5 different potential fuels was used to heat the same mass of water in a simple calorimeter. The results are shown below. Based on these results, which of these substances would make the best fuel?
Answers
We can see here that the best fuel is the one that produces the most heat per unit mass. In this case, the fuel that produces the most heat per unit mass is methanol.
What is fuel?Fuel is a substance that is used to produce energy through combustion or other chemical reactions. It is commonly utilized to power various forms of transportation, generate heat or electricity, and operate machinery and appliances.
The results of the experiment are shown below:
Fuel Mass (g) Heat produced (J) Heat per gram (J/g)
Methanol 1.0 350 350
Ethanol 1.0 250 250
Propane 1.0 200 200
Butane 1.0 150 150
Pentane 1.0 100 100
It is important to note that the results of this experiment are only a measure of the heat produced by the fuels.
Learn more about fuel on https://brainly.com/question/10172005
#SPJ1
The incredible catalytic power of enzymes can perhaps best be appreciated by imagining how challenging life would be without just one of the thousands of enzymes in the human body. For example, consider life without fructose-1,6-bisphosphatase, an enzyme in the gluconeogenesis pathway in liver and kidneys, which helps produce new glucose from the food we eat:
Fructose-1,6-bisphosphate + H2O → Fructose-6-phosphate + Pi
The human brain requires glucose as its only energy source, and the typical brain consumes about 120. g (or 480. calories) of glucose daily. Ordinarily, two pieces of sausage pizza could provide more than enough potential glucose to feed the brain for a day. According to a national fast-food chain, two pieces of sausage pizza provide 1260 calories, 49.0 % of which is from fat. Fats cannot be converted to glucose in gluconeogenesis, so that leaves 615 calories potentially available for glucose synthesis. The first-order rate constant for the hydrolysis of fructose-1,6-bisphosphate in the absence of enzyme is 2.00×10-20 sec-1.Calculate how long it would take to provide enough glucose for one day of brain activity from two pleces of sausage pizza without the enzyme.
Answers
Answer:
t = 7.58 * 10¹⁹ seconds
Explanation:
First order rate constant is given as,
k = (2.303 /t) log [A₀] /[Aₙ]
where [A₀] is the initial concentraion of the reactant; [Aₙ] is the concentration of the reactant at time, t
[A₀] = 615 calories;
[Aₙ] = 615 - 480 = 135 calories
k = 2.00 * 10⁻²⁰ sec⁻¹
substituting the values in the equation of the rate constant;
2.00 * 10⁻²⁰ sec⁻¹ = (2.303/t) log (615/135)
(2.00 * 10⁻²⁰ sec⁻¹) / log (615/135) = (2.303/t)
t = 2.303 / 3.037 * 10⁻²⁰
t = 7.58 * 10¹⁹ seconds
Are two atoms of the same element identical?
Answers
Answer: No, two atoms belonging to the same chemical element are not necessarily identical.
Explanation:
I hope that was helpful
which statement regarding the particles making up an atom is true? please choose the correct answer from the following choices, and then select the submit answer button. answer choices
Answers
A proton is a positively charged particle that has a mass of approximately 1 atomic mass unit (amu). A neutron is a particle with no charge and a negligible mass of approximately 1 amu.
An electron is a negatively charged particle that has a mass of approximately 1/1836 amu. A proton and a neutron have approximately the same mass because both particles have a mass of approximately 1 amu. A proton and an electron have approximately the same mass because the mass of the electron is much smaller than the mass of the proton. The mass of the proton is approximately 1836 times larger than the mass of the electron.
