for the ibr2- lewis structure, the number of pairs of nonbonding electrons on the central iodine atom is?
Answers
\(IBr^{-}_{2}\) has 3 pairs of non-bonding electrons as shown in the picture.
\(IBr^{-}_{2}\) has a linear structure and includes three lone pairs of electrons in an equatorial location. This decreases the lone pair repulsions, and the two bromine atoms are in an axial position. Therefore, the structure \(IBr^{-}_{2}\) has a linear organization. \(IBr^{-}_{2}\) has an iodine atom that possesses two bond pairs as well as three lone pairs of electrons. Both bond pairings are formed using atoms containing two bromines.
To know more about electrons click on the link below:
https://brainly.com/question/13998346
#SPJ4

Related Questions
in the warm-up activity, you observed how the reaction inside the chamber affected the temperature of the surrounding water. based on what happens to the surrounding water, do you think heat energy (enthalpy) is absorbed in the reaction or released? explain.
Answers
Based on the observation in the warm-up activity, where the temperature of the surrounding water increased during the reaction inside the chamber, it can be inferred that the reaction released heat energy (enthalpy).
This is because the temperature of the surrounding water increased, indicating that energy was transferred from the system (reaction inside the chamber) to the surroundings (water).
According to the first law of thermodynamics, the total energy of a closed system is conserved, meaning that energy cannot be created or destroyed but can only be transferred between the system and the surroundings. In exothermic reactions, energy is released by the system to the surroundings, resulting in an increase in the temperature of the surroundings.
Therefore, the observation of an increase in temperature of the surrounding water suggests that the reaction inside the chamber is exothermic and releases heat energy.
To learn more about enthalphy refer to:
brainly.com/question/30055078
#SPJ4
he long run equilibrium condition for perfect competition is:
a. P=AVC=MR=MC.
b. Q=AVC=MR=MC.
c. Q=ATC=MR=MC.
d. P=ATC=MR=MC.
Answers
Option (d), P=ATC=MR=MC, accurately represents the long-run equilibrium condition for perfect competition, reflecting the balance between price and cost for firms operating in a competitive market.
The long-run equilibrium condition for perfect competition is that price (P) is equal to average total cost (ATC), which is also equal to marginal cost (MC), and marginal revenue (MR).
Option (d), P=ATC=MR=MC, best represents the long-run equilibrium condition for perfect competition. In perfect competition, firms operate at the minimum point of their average total cost curve, where price equals both average total cost and marginal cost. This condition ensures that firms are earning zero economic profit and are producing at an efficient level.
In the long run, if firms are earning economic profit, new firms will enter the market, increasing competition and driving prices down. Conversely, if firms are experiencing losses, some firms may exit the market, reducing competition and causing prices to rise. This process continues until firms reach a state where price equals average total cost, marginal cost, and marginal revenue, ensuring a long-run equilibrium.
Therefore, option (d), P=ATC=MR=MC, accurately represents the long-run equilibrium condition for perfect competition, reflecting the balance between price and cost for firms operating in a competitive market.
Know more about Equilibrium here:
https://brainly.com/question/30694482
#SPJ11
a single-stage extraction is performed in which 400 kg of a solution containing 35 wt % acetic acid in water is contacted with 400 kg of pure isopropyl ether. calculate the amounts and compositions of the extract and raffinate layers. solve for the amounts both algebraically and by the lever-arm rule. what percent of the acetic acid is removed? use equilibrium data from appendix a.3.
Answers
As per the given problem, 400 kg of a solution containing 35 wt % acetic acid in water is contacted with 400 kg of pure isopropyl ether. We need to calculate the amounts and compositions of the extract and raffinate layers, and the percentage of acetic acid removed.
Using the lever-arm rule, we can determine the amounts of the extract and raffinate layers. Since the two solvents are being mixed in equal quantities, the total weight of the system is 800 kg. The lever arm for the isopropyl ether is 1, and for the acetic acid/water solution is (0.35/0.65) = 0.538. Therefore, the weight of the extract can be calculated as (1/1.538) × 400 kg = 259.97 kg, and the weight of the raffinate can be calculated as (0.538/1.538) × 400 kg = 140.03 kg.
