For molecules of general formula AXIn (where n > 2), how do you determine if a molecule is polar?
Answers
In large molecules, the molecular polarity molecular polarity depends on both the bond shape and bond polarity. When there is a polar bond, it doesn't necessarily mean that the molecule is polar; the shape and the atoms around the central atom must be considered. Example for AX\(_{2}\): BeCl\(_{2}\) - both Be-Cl bonds in this molecule are polar because of the difference in electronegativity, but the molecule has no polarity because the molecule shape is linear (180∘∘) and both polarities cancel each other.
Example for AXn (n >> 2):SO\(_{2}\) (AX\(_{2}\)E) - we can see that there is assymetric distribution of electron between S and O atoms; there are more electrons around more electronegative O atom. This makes permanent dipole moment, which makes SO\(_{2}\) molecule. Polarities doesn't cancel because the molecule is V shaped.
When there is a lone electron pair around central atom, that changes the molecule shape in a way that polarities cannot cancel each other, then the molecule is polar - AXnEn
to know more about polarity
brainly.com/question/3184550
#SPJ4
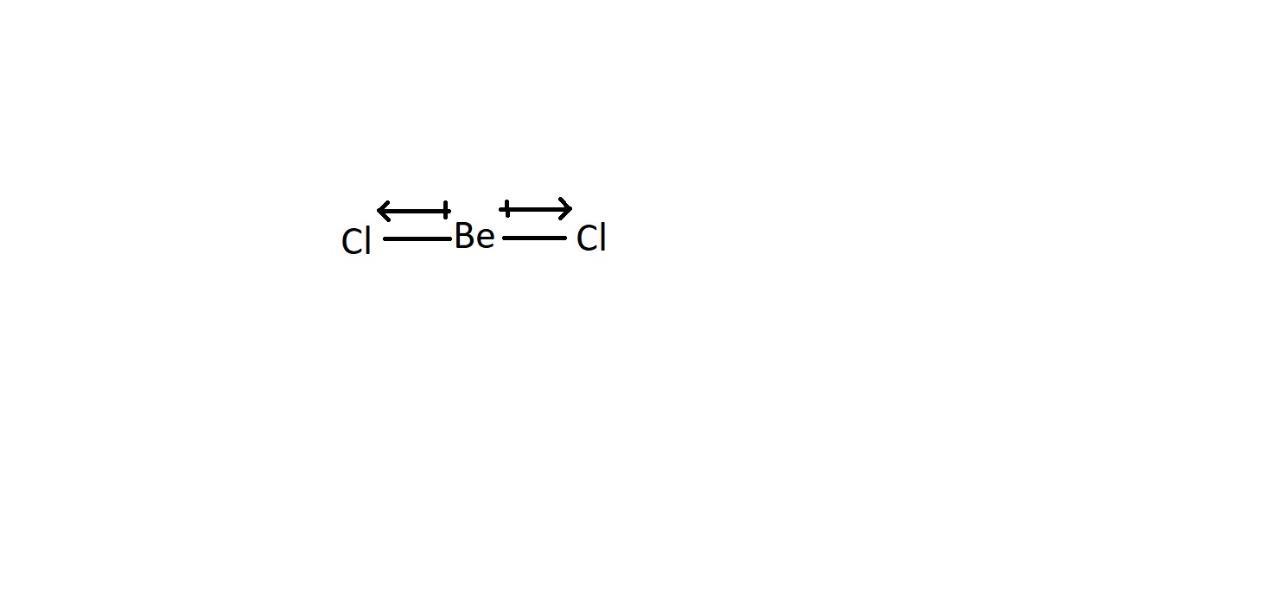

Related Questions
Write the balanced complete ionic equations and net ionic equations for the reactions that occur when each of the following solutions are mixed. (Type your answers using the format [NH4]+ for NH4+ or Ca3(PO4)2 for Ca3(PO4)2. Use the lowest possible coefficients.)(a) Cr2(SO4)3(aq) and (NH4)2CO3(aq)complete ionic equation:(aq) + CO32-(aq) + Cr3+(aq) + SO42-(aq) (s) + NH4+(aq) + (aq)net ionic equation:Cr3+(aq) + (aq) (s)(b) FeCl3(aq) and Ag2SO4(aq)complete ionic equation:(aq) + Cl-(aq) + Ag+(aq) + SO42-(aq) (s) + Fe3+(aq) + (aq)net ionic equation:Ag+(aq) + (aq) (s)(c) Al2(SO4)3(aq) and K3PO4(aq)complete ionic equation:(aq) + PO43-(aq) + Al3+(aq) + SO42-(aq) (s) + K+(aq) + (aq)net ionic equation:Al3+(aq) + (aq) (s)
Answers
(a) \(Cr_2(SO_4)_3\)(aq) and \((NH_4)_2CO_3\)(aq)
Complete ionic equation: \(Cr^{3+\)(aq) + \(3SO_{42}\)-(aq) + 2\(NH_4\)+(aq) + \(CO_3^{2-}\)(aq) → \(Cr_2(CO_3)_3\)(s) + 6\(NH^{4+}\)(aq) + 6\(SO_4^{2-}\)(aq)
Net ionic equation: \(Cr^{3+\)(aq) + 3 \(CO_3^{2-}\)(aq) → \(Cr_2(CO_3)_3\)(s)
(b) \(FeCl_3\)(aq) and\(Ag_2SO_4\)(aq)
Complete ionic equation: \(Fe^{3+\)(aq) + 3Cl-(aq) + 2Ag+(aq) + \(SO_4^{2-}\)(aq) → 2AgCl(s) + \(Fe^{3+\)(aq) + \(SO_4^{2-}\)(aq)
Net ionic equation: 2Ag+(aq) + 2Cl-(aq) → 2AgCl(s)
(c) \(Al_2(SO_4)_3\)(aq) and \(K_3PO_4\)(aq)
Complete ionic equation: \(2Al^{3+\)(aq) + 6\(SO_4^{2-}\)(aq) + 6K+(aq) + 2\(PO_4^{3-}\)(aq) → \(Al_2(PO_4)_3\)(s) + 6K+(aq) + 6\(SO_4^{2-}\)(aq)