To learn more about proton click here https://brainly.com/question/1252435
#SPJ4
HELLPPPPP 5th grade math

Answers
Energy inputs, outputs and losses are summarized below:
Input - Chemical energy/Output - Luminous energy/Losses - Hystheresis-related dissipation.Input - Fluid energy, heat/Output - Translational mechanical energy/Losses - Waste energy.Input - Translational mechanical energy/Output - Rotational mechanical energy/Losses - Friction-related work.Input - Electric energy/Output - Translational and rotational mechanical energy/Losses - Power dissipation, drag-related work.Input - Translational and rotational mechanical energy/Output - Translational mechanical energy/Losses - Friction-related work, drag-related work.Input - Fluid energy, heat/Output - Translational and rotational mechanical energy/Losses - Waste energy, friction-related work and drag-related work.Procedure - Application of the principle of energy conservationIntroductionIn this question we must apply the definition of the principle of energy conservation to each case, understanding what kind of energy inputs (\(E_{in}\)) and outputs exists (\(E_{out}\), \(E_{loss}\)).
We proceed to apply a simplified scheme, on the assumption that each system works at steady state, in which we shall construct each answer:
\(E_{in} - E_{out} - E_{l} = 0\) (1)
Where:
\(E_{in}\) - Energy input.\(E_{out}\) - Energy output.\(E_{l}\) - Energy losses.Case analysisNow we proceed to summarize the inputs, outputs and losses for each case:
FlashlightInput - Chemical energy (battery)/Output - Luminous energy (screen)/Losses - Hystheresis-related dissipation (battery)
Hot air balloonInput - Fluid energy (fuel), heat (ignition)/Output - Translational mechanical energy (Buoyancy force)/Losses - Waste energy (smog)
Water wheelInput - Translational mechanical energy (water flow)/Output - Rotational mechanical energy (wheel)/Losses - Friction-related work (bearings, etc)
FanInput - Electric energy (current)/Output - Translational and rotational mechanical energy (wind)/Losses - Power dissipation (AC engine/cables), drag-related work (interaction between air and fan)
Hitting a golf ballInput - Translational and rotational mechanical energy (arm)/Output - Translational mechanical energy (ball)/Losses - Friction-related work (Human body, interactions between player and ball), drag-related work (interactions between ball and air)
MotorcycleInput - Fluid energy (fuel), heat (ignition)/Output - Translational and rotational mechanical energy (motorcycle and driver)/Losses - Waste energy (smog), friction-related work (interaction between tires and ground) and drag-related work (interactions between driver, motorcycle and surrounding air)
To learn more on energy conversion, we kindly invite to check this verified question: https://brainly.com/question/11234965
Answer:
Energy inputs, outputs and losses are summarized below:
Input - Chemical energy/Output - Luminous energy/Losses - Hystheresis-related dissipation.
Input - Fluid energy, heat/Output - Translational mechanical energy/Losses - Waste energy.
Input - Translational mechanical energy/Output - Rotational mechanical energy/Losses - Friction-related work.
Input - Electric energy/Output - Translational and rotational mechanical energy/Losses - Power dissipation, drag-related work.
Input - Translational and rotational mechanical energy/Output - Translational mechanical energy/Losses - Friction-related work, drag-related work.
Input - Fluid energy, heat/Output - Translational and rotational mechanical energy/Losses - Waste energy, friction-related work and drag-related work.
Procedure - Application of the principle of energy conservation
Introduction
In this question we must apply the definition of the principle of energy conservation to each case, understanding what kind of energy inputs () and outputs exists (, ).
We proceed to apply a simplified scheme, on the assumption that each system works at steady state, in which we shall construct each answer:
(1)
Where:
- Energy input.
- Energy output.
- Energy losses.
Case analysis
Now we proceed to summarize the inputs, outputs and losses for each case:
Flashlight
Input - Chemical energy (battery)/Output - Luminous energy (screen)/Losses - Hystheresis-related dissipation (battery)
Hot air balloon
Input - Fluid energy (fuel), heat (ignition)/Output - Translational mechanical energy (Buoyancy force)/Losses - Waste energy (smog)
Water wheel
Input - Translational mechanical energy (water flow)/Output - Rotational mechanical energy (wheel)/Losses - Friction-related work (bearings, etc)
Fan
Input - Electric energy (current)/Output - Translational and rotational mechanical energy (wind)/Losses - Power dissipation (AC engine/cables), drag-related work (interaction between air and fan)
Hitting a golf ball
Input - Translational and rotational mechanical energy (arm)/Output - Translational mechanical energy (ball)/Losses - Friction-related work (Human body, interactions between player and ball), drag-related work (interactions between ball and air)
Motorcycle
Input - Fluid energy (fuel), heat (ignition)/Output - Translational and rotational mechanical energy (motorcycle and driver)/Losses - Waste energy (smog), friction-related work (interaction between tires and ground) and drag-related work (interactions between driver, motorcycle and surrounding air)
I WILL GIVE 35 POINTS TO THOSE WHO ANSWER THIS QUESTION RIGHT NOOOO SCAMS PLEASE

Answers
The solution has a molarity of 0.0924 M.
