For an atom of chlorine-35, what number would you put as the SUPERSCRIPT in the atomic notation?
Answers
Related Questions
why does benzophenone dissolve in methanol
Answers
Benzophenone dissolves in methanol because it forms intermolecular hydrogen bonds with the methanol molecules.
Methanol is a polar solvent, meaning it has a positive and negative end. Benzophenone is also polar, with the carbonyl group (C=O) being the most polar part of the molecule. The oxygen atom in the carbonyl group has a partial negative charge, and the hydrogen atoms in the methanol molecule have a partial positive charge.
This allows for the formation of intermolecular hydrogen bonds between the benzophenone and methanol molecules. These hydrogen bonds increase the solubility of benzophenone in methanol, allowing it to dissolve.
In summary, benzophenone dissolves in methanol due to the formation of intermolecular hydrogen bonds between the polar molecules.
To know more about Benzophenone click here:
https://brainly.com/question/30227023#
#SPJ11
Name this compound. Please help
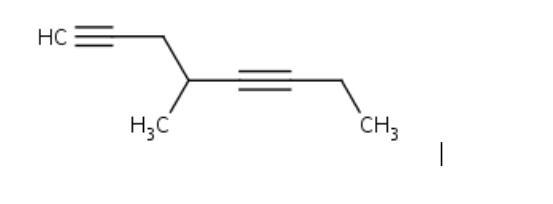
Answers
Answer:
mitosis
Explanation:
Pls help I will give u points pls

Answers
Answer:
i think D
Explanation:
Jessica is holding a ball in her hand and then she drops the ball. Which energy transformation is happening?
A. stored energy to motion energy
B. stored energy to sound energy
C. magnetic energy to stored energy
D. electrical energy to motion energy
Answers
Answer:
The 1-kg ball accelerates twice as much as the 2-kg ball.
Explanation:
please add me as brainliest.....
What mass of sucrose needs to be dissolved into water in order to prepare a 15% by mass solution with a total mass of 650 g
Answers

The mass of sucrose needs to be dissolved will be "97.5 g".
SucroseA cube of sugar or sweetener that is composed of yet another molecule of glucose as well as one component of fructose linked simultaneously.
According to the question,
Mass percentage = 15%
Total mass = 650 g
Let,
The mass of sucrose be "x".
Now,
The mass of sucrose will be:
→ 0.15 = \(\frac{x}{650}\)
By applying cross-multiplication, we get
x = 0.15 × 650
= 97.5 g
Thus the above answer is appropriate.
Find out more information about sucrose here:
https://brainly.com/question/211758
What happens when two objects with different masses collide?
Answers
Answer:
if the colliding objects have unequal mass, they will have unequal accelerations as a result of the contact force that results during the collision.
Help me respond this question please

Answers
Answer:
It has two valence electrons
an hno3(aq) solution has a ph of 1.75. what is the molar concentration of the hno3(aq) solution?
Answers
The molar concentration of the HNO3(aq) solution with a pH of 1.75 is approximately 0.0177827941 M.
The pH of a solution is a measure of its acidity or alkalinity. In the case of the HNO3(aq) solution with a pH of 1.75, it indicates that the solution is strongly acidic. To determine the molar concentration of the HNO3(aq) solution, we need to understand the relationship between pH and the concentration of hydrogen ions (H+).
To find the molar concentration of the HNO3(aq) solution, we can use the relationship between pH and the concentration of H+ ions. The equation is:
[H+] = 10^(-pH)
Substituting the given pH value of 1.75 into the equation:
[H+] = 10^(-1.75)
Calculating this expression, we find that [H+] ≈ 0.0177827941.
Since HNO3 is a strong acid and dissociates completely in water, the concentration of HNO3 in the solution is equal to the concentration of H+ ions. Therefore, the molar concentration of the HNO3(aq) solution is approximately 0.0177827941 M.
Know more about Molar Concentration here:
https://brainly.com/question/21841645
#SPJ11
Why is there not a constant molar volume for solids and liquids?
a Solid and liquid particles are packed close together.
b The densities of solids and liquids are variable.
C The volume of a solid or a liquid has very little empty space.
d All of the above
Answers
Answer:D
Explanation:
If a dextrose solution had an osmolarity of 100 mosmol/l, what percentage (w/v) of dextrose (mw = 198.17) would be present?
Answers
The percentage (w/v) of dextrose in the solution is approximately 1.9817%. To determine the percentage (w/v) of dextrose in a solution with a given osmolarity, we need to calculate the amount of dextrose present in 100 mL of the solution.