Net ionic equation: \(2Al^{3+\)(aq) + 2\(PO_4^{3-}\)(aq) → \(Al_2(PO_4)_3\)(s)
These are examples of double displacement or precipitation reactions, where two solutions containing ionic compounds are mixed and an insoluble product (precipitate) is formed.
The complete ionic equation shows all the ions present in the solution before and after the reaction, while the net ionic equation only includes the ions that participate in the formation of the precipitate.
In each reaction, the cations and anions switch partners to form new compounds. In the complete ionic equation, each ion is shown as either aqueous (aq) or solid (s) based on whether it remains in solution or forms a solid precipitate.
In the net ionic equation, only the ions that form the solid product are included, and any spectator ions that do not participate in the reaction are removed.
To know more about "Solid" refer here:
https://brainly.com/question/5654381#
#SPJ11
Pls help with this question!!
Zinc sulfate is an ionic compound formed between one atom of zinc and one copy of the polyatomic ion sulfate, which contains one atom of sulfur and four atoms of oxygen. The molar mass of zinc sulfate is calculated above.
What percent of the mass in a sample of zinc sulfate comes from the zinc?

Answers
The percent of the mass in a sample of zinc sulfate that comes from zinc would be 40.50%. Option 5.
Percent CompositionThe percent composition of a component in a substance is the ratio of the mass of the component and the mass of the substance itself.
This can be mathematically expressed as;
Pecent composition= mass of component/mass of substance x 100%
In this case, the substance is zinc sulfate, a compound that contains zinc, sulfur, and oxygen in a ratio 1:1:4.
The molar weight of zinc is 65.38 while the molar mass of the entire zinc sulfate is 161.442.
Thus, the percent of the mass of any sample of zinc sulfate that comes from zinc would be:
Percent zinc = 65.38/161.442 x 100
= 40.50%
In other words, the percent of zinc in any zinc sulfate sample would be 40.50%
More on percent composition can be found here: https://brainly.com/question/17505281
#SPJ1
what happpens when nitrogen fills its valence shell?
A. Three electrons are lost, creating N+3
B. Three electrons are gained, creating N-3
C. Three electrons are gained creating N+3
D. Three electrons are lost, creating N-3
Answers
Nitrogen needs to gain 3 electrons to have a full valence shell so whenever its given those three it becomes N-3
CER: Momentum and Collisions - Preventing Concussions in Football Players and
Woodpeckers
Using the question below, develop a claim-evidence-reasoning to fully answer and explain
every part of the phenomenon we looked at in this part of the Unit.