To determine the compositions of the extract and raffinate layers, we use the lever-arm rule and the equilibrium data from Appendix A.3. For the extract layer, the lever-arm for isopropyl ether is 1, and for acetic acid, it is (0.90/0.10) = 9. Therefore, the weight percent of acetic acid in the extract layer is (1/10) × 35% = 3.5%. Similarly, for the raffinate layer, the lever arm for isopropyl ether is 0, and for acetic acid, it is (0.04/0.96) = 0.042. Therefore, the weight percent of acetic acid in the raffinate layer is (0.042/1.042) × 35% = 1.48%.
To determine the percentage of acetic acid removed, we need to calculate the amount of acetic acid that remains in the raffinate layer. The weight of acetic acid in the original solution is 0.35 × 400 kg = 140 kg. The weight of acetic acid in the raffinate layer is 0.0148 × 140.03 kg = 2.07 kg. Therefore, the amount of acetic acid removed is 140 kg - 2.07 kg = 137.93 kg. The percentage of acetic acid removed is (137.93 kg/140 kg) × 100% = 98.52%.
To learn more about Acetic acid click here
https://brainly.com/question/26169392
#SPJ11
A solution contains 35.00 g of sodium chloride in 100. G of water at 45.0C. How could this solution be described
Answers
Answer:
It is unsaturated
Explanation:
A solution is said to be unsaturated when it contains less solute than it can normally hold at a given temperature.
The solubility of NaCl remains fairly independent of temperature hence the line for the solubility of NaCl remains fairly flat in the solubility curve.
The solubility of NaCl is about 36 g of NaCl in 100g of water. Hence a solution that contains 35.00 g of sodium chloride in 100 g of water at 45.0C is unsaturated.
How many grams of diphosphorus trioxide, P2O3, are required to produce 10.2 moles of phosphorous acid, H3PO3?

Answers
Total, 561 grams of diphosphorus trioxide are required to produce 10.2 moles of phosphorous acid.
The balanced chemical equation for the reaction between diphosphorus trioxide and water to produce phosphorous acid is;
P₂O₃ + 3H₂O → 2H₃PO₃
From this equation, we can see that 1 mole of P₂O₃ produces 2 moles of H₃PO₃.
Therefore, the number of moles of P₂O₃ required to produce 10.2 moles of H₃PO₃ is;
10.2 moles H₃PO₃ × 1 mole P₂O₃/2 moles H₃PO₃ = 5.1 moles P₂O₃
To convert from moles to grams, we need to use the molar mass of P₂O₃, which is;
2 × atomic mass of P + 3 × atomic mass of O = 2 × 31.0 g/mol + 3 × 16.0 g/mol = 110.0 g/mol
Therefore, the mass of P₂O₃ required is;
5.1 moles P₂O₃ × 110.0 g/mol = 561 g
Therefore, 561 grams of P₂O₃ is required.
To know more about diphosphorus trioxide here
https://brainly.com/question/420164
#SPJ1
How do magnetic stripes show the history of Earth's magnetic field?
A. As you move away from an ocean ridge, the rocks get older.
B. As you move away from a coast, the rocks get younger.
C. As you move down through the seafloor, rocks get older.
D. As you move eastward along a continent, rocks get older.
Answers
The way that magnetic stripes show the history of the Earth's magnetic field is A. As you move away from an ocean ridge, the rocks get older.
What do magnetic strips show of Earth's history ?By looking at how our planet's magnetic field changes throughout time, scientists may estimate the age of the seafloor. A geomagnetic reversal is the periodic reversal of the currents in the liquid core that generate the Earth's magnetic field. Throughout Earth's history, this has occurred frequently.
When researchers examined the seafloor's magnetic characteristics, they found normal and reversed magnetic stripes of varying widths. These magnetic patterns are symmetrical on both sides and parallel to the mid-ocean ridges.
Away from midocean ridges, they observed that the age of the bottom became progressively older.
Find out more on magnetic stripes at https://brainly.com/question/15010317
#SPJ1
Given 450 grams of a 0.75molal solution of acetone dissolved in water.Solve for the number of grams of acetone that are in solution.