First, we convert the osmolarity from mosmol/L to mosmol/100 mL:
100 mosmol/L = 100 mosmol/100 mL
Next, we calculate the number of moles of dextrose present in 100 mL of the solution:
Number of moles = Osmolarity (in mosmol/100 mL) / 1000
Number of moles of dextrose = 100 mosmol/100 mL / 1000 = 1 mosmol/100 mL
Now, we can calculate the mass of dextrose present in 100 mL of the solution:
Mass of dextrose = Number of moles of dextrose * Molecular weight of dextrose
Mass of dextrose = 1 mosmol/100 mL * 198.17 g/mol = 1.9817 g/100 mL
Finally, we can calculate the percentage (w/v) of dextrose:
Percentage (w/v) = (Mass of dextrose / Volume of solution) * 100
Percentage (w/v) = (1.9817 g/100 mL / 100 mL) * 100 = 1.9817%
Therefore, the percentage (w/v) of dextrose in the solution is approximately 1.9817%.
Learn more about osmolarity here:
https://brainly.com/question/32470302
#SPJ11
what are the subatomic particles by which atom made of?
Answers
Answer:
The three main subatomic particles that form an atom are protons, neutrons, and electrons.
Explanation:
Answer:
The three main subatomic particles that form an atom are protons, neutrons, and electron
The Kw for water at 40°C is 2.92 x 10-14 What is the pH of a 0.12M solution of an acid at this temperature, if the pKb of the conjugate base is 6.3? 04.08 4.37 O 5.21 O 3.85 O 4.96
Answers
4.96 is the pH of a 0.12M solution of an acid at this temperature, if the pKb of the conjugate base is 6.3.
To answer this question, we need to use the relationship between the pH, pKb, and the concentration of the acid. First, we need to find the pKa of the acid, which is equal to 14 - pKb. So, pKa = 14 - 6.3 = 7.7.
Next, we can use the Henderson-Hasselbalch equation, which is pH = pKa + log([conjugate base]/[acid]). We know the pKa, but we need to find the concentration of the conjugate base. To do this, we can use the fact that Kw = [H+][OH-] = 2.92 x 10^-14. At 40°C, [H+] = [OH-] = 1.70 x 10^-7 M.
Since the acid is not the same as the conjugate base, we need to use stoichiometry to find the concentration of the conjugate base. Let x be the concentration of the acid that dissociates. Then, the concentration of the conjugate base is also x, and the concentration of the remaining undissociated acid is 0.12 - x.
The equilibrium equation for the dissociation of the acid is HA + H2O ↔ H3O+ + A-. The equilibrium constant is Ka = [H3O+][A-]/[HA]. At equilibrium, the concentration of H3O+ is equal to x, the concentration of A- is also equal to x (since they have a 1:1 stoichiometry), and the concentration of HA is 0.12 - x. So, Ka = x^2/(0.12 - x).
Using the definition of Ka and the given value of Kw, we can set up the following equation:
Ka * Kb = Kw
(x^2/(0.12 - x)) * (10^-14/1.70 x 10^-7) = 2.92 x 10^-14
Simplifying, we get:
x^2 = 5.7552 x 10^-6
x = 7.592 x 10^-3 M
Now we can use the Henderson-Hasselbalch equation to find the pH:
pH = 7.7 + log(7.592 x 10^-3/0.12)
pH = 4.96
Therefore, the answer is 4.96.
To know more about Conjugate base visit:
https://brainly.com/question/30225100
#SPJ11
Jane made this picture to represent a chemical reaction
Which of the following statements best explains the type of chemical reaction represented by Jane's picture?
It represents a synthesis reaction because one combined reactant forms multiple products
Olt represents a synthesis reaction because the same atoms are present in the reactants and products
It represents a decomposition reaction because one reactant breaks apart and forms two products
It represents a decomposition reaction because the total mass of the products is less than the mass of the reactant.

Answers
Answer:
It represents a decomposition reaction because one reactant breaks apart and forms two products
Explanation:
Your welcome
To solve such this we must know the concept of redox reaction. Therefore, the correct option is option C. It represents a decomposition reaction because one reactant breaks apart and forms two products.
What is chemical reaction?Chemical reaction is a process in which two or more than two molecules collide in right orientation and energy to form a new chemical compound. The mass of the overall reaction should be conserved. There are so many types of chemical reaction reaction like combination reaction, double displacement reaction.