Question: Why do football players get concussions, but woodpeckers don't?
Claim (your answer to the question; 1 sentence):
Evidence (values, descriptions, and observations that support your claim; 2-3
sentences):
Reasoning (explanation of the evidence to further support your claim; 3-4 sentences):
Answers
Claim: Woodpeckers don't get concussions while football players do because woodpeckers have evolved several adaptations that protect their brains from high impact forces that occur during pecking, while football helmets are not designed to prevent concussions.
Evidence: Woodpeckers have several adaptations that protect their brains, including a thick skull, a small brain cavity, and a specialized beak that absorbs shock. Additionally, woodpeckers have a hyoid bone that wraps around their skull, which acts as a shock absorber. In contrast, football helmets are designed to prevent skull fractures, but not concussions.
Reasoning: The adaptations in woodpeckers' skulls and beaks allow them to absorb and distribute the impact forces of their pecking, protecting their brains from injury. The hyoid bone in particular is able to compress and expand, reducing the force of the impact on the brain. Football helmets, on the other hand, are designed to prevent fractures by spreading out the force of the impact over a larger area. However, they are not effective in preventing concussions because they do not absorb or distribute the forces in the same way as woodpecker adaptations do. Therefore, while football helmets provide some protection, they are not enough to prevent concussions in players.
How many oxygen ATOMS are needed to produce 2 molecules of water (H2O)?

Answers
Answer:
2..............
................
Answer:
A. 2
Explanation:
relate the concepts of isotope and mass number
Answers
Answer:
Explanation:
Forms of the same atom that differ only in their number of neutrons are called isotopes. Together, the number of protons and the number of neutrons determine an element's mass number: mass number = protons + neutrons. ... A property closely related to an atom's mass number is its atomic mass.
Indicate the electron pair geometry and the molecular geometry for each of the six compounds.
Compound Electron pair geometry Molecular geometry
a) A sulfur atom is double bonded to an oxygen atom on the left and the right, and has a lone pair. Each oxygen atom has two lone pairs.
b) A sulfur atom is bonded to a chlorine atom on the left and the right, and has two lone pairs. Each chlorine atom has three lone pairs.
tetrahedral
c) A beryllium atom is bonded to two chlorine atoms 180 degrees apart. Each chlorine atom has three lone pairs.
d)A phosphorous atom is bonded to a fluorine atom on the left, the right, and the bottom, and has one lone pair. Each fluorine atom has three lone pairs.
e)A boron atom is bonded to a fluorine atom on the left, the right, and the bottom. Each fluorine atom has three lone pairs.
f)A carbon atom is bonded to a hydrogen atom on the left, the right, the top, and the bottom.
Answers
a) The electron pair geometry of the compound with a sulfur atom double bonded to an oxygen atom on the left and the right and has a lone pair is tetrahedral, whereas its molecular geometry is bent. Each oxygen atom has two lone pairs.b) The electron pair geometry of the compound with a sulfur atom bonded to a chlorine atom on the left and the
right and has two lone pairs is tetrahedral, whereas its molecular geometry is bent. Each chlorine atom has three lone pairs.c) The electron pair geometry of the compound with a beryllium atom bonded to two chlorine atoms 180 degrees
apart, each chlorine atom having three lone pairs, is linear. Its molecular geometry is also linear.d) The electron pair geometry of the compound with a phosphorous atom bonded to a fluorine atom on the left, the right, and the bottom,
and has one lone pair is tetrahedral. Its molecular geometry is trigonal pyramidal. Each fluorine atom has three lone pairs.e) The electron pair geometry of the compound with a boron atom bonded to a fluorine atom on the left, the right, and the bottom, and each fluorine atom having three lone pairs is trigonal planar. Its molecular geometry is also trigonal planar.f) The electron pair geometry of the compound with a carbon atom bonded to a hydrogen atom on the left, the right, the top, and the bottom is tetrahedral. Its molecular geometry is also tetrahedral. The bond angle between the carbon and hydrogen atoms is 109.5 degrees.