Answers
The number of grams of acetone that are in solution is \(208.56g\).
Acetone is an organic compound with the chemical formula C3H6O. It is a colorless, highly flammable liquid with a characteristic sweet odor. Acetone is the simplest and smallest ketone, and is widely used as a solvent in a variety of industrial and household applications. The number of grams of acetone that are in solution can be calculated using the molar mass of acetone and the molarity of the solution. The molar mass of acetone is 58.08 g/mol, and the molarity of the solution is 0.75 moles/L. We can calculate the number of grams of acetone that are in solution using the following equation:
Number of grams of acetone = \((Molarity of solution *Volume of solution * Molar mass of acetone)\)
Number of grams of acetone = \((0.75 mol/L * 450 g/L * 58.08 g/mol)\)
Number of grams of acetone = \(208.56 g\)
learn more about Acetone Refer:brainly.com/question/13334667
#SPJ4
If Delta G degree of the following reaction is -110 kJ/mol. what is E degree_cell? (F = 96.500 C middot mol^-1) A^3-(aq) + 3B (s) rightarrow A (s) + 3B^- (aq)+ 0.38 V - 0.09 V- 0 38 V+ 0.00038 V+ 0.09 V
Answers
The E°_cell for the given reaction is approximately 0.377 V.
I understand that you want to find the E°_cell for a reaction with a given ΔG° and the Faraday constant (F). The Faraday constant is a physical constant that relates the amount of electric charge carried by one mole of electrons to the magnitude of the electric charge on a single electron. Its value is approximately 96,485.3329 coulombs per mole (C/mol).The Faraday constant is named after the English physicist and chemist Michael Faraday, who made important contributions to the study of electromagnetism and electrochemistry in the 19th century. It is used in a variety of fields, including electrochemistry, physics, and engineering, to calculate the amount of electrical charge involved in various processes.The Faraday constant can be derived from the Avogadro constant, which relates the number of particles (atoms or molecules) in one mole of a substance to the actual number of particles. The relationship between the Faraday constant and the Avogadro constant is given by:
F = N_A * e
where F is the Faraday constant, N_A is the Avogadro constant, and e is the elementary charge, which is the magnitude of the charge on a single electron (approximately 1.602 × 10^-19 coulombs).
Given: ΔG° = -110 kJ/mol, F = 96,500 C/mol
First, let's convert ΔG° to J/mol: ΔG° = -110,000 J/mol
Now, we can use the relationship between ΔG°, E°_cell, and F:
ΔG° = -nFE°_cell
We know that 3 electrons are transferred in this reaction (from A^3- to A and from B to 3B^-), so n = 3.
Rearrange the equation to solve for E°_cell:
E°_cell = -ΔG° / (nF) = -(-110,000 J/mol) / (3 * 96,500 C/mol)
E°_cell ≈ 0.377 V
Therefore, the E°_cell for the given reaction is approximately 0.377 V.
To know more about Faraday constant visit:
https://brainly.com/question/31604460
#SPJ11
an atom of 206pb has a mass of 205.974440 amu. calculate its binding energy in mev per nucleon. enter your answer with 4 significant figures and no units.use the masses:mass of 1h atom
Answers
The nucleus in question has a binding energy of 7.88 MeV/nucleon.
The subatomic particles that make up an atom's nucleus are known as nucleons. Protons and neutrons are nuclear particles.
We are given a nucleus having representation: 206 Pb
Number of protons = 82
Number. of neutrons. = 206 - 82 = 124
Δm = [(np × mp) + (nn × mn) - M
where,
np = number of protons = 82
mp = mass of one proton = 1.007825 amu
nn = number of neutrons = 124
mn = mass of one neutron = 1.008665 amu
M = nuclear mass = 205.974440 amu
Δm = [(82 × 1.0078725) + (124 × 1.008665)] - 205.974440
Δm = 1.74167amu
the binding energy of the nucleus, we use the equation:
E = Δm\(c^{2}\)
E = (1.74167u) × \(c^{2}\)
E = 1622.36MeV
1u = 931.5 MeV/\(c^{2}\)
Number of nucleons in Fe atom = 206
the number of nucleons divided by the binding energy per nucleon yields:
Binding energy per nucleon = binding energy /nucleons = 7.88MeV/nucleon
binding energy is the amount of energy needed to separate a particle from a system of particles or to disperse all the particles in the system. Particularly relevant cases of binding energy include subatomic particles in atomic nuclei, electrons attached to atoms' nuclei, and atoms and ions bonded together in crystals.