Decomposition reaction is a chemical reaction where one reactant breaks down into two or more than two products on supplying energy in form of heat or electricity.
Therefore, the correct option is option C. It represents a decomposition reaction because one reactant breaks apart and forms two products.
Learn more about the chemical reactions, here:
brainly.com/question/3461108
#SPJ2
How many total electrons must be transferred to form one formula unit of Al2O3?
Answers
To form one formula unit of Al2O3, a total of 12 electrons (6 from aluminum and 6 from oxygen) must be transferred.
The compound Al2O3, commonly known as aluminum oxide, is formed when two aluminum atoms and three oxygen atoms bond. To determine the number of total electrons that must be transferred to form one formula unit of Al2O3, we first need to find the number of electrons that are present in one formula unit of Al2O3.
Aluminum has an atomic number of 13, which means it has 13 protons and 13 electrons in a neutral atom. Each aluminum atom in Al2O3 contributes 3 electrons, and since there are two aluminum atoms in one formula unit of Al2O3, the total number of electrons contributed by aluminum atoms is 6.
Oxygen has an atomic number of 8, which means it has 8 protons and 8 electrons in a neutral atom. Each oxygen atom in Al2O3 contributes 2 electrons, and since there are three oxygen atoms in one formula unit of Al2O3, the total number of electrons contributed by oxygen atoms is 6.
Therefore, to form one formula unit of Al2O3, a total of 12 electrons (6 from aluminum and 6 from oxygen) must be transferred.
To learn more about electrons click on the given link:
https://brainly.com/question/860094
#SPJ11
What would be the final temperature of 60.0 g of benzene if you added 1530 cal of heat to it? Assume its initial temperature was 25° c, and the specific heat of benzene is 0.42 cal/g ·°c.
Answers
Adding 1530 cal of heat to 60.0 g of benzene results in a final temperature of approximately 86.4°C.
To calculate the final temperature of benzene after adding heat, we can use the equation:
q = m * c * ΔT
where q is the heat added, m is the mass of the substance, c is the specific heat capacity, and ΔT is the change in temperature.
Rearranging the equation, we can solve for ΔT:
ΔT = q / (m * c)
ΔT = 1530 cal / (60.0 g * 0.42 cal/g·°C)
ΔT ≈ 61.4°C
To find the final temperature (T₂), we add ΔT to the initial temperature:
T₂ = T₁ + ΔT
T₂ = 25°C + 61.4°C
T₂ ≈ 86.4°C
Therefore, the final temperature of the benzene would be approximately 86.4°C after adding 1530 cal of heat.
To know more about benzene refer here :
https://brainly.com/question/9907481#
#SPJ11
Identify indicators of a chemical reaction. Check all of the boxes that apply.
Two clear liquids are combined. A green solid forms.
color change
absorption of heat
formation of precipitate
formation of gas
Blobs of green solid are shown in clear liquid in a test tube.
Answers
Answer:color change and formation of precipitate.
Explanation: The reaction of products of the chemical reaction may have different properties
According to VSEPR theory, if there are two electron domains on a central atom, they will be arranged such that the angles between the domains are __________. a. 360° b. 120° c. 90° d. 180° e. 109.5°
Answers
Answer:
Option d is correct
Explanation:
The basis of the VSEPR model of molecular bonding is that to minimize repulsions, electron domains in the valence shell of an atom arrange on their own.
According to VSEPR theory, if there are two electron domains on a central atom, they will be arranged such that the angles between the domains are 180°
How does sunlight affect seasons?
Answers
Answer:
Seasons are caused by the Earth’s revolution around the Sun, as well as the tilt of the Earth on its axis. The hemisphere receiving the most direct sunlight experiences spring and summer, while the other experiences autumn and winter. During the warmer months, the Sun is higher in the sky, stays above the horizon for longer, and its rays are more direct.
What is the atomic mass of Carbon?
Answers
Answer:
Ar = 12
Explanation:
I think at GCSE level it's 12. It might be more specific in A-Level and further.
Answer:
atomic mass of carbon is
12.0107 u mark me as brainlist
]
Consider the following intermediate chemical equations.
C(s) +
CO(g) +
O₂(g) → CO(g)
How will oxygen appear in the final chemical equation?
O O2(g) as a product
O O2(g) as a reactant
OO(g) as a product
O 20(g) as a reactant
O₂(g) → CO₂(g)
Answers
In the above intermediate chemical equation, oxygen will appear as follows: O₂(g) as a reactant (option B).