The electron pair geometry of each compound is determined by its molecular geometry, which depends on the number of bonds and lone pairs of electrons around the central atom. When there are two bonded atoms and no lone pairs, the molecular geometry is linear. When there are three bonded atoms and no lone pairs, the molecular geometry is trigonal planar. When there are two bonded atoms and one lone pair, the molecular geometry is bent or angular. When there are four bonded atoms and no lone pairs, the molecular geometry is tetrahedral. When there are three bonded atoms and one lone pair, the molecular geometry is trigonal pyramidal.
For more similar questions on topic Indicate the el
https://brainly.com/question/29470595
#SPJ11
The electron pair geometry of all six compounds is tetrahedral, while the molecular geometry of (b), (d), and (e) is trigonal planar, and the molecular geometry of (a), (c), and (f) is tetrahedral.
On the other hand, molecular geometry refers to the actual arrangement of atoms in space.
a) In the case of sulfur dioxide the electron pair geometry is trigonal planar because the sulfur atom has three electron pairs and one lone pair, resulting in a bent or V-shaped molecular geometry.
b) For sulfur trichloride, the electron pair geometry is tetrahedral because the sulfur atom has four electron pairs and one lone pair, resulting in a trigonal pyramidal molecular geometry.
c) In beryllium chloride the electron pair geometry is linear because the central atom has only two electron pairs and no lone pairs, resulting in a linear molecular geometry.
d) For phosphorus trifluoride, the electron pair geometry is tetrahedral because the central atom has four electron pairs and one lone pair, resulting in a trigonal pyramidal molecular geometry.
e) In boron trifluoride, the electron pair geometry is trigonal planar because the central atom has only three electron pairs and no lone pairs, resulting in a trigonal planar molecular geometry.
f) Finally, for methane, the electron pair geometry is tetrahedral because the central atom has four electron pairs and no lone pairs, resulting in a tetrahedral molecular geometry.
Learn more about electron pair geometry
https://brainly.com/question/24232047
#SPJ4
What is the molarity of 4 mol of NaOH dissolved in 2 L of water? O A. 0.5 M OB. 8 M O C. 2M D. 4 M
Answers
Answer:
concentration = mol/volume = 4/2 = 2M
Each of the following alkyl benzenes are reacted with
HNO3
in
H2SO
, and the resulting product mixtures studied by
GC
. Considering what we learned in this experiment, rank the molecules from highest percentage of para product to lowest percentage of para product.
Answers
When alkyl benzenes are reacted with HNO3 in H2SO4, they undergo nitration to form a mixture of ortho, meta, and para isomers. The ratio of these isomers depends on the electronic properties of the substituent on the benzene ring.
The substituents that are electron-donating (such as -CH3) increase the electron density on the benzene ring, making the ring more susceptible to attack by the electrophilic nitronium ion. This leads to a higher percentage of para product formation.
On the other hand, electron-withdrawing substituents (such as -NO2) decrease the electron density on the ring, making it less susceptible to attack by the nitronium ion. This leads to a lower percentage of para product formation.
Therefore, the rank of the alkyl benzenes from highest percentage of para product to lowest percentage of para product would be:
Toluene (-CH3)
Ethylbenzene (-C2H5)
Cumene (-C(CH3)3)
Nitrobenzene (-NO2)
Toluene would have the highest percentage of para product because the methyl group is an electron-donating group, increasing the electron density on the benzene ring. Nitrobenzene would have the lowest percentage of para product because the nitro group is an electron-withdrawing group, decreasing the electron density on the benzene ring.
For more details about electronic click here:
https://brainly.com/question/1255220#
#SPJ11
The reaction between calcium carbonate (CaCO3) and HCl produces calcium chloride (CaCl2), carbon dioxide (CO2), and water (H2O).What happens when the concentration of hydrogen chloride (HCl) molecules is doubled in this reaction?
CaCO3 + 2HCl → CaCl2 + CO2 + H2O
When the hydrogen chloride concentration doubles, the number of collisions between the reactants
, which causes the rate of the forward reaction to
.
Answers
When the hydrogen chloride concentration doubles, the number of collisions between the reactants becomes increased, which causes the rate of the forward reaction to be faster.
What is the rate of a reaction?The rate of a reaction is how fast a chemical reaction occurs i.e. the conversion of reactants to products.