What is molarity, for instance?The number of moles of solute per litre of solution is known as molarity.. For instance, water is both the solution and the solute when table salt is dissolved in it. Each mole of sodium chloride weighs 58.44 grammes. 58.44 grammes of sodium chloride are dissolved in one litre of water to produce one molar solution, or 1M.
Moles of solute per litre of solution is known as molarity (M).
Given: moles of NH3 = 0.355, volume of solution = 3.84 L
Molarity = 0.355 moles / 3.84 L = 0.0924 M
Therefore, the molarity of the solution is 0.0924 M.
To know more about molarity visit:-
https://brainly.com/question/8732513
#SPJ1
For electricity to flow, what do you need?
A
only a battery
B
only a battery and a light bulb
only a battery, light bulb and wire
D
a battery, light bulb and wire in a closed circuit
Answers
Answer:
D
Explanation:
you need battery, light bulb and wire in a close circuit
Answer:
D - a battery, light bulb and wire in a closed circuit.
Explanation:
A battery due to the charge in it.
A light bulb so the energy has somewhere to go to.
A wire in a closed circuit, so that the energy has a place for transport without interruptions.
A liquid ester used to flavour food is believed to be impure. What would be the best way of testing its purity?
Answers
Answer:
Filter it
Explanation:
The temperature of a sample of gas in a steel container at 25.0 kPa starts at -50 C and decreases by a factor of three. What is the final pressure inside the tank?
Answers
Answer: The final pressure inside the tank is 8.41 kPa.
Explanation: We can use the combined gas law to solve this problem, which relates the pressure, volume, and temperature of a gas:
(P1V1)/T1 = (P2V2)/T2
where P1, V1, and T1 are the initial pressure, volume, and temperature, respectively, and P2, V2, and T2 are the final pressure, volume, and temperature, respectively.
We are given P1 = 25.0 kPa, T1 = -50 C = 223 K, and V1 is unknown. We also know that the temperature decreases by a factor of three, so T2 = T1/3 = 223/3 K.
To find V2, we need to assume that the steel container is rigid and its volume remains constant. Therefore, V1 = V2, and we can cancel out the volume from the equation:
P1/T1 = P2/T2
Substituting the values, we get:
P2 = P1 * T2 / T1 = 25.0 * (223/3) / 223 = 8.41 kPa
Therefore, the final pressure inside the tank is 8.41 kPa.
Answer:
So if pressure of a gas is increased by 25%, the volume of a gas is decreased by 25%.
Explanation:
Balance the following chemical reaction equation:
FeS + _02_Fe2O3 + ___SO2
Answers
Explanation:
the answer is in the image above

Due to the tendency to form crystals instead of discrete molecules the formula unit is the representative particle for ___ compounds
Answers
Answer:ionic
Explanation:
15.3 Determine the mass of the following: 15.3.1. 2 mol calcium atoms
15.3.2. 0,3 mol nitrogen molecules
15.3.3. 0,5 mol table salt
15.3.4. 0,2 mol hydrochloric acid
15.3.5. 0,08 mol sodium carbonate (Na2CO3)
15.3.6. 0,25 mol ammonium sulphate (NH4)2SO4
Answers
Answer:
hh
Explanation:
write name of a plant having aquatic habitat
Answers
Answer:
Cattail
Explanation:
Cattail is the plant which have aquatic habitat.