What is a chemical equation?A chemical equation in chemistry is a symbolic representation of a chemical reaction where reactants are represented on the left, and products on the right.
According to this question, an intermediate chemical equation is presented as follows:
CO(g) + O₂(g) → CO(g)C(s) + O₂(g) → CO₂(g)As observed in the above chemical equation, oxygen will react in its gaseous form i.e. as a reactant.
Learn more about chemical equation at: https://brainly.com/question/28294176
#SPJ1
What is the concentration of ALL ions in a 0.20 M solution of Na3PO4
Answers
Answer:
0.80 M ion concentration
Explanation:
Step 1: Define
0.20 M solution of Na₃PO₄
Step 2: Find ions (Decomposition RxN)
Na₃PO₄ → 3Na⁺ + PO₄³⁻
Step 3: Find ion concentration
3Na⁺ - 3(0.20 M) = 0.6 M Na⁺ ion
PO₄³⁻ - 1(0.20 M) = 0.2 M PO₄³⁻ ion
Step 4: Add ion concentrations
0.6 M + 0.2 M = 0.80 M ion concentration
Identify the element that matches this orbital diagram

Answers
Answer:
Nitrogen
Explanation:
The electron configuration of nitrogen is:
1s2 2s2 2p3
which satisfies the diagram.
read each of the sentences an associate it with the correct number of genes affecting phenotypes. place each sentence into the correct box (HINT: one sentence should be sorted into both boxes.)

Answers
Numerous genes: Multiple genes and environmental factors frequently interact to cause genetic diseases. Mutations in a single gene are what cause sickle cell anemia.
What is anemia?Anemia is a condition in which there are not enough healthy red blood cells in the body. Oxygen is transported throughout the body by red blood cells from the lungs. Fatigue, weakness, dizziness, coldness in the extremities, pale skin, and difficulty concentrating are all signs of a lack of these cells in the body. An underlying medical condition, such as an iron or vitamin deficiency or chronic disease, frequently causes anemia. The root cause of anemia will determine the course of treatment.
Learn more about anemia :
brainly.com/question/822235
#SPJ1
Which of the following terms refers to the area immediately around the eye
Answers
Answer:
the eye socket
Explanation:
the area around the eye is called the eye socket or the eye orbit.
which is activation energy?
A-The kinetic energy of the reactants of a system after the reaction has begun
B-the energy released when atomic bonds are broken
C-the energy that must be added to a system to break the bonds of the reactants
D-the total potential energy of the bonds of the reactants in a system
Answers
As activation energy is the required amount of energy needed for a reaction to occur.
The activation energy of a molecule or compound is the minimum amount of energy that must be added to to a system to break the bonds of the reactants. Hence, option C is correct.
What are reactants?Reactants are reacting species in a chemical reaction, that gives new products by regrouping atom in reactants. The valence electrons of reactants are participated in a chemical reaction.
Reactants needs some energy to activate themselves lead to effective collision between reactants to result in products. This additional energy required by reactants is called the activation energy.
This energy is needed weaken the intermolecular attraction and electrostatic forces within the atoms of reactants and enabling them to be reactive.
Find more on activation energy:
https://brainly.com/question/11334504
#SPJ2
Which of the following statements concerning the density of a gas is true?
Answers
Answer:
can u give us the options
could someone help me!
Consider a cup made of plastic. What are the pros of using plastic?
Answers
Answer:
It's cheap to make, and its recyclable
If D is neutral liquid, which of the following is C
heat
MgCO3
(A) + (B)
C с
MgCl2 + (D)
Answers
Answer:
Cl2
Explanation:
Bcs in product there is chorine
2KClO3 Right arrow. 2KCl + 3O2
How many moles of oxygen are produced when 2 mol of potassium chlorate (KClO3) decompose?
1
2
3
6
Answers
Answer:
3 moles
Explanation:
The balanced chemical equation for the decomposition of potassium chlorate is:
2KClO3 → 2KCl + 3O2
This means that for every 2 moles of KClO3 that decompose, 3 moles of O2 are produced. We are given that 2 moles of KClO3 decompose, so we can use the stoichiometry of the balanced equation to determine the amount of O2 produced.
2 moles KClO3 × (3 moles O2 / 2 moles KClO3) = 3 moles O2
Therefore, 3 moles of O2 are produced when 2 moles of KClO3 decompose.
how is a solute and a solvent different