The rate of a chemical reaction can be influenced by certain factors including the concentration of reactants.
The more the concentration of a reactant, the more the collision between the reactants and hence, the faster the reaction will proceed.
Therefore, when the hydrogen chloride concentration doubles, the number of collisions between the reactants becomes increased, which causes the rate of the forward reaction to be faster
Learn more about rate of reaction at: https://brainly.com/question/8592296
#SPJ1
Answer:1. increases 2. increases
Explanation:
Got it right on plato!
Complete the following sentences by writing in the correct word.
In a neutral atom, the number of electrons must be the same as the number of
.
The subatomic particle in an atom with no charge is the
.
The number of protons in an atom is the
number.
The total number of protons and neutrons in an atom is the
number.
Answers
1)proton number
2)neutron
3)(Z)
4)mass
This equation represents cellular respiration.
C6H12O6 +602
6 CO2 + 6H2O + energy
Which table lists all the atoms in reactants and products of cellular respiration?
Reactants 6 C 12 H 18 O
ΟΑ.
Products 6 C 12 H 18 O
Reactants 6 C 6H 2
Ов.
Products 6 C 12 H 120
Reactants 6 C 12 H 120
C.
Products 6 C 12 H 30
Reactants 6 C 12 H 18 0
D.
Products 1C6H 18 O
Please hurry

Answers
if you work it put you will get the 3rd one I think
Measurements of half-life make radioactive isotopes useful for
Answers
What is the formula for Tetracarbon octahydtide?
Answers
Answer:
Tricarbon octahydride has the formula C 3 H 8.This means it has three carbon atoms and eight hydrogen atoms.
Explanation:
Answer:
C3 H8
uR wElCOme
What is the pH of a solution with a [H+] = 1.0 x 10-5
Answers
Answer:
5Explanation:
The pH of a solution can be found by using the formula
\(pH = - log ([ {H}^{+} ])\)
From the question we have
\(pH = - log(1.0 \times {10}^{ - 5} ) \\ = 5\)
We have the final answer as
5Hope this helps you
the property of water molecules that is responsible for all the other physical and chemical properties is
Answers
The hydrogen bonding of water is one of the main property of water which makes it responsible for all the other physical and chemical properties.
Water molecules are polar in nature and they form hydrogen bonds. Hence, they have a high boiling point , high specific heat and density. Water can exhibit the properties of an acid, as well as a base (amphoteric character). The water molecules are constantly moving and the hydrogen bonds continuously breaks and forms again. These hydrogen bonds are strong, which is the reason for the unique properties of water.
To learn more about properties of water :
https://brainly.com/question/15395979
#SPJ4
) a solution is prepared by adding 1.50 g of solid nacl to 50.0 ml of 0.100 m cacl2. what is the molarity of chloride ion in the final solution? assume that the volume of the final solution is 50.0 ml.
Answers
The molarity of chloride ions in the solution is 0.712 M.
What is molarity?Molarity (M) is the amount of a substance in a certain volume of solution.
Molarity is also known as the molar concentration of a solution.
For NaCl, we use the following equation to determine the number of moles:
Given mass / Molar mass = number of moles
NaCl is assumed to weigh 1.50 g.
NaCl has a molar mass of 58.5 g/mol.
Using the values in the equation above, we obtain:
NaCl moles are equal to 1.50g/58.5g/mol, or 0.0256mol.
Both sodium and chloride ions are produced in one mole of sodium chloride (NaCl).
0.0256mol = moles of chloride ions
Regarding calcium chloride:
The following equation is used to determine the number of moles for a given molarity:
Molarity of the solution is calculated as follows: moles of solute * 100 / volume of solution (in ML).
(1)
Calcium chloride solution has a molarity of 0.100 M.
50.0 mL is the solution's volume.
Equation 1 is solved with the following values: 0.100M = Moles of CaCl2 * 1000 / 50
CaCl2 moles = 0.100M x 50
Moles of CaCl2 are equal to 0.100M*50/1000, or 0.005 mol.
One mole of calcium chloride yields two moles of chloride ions in addition to one mole of calcium ions.
Chloride ion moles equal (2 x 0.005) = 0.01 moles.