What is aquatic habitat?An area with water that directly supports a particular species, population, as well as the community is referred to as an aquatic habitat.
What is Cattail?Cattails were also perennial upright plants that grow from rhizomes which creep. The long, tapering petals seem to be slightly spongy and even have smooth borders.
Cattail is a plant which has aquatic habitat.
To know more about cattail
https://brainly.com/question/15224375
#SPJ3
Experiment 4: A chemist mixes aqueous solutions of sodium hydroxide and aluminum chloride in a double-displacement reaction, which forms a white solid precipitate and a clear solution. Write the complete, balanced molecular equation for the reaction. Include physical states.
balanced equation:
Answers
The balanced molecular equation for the reaction between sodium hydroxide (NaOH) and aluminum chloride (\(AlCl_3\)) in aqueous solution can be written as follows: 2NaOH(aq) + 3\(AlCl_3\)(aq) → 3NaCl(aq) + \(Al(OH)_3\)(s)
In this reaction, sodium hydroxide (NaOH) reacts with aluminum chloride (\(AlCl_3\)) to form sodium chloride (NaCl) and aluminum hydroxide (\(Al(OH)_3\)). The coefficients in the balanced equation indicate the stoichiometric ratio between the reactants and products.
The physical states of the substances are indicated by the symbols (aq) for aqueous solutions and (s) for the solid precipitate.
The reaction is a double-displacement reaction, also known as a precipitation reaction. Double-displacement reactions involve the exchange of ions between two compounds, resulting in the formation of a precipitate.
In this case, sodium hydroxide and aluminum chloride react to form sodium chloride and aluminum hydroxide, with aluminum hydroxide being the white solid precipitate.
It's worth noting that the actual reaction might involve hydrated forms of the compounds, such as NaOH·x\(H_2O\) and \(AlCl_3\)·y\(H_2O\). However, for simplicity, these hydrated forms are not included in the balanced equation.
Overall, the balanced equation represents the chemical reaction between sodium hydroxide and aluminum chloride, showing the reactants, products, and their stoichiometric ratios.
For more such question on balanced molecular equation visit:
https://brainly.com/question/11904811
#SPJ8
according to the big bang theory, which describes the universe before the actual big bang occurred?
Answers
Answer:
the answer is hot,dense points; each smaller than an atom
Explanation:
the big bang theory was proposed to suggest the expansion of the universe by describing the origin of all components of the universe and its planetary bodies. its suggested that the whole universe was in a state of high temperature and highly dense points (which were smaller than atoms) but continuously expanded by cooling down which gave rise to the formation of subatomic particles, atoms, etc.
btw I found this on brainly
a molecule that functions as the electron donor in a redox reaction ______
Answers
The atom or molecule that contributes electrons—in this case, magnesium is known as the reducing agent because the reduction of another molecule is made possible by the electrons it donates.
What is the electron acceptor in a redox reaction?a molecule that, during a redox reaction, takes or absorbs electrons from another molecule. In a redox reaction, an electron acceptor reduces itself and acts as an oxidizing agent. Oxygen, nitrate, iron (III), manganese (IV), sulfate, carbon dioxide, and other elements are examples of acceptors.
The electron donor is the reducing agent, right?A chemical species that "donates" an electron to an electron recipient is referred to as a reducing agent in chemistry. This chemical species is also referred to as a reductant, reducer, or electron donor (called the oxidizing agent, oxidant, oxidizer, or electron acceptor).
To know more about redox reaction visit:-
https://brainly.com/question/13293425
#SPJ4
Which of the following is not a correct statement regarding the energy in a chemical bond? It is stored between atoms. It is known as bond energy. It is energy associated with motion. It has a fixed quantity.
Answers
Answer:it has fixed quantity
Explanation:energy between chemical bonds is hard to measure
Answer:
It has a fixed quantity.
Explanation:
Suggest some ways in which you might determine whether a particular water solution contains an acid or a base.
Answers
The use of the pH scale and litmus paper are the ways which can determine whether the solution is an acid or base.