Now, we use equation 1 to determine the molarity of chloride ions in the final solution:
(0.0256 + 0.01) = 0.0356 moles of chloride ions are present in total in the solution.
50.0 mL is the total amount of solution.
Equation 1 is solved for the following values:
Chloride ion molecular weight = 0.0356 * 1000 / 50.0
The solution's molarity is 0.712M.
Consequently, the solution's molarity of chloride ions is 0.712 M.
To learn more about molarity refer to:
https://brainly.com/question/26873446
#SPJ4
Solid zinc(lt) sulfide reacts with aqueous hydrobromic acid (HBr) to form aqueous zinc(II) bromide and dihydrogen sulfide gas Express your answer as a chemical equation. Identify all of the phases in your answer. ΑΣΦ ? Pb$(s) + 2HBr(aq) →PBr_(s) +H, S(g)
Answers
the chemical equation for the reaction between solid zinc sulfide and aqueous hydrobromic acid is ZnS(s) + 2HBr(aq) → ZnBr2(aq) + H2S(g), where "s" represents a solid state, "aq" represents an aqueous or liquid state, and "g" represents a gaseous state.
The correct chemical equation for the reaction of solid zinc sulfide with aqueous hydrobromic acid to form aqueous zinc(II) bromide and dihydrogen sulfide gas is:
ZnS(s) + 2HBr(aq) → ZnBr2(aq) + H2S(g).In this equation, "s" represents a solid state, "aq" represents an aqueous or liquid state, and "g" represents a gaseous state. The reactants of the equation are solid zinc sulfide and aqueous hydrobromic acid, while the products are aqueous zinc(II) bromide and dihydrogen sulfide gas. The reaction can be explained by the displacement of hydrogen from hydrobromic acid by zinc sulfide, which results in the formation of zinc bromide and hydrogen sulfide gas. The balanced equation shows that one molecule of zinc sulfide reacts with two molecules of hydrobromic acid to form one molecule of zinc bromide and one molecule of hydrogen sulfide gas.
In summary, the chemical equation for the reaction between solid zinc sulfide and aqueous hydrobromic acid is ZnS(s) + 2HBr(aq) → ZnBr2(aq) + H2S(g), where "s" represents a solid state, "aq" represents an aqueous or liquid state, and "g" represents a gaseous state.
learn more about reaction here
https://brainly.com/question/29997907
#SPJ11
what is one of the three goals of environmental science as proposed by your text
Answers
One of the three goals of environmental science, as proposed by various texts, is to understand how natural systems function.
This involves studying the interrelationships between living organisms and their environment, including the physical, chemical, and biological processes that shape the world around us. By gaining a deeper understanding of how these systems work, environmental scientists can develop better ways to manage and protect natural resources, minimize environmental impacts, and promote sustainable development.
Other goals of environmental science include identifying and addressing environmental problems, such as pollution and climate change, and finding solutions to these issues through scientific research and policy development.
Ultimately, the overarching goal of environmental science is to promote a healthier, more sustainable planet for all living beings.
To learn more about environmental science visit:
https://brainly.com/question/1186120
#SPJ11
How long would it take to count 6.02 × 10^23 raisins, if you counted at a rate of one raisin per second?
(Please I need it quick)
Answers
NEED THIS NEED TO GET OFF ITS MY BRO BDAY NEED IT FOR A BIG GRADE NEed it SO BAD

Answers
Answer:
he is right, top right
Explanation:
How many grams of water are produced from the decomposition of 63 grams of lithium hydroxide
(LiOH)?
Answers
Answer:
18.65004 grams H2O
Explanation:
First, we need to write down the balanced chemical equation for the decomposition reaction:
2LiOH -> H2O + Li2O
Since we have grams of LiOH and we need to know the grams of water, we need to convert to moles since we can only compare moles to moles.
The amu of LiOH is 23.947.
The given grams of LiOH is 63.. To convert to moles, we will divide 63 by 23.947..
This gives us 2.6310 moles LiOH..
To convert to moles of H2O (and later grams of H2O), we will use the mole fractions from the balanced equation...
When we look at the balanced equation we can see that 2 moles of LIOH can produce 1 mol of Water, so:
2.6310 moles \(* \frac{1 molH_{2}O}{2 mol LiOH}\) = 1.3155 moles H2O
Now we will convert from moles to grams (we must multiply by the amu)
1.3155 moles H2O = 18.65 grams H2O
According to stoichiometry of the chemical equation of decomposition of lithium hydroxide 23.6 g of water are produced from the decomposition of 63 grams of lithium hydroxide.
What is stoichiometry?Stoichiometry is the determination of proportions of elements or compounds in a chemical reaction. The related relations are based on law of conservation of mass and law of combining weights and volumes.
Stoichiometry is used in quantitative analysis for measuring concentrations of substances present in the sample.It is useful in balancing chemical equations.
In the given example, as 47.9 g of lithium hydroxide produces 18 g water ,therefore 63 of lithium hydroxide will produce 63×18/47.9=23.6 g of water.
Thus, 23.6 g of water are produced from the decomposition of 63 grams of lithium hydroxide.
Learn more about stoichiometry,here:
https://brainly.com/question/9743981
#SPJ2
PLZ HELP ITS DUE IN 17 MINUTES
When water is changed from a liquid to a gas, what occurs
A. The atoms lose electrons.
B. A new compound is formed.
C. Molecules spread farther apart.
D. Hydrogen is separated from oxygen
Answers
Answer:
c
Explanation:
they spread farther apart as they lose their structure
What is the molarity of a solution with a volume of 2.3 Liters and 9.7 moles?
Answers
Answer:
Explanation:
molarity = (moles of solute)/(liters of solution) = 9.7/2.3 = 4.2
Ca(OH)2 + 2 H2SO4 → CaSO4 + 2 H2O
Answers
Answer:
hope this helped
Calcium Hydroxide + Sulfuric Acid = Calcium Sulfate + Water
Explanation:
Person above is correct
I need to know how to do this but if you could just answer it it's fine

Answers
Answer:
im not entirely sure what needs to be done but the 2NH3 is the product and the other half is the reactants .
if any elements are added to others it is the reactants
this is a breakdown of a compound because the arrow always indicates the direction of the reaction .
Describe what has to happen when matter is heated by convection
Answers
Answer:
Here^
Explanation:
The matter starts heating, up as if it's ice , turns into a liquid.
What is the molar concentration of 2.21 mil of KCI dissolved in 0.6068L of solution? explain why
Answers
Answer:
3.64M is the molar concentration of the solution
Explanation:
Molarity (Molar concentration, M) is an unit of concentration widely used in chemistry defined as the ratio between the moles of solute (In this case, KCl) and the volume of the solution in liters.
As you can see, the moles of KCl are 2.21 mol, and the volume of the solution is 0.6068L. The molarity is:
2.21mol / 0.6068L =
3.64M is the molar concentration of the solution
QUESTION 10 How much heat is required to raise 50 grams of water from 13°C to 88°C? Specific Heat Capacities H₂O(s) = 2.108 J/g. K H₂O(1) = 4.184 J/g.K H₂O(g) = 1.996 J/g. K O 16 kJ O-16 kJ 8 kJ 4
Answers
Total, 15,780 Joules of heat to raise 50 grams of water from 13°C to 88°C.
To calculate the amount of heat required to raise the temperature of 50 g of water from 13°C to 88°C, we need to use the specific heat capacity of water;
q = m × c × ΔT
where;
q = heat required (in Joules)
m = mass of water (in grams)
c = specific heat capacity of water (in Joules/gram-Kelvin)
ΔT = change in temperature (in Kelvin)
The specific heat capacity of liquid water (H₂O(1)) is 4.184 J/g.K. So, we can substitute the given values into the above equation;
q = 50 g × 4.184 J/g.K × (88°C - 13°C)
q = 50 g × 4.184 J/g.K × 75 K
q = 15,780 J
Therefore, it requires 15,780 Joules of heat.
To know more about specific heat capacity here
https://brainly.com/question/28941910
#SPJ4
A glass of unsweetened lemonade has a mass of 255 grams. A spoonful of sugar is massed before stirring It Into the lemonade and you find that
It has a mass of 25 grams.
• How much mass will the sweetened lemonade have once you stir in the sugar?
• State and explain the science concept that leads you to this answer.
Answers
Answer:
270
Explanation:
Once you add more mass to something the mass doesn’t go away you add more mass.
hope this helps!