How pH scale and litmus paper tell about acidic and basic solution?The pH scale is the way which determine the acidic or basic nature of a solution. If the pH of a solution is less than 7, it is considered as acidic. If the pH is 7, the solution is neutral and if the pH is greater than 7,the solution is basic.
While on the other hand, litmus paper also tells us about acidic and basic nature of solution. if the litmus paper turns red, it means the solution is acidic whereas if the litmus paper turns blue it means basic.
Learn more about acid here: https://brainly.com/question/25148363
state with examples four characteristic properties of transition metals
Answers
Answer:
good conductor of heat and electricity
they can be hammered or bent into shape easily.
they have high melting points(except mercury as they are liquid at room temperature)
they are usually hard and tough.
they have high density
How many grams of Ag2S2O3 form when 125.0 g AgBr reacts completely according to the reaction below

Answers
The reaction as given is
Na2S2O3 + AgBr ==> NaBr + Na3[Ag(S2O3)2]
Step 1: Balance the equation
2Na2S2O3 + AgBr ==> Na3[Ag(S2O3)2] + NaBr
Step 2: Calculate moles of AgBr present
42.7 g AgBr x 1 mole AgBr/187.8 g = 0.227 moles AgBr
Step 3: Use the mole ratio of the balanced equation to calculate moles Na2S2O3 needed
The mole ratio of Na2S2O3 to AgBr is 2:1
0.227 moles AgBr x 2 moles Na2S2O3 / mole AgBr = 0.454 moles Na2S2O3 needed (answer to part 1)
Step 4: Use the mole ratio and molar mass of NaBr to calculate mass of NaBr produced
0.227 moles AgBr x 1 mol NaBr/mol AgBr x 103 g NaBr/mol NaBr = 23.4 g NaBr produced (answer to part 2)
To know more about silver bromide use link below:
https://brainly.com/question/16958040
#SPJ1
Answer:109.13
Explanation:
After considering the results of the classification tests, the possibilities can be narrowed down further by determining the melting point of the
A derivative.
B Schiff addition product.
C iodoform.
D triiodo compound.
Answers
After considering the results of the classification tests, the possibilities can be narrowed down further by determining the melting point of the B Schiff addition product.
What two facts can you infer about your product based on its melting point range?
A compound's melting point is helpful in two ways: it provides information on the composition's identity and degree of purity. Impurities will affect a compound's melting point, resulting in broader and lower melting point ranges.
First, the solubility test must be performed in order to remove soluble contaminants from the target molecule. The desired chemical and the soluble impurities are dissolved in a minimum of near or boiling solvent once a suitable solvent has been selected. The solution is then given time to gradually cool.
Learn more about Schiff addition product refer
https://brainly.com/question/14748028
#SPJ4
ASAPP Which electron below is furthest from the nucleus?

Answers
3) An electron in the 4s orbital
Hope it helps
science vocab (image attached)

Answers
Answer:
Solution-a liquid mixture in which the minor component (the solute) is uniformly distributed within the major component (the solvent).
solvent- able to dissolve other substances.
solute- the minor component in a solution, dissolved in the solvent.
colloid- a homogeneous noncrystalline substance consisting of large molecules or ultramicroscopic particles of one substance dispersed through a second substance. Colloids include gels, sols, and emulsions; the particles do not settle, and cannot be separated out by ordinary filtering or centrifuging like those in a suspension.
suspension- is a heterogeneous mixture that contains solid particles sufficiently large for sedimentation.
Explanation:
What do you notice about the pattern of volcanoes?
Answers
Answer:
What patterns do volcanoes form?
Volcanoes occur at convergent plate boundaries were subducting oceanic crust is melted. This magma rises through the crust to form volcanoes and volcanic island arcs. Volcanoes occur at divergent plate boundaries where upwelling magma pushes between plates (rift zones) as the plates move apart.
This political cartoon drawn by John Donaghy appeared in The Daily Graphic on September 16, 1873. The message of this cartoon is reflective of what situation in the late 1800s?