Learn more about binding energy here:
https://brainly.com/question/13025424
#SPJ4
5. Predict You are floating motionless on a
rubber raft in the middle of a pool. A friend
forms a wave by slapping the water every
second. Will the wave carry you to the edge
of the pool? Explain your answer.
Answers
When the pool's center is occupied by the rubber raft. Think of it as the raft's starting position.
Once it reaches the rubber raft, the raft will start to rise when the friend creates a wave by slapping the water.
The raft will really gain energy from the wave, moving upward.
The raft will return to its original place once the wave passes.
The raft will return to its original place where it was before the wave hits it because it is indicated in the question that it is immobile.
As a result, the wave won't take it to the pool's edge.
Because you are only lightly lifted by the waves or because Lee cannot be pushed to the other side of the pool, the wave won't drive the person on the raft over the edge of the pool.
To know more about waves, click on the link below:
https://brainly.com/question/11350613
#SPJ9
1. How many moles of NaCl would be contained in .750 L solution with a molarity of
0.45M?
Answers
Molarity = no. of moles /volume of solution in L
Given, 0.45M= no. of moles/0.750L
Therefore, no. of moles = 0.45M x 0.750L= 0.3375
Answer:
\(\boxed {\boxed {\sf 0.3375 \ mol \ NaCl}}\)
Explanation:
Molarity is found by dividing the moles of solute by liters of solution.
\(M=\frac{moles \ of \ solute }{liters \ of \ solution}\)
We know the molarity is 0.45 M and there are 0.750 liters of solution. The solute is NaCl (sodium chloride). We can substitute the values into the formula.
\(0.45 \ M = \frac{ moles \ of \ NaCl}{ 0.750 \ L }\)
The molarity (M) can also be represented by mol/L
\(0.45 \ mol/L = \frac{ moles \ of \ NaCl}{ 0.750 \ L }\)
We are solving for the moles of solute, so we must isolate the numerator. It is being divided by 0.750 liters. The inverse operation is multiplication, so multiply both sides of the equation by 0.750 L.
\(0.750 \ L * 0.45 \ mol/L = \frac{ moles \ of \ NaCl}{ 0.750 \ L }*0.750 \ L\)
The liters will cancel out.
\(0.750 * 0.45 \ mol = { moles \ of \ NaCl}\)
\(0.3375 \ mol \ = moles \ of NaCl\)
There are 0.3375 moles of NaCl in a 0.750 liter solution with a molarity of 0.45 M.
How does the respitatory system work with the muscular system?
A: Filters out waste from food and pushes it through intestines and out the body (and you know how and where it gets out
B: Respiratory System and the Muscles of Inhalation and Exhalation.
C: It doesn't
D: Supplies oxygen to the blood and removes carbon dioxide.
Anyone help please I’m failing :(
Answers
Answer:
B
Explanation:
The muscular and nervous systems enable the involuntary breathing mechanism. The main muscles in inhalation and exhalation are the diaphragm and the intercostals (shown in blue), as well as other muscles. Exhalation is a passive action, as the lungs recoil and shrink when the muscles relax.
a) is the energy required to heat air from 295 to 305 K the same as the energy required to heat it from 345 to 355 K? Assume the pressure remains constant in both cases. [1 mark] b) What is enthalpy? [1 mark] c) is it possible to compress an ideal gas isothermally in an adiabatic piston-cylinder device? Explain. [2 marks] d) A gas is expanded from an initial volume of 0.3 m³ to a final volume of 1.2 m³. During the quasi-equilibrium process, the pressure changes with volume according to the relation P=a+bV+cV², where a= 1080 kPa, b = -500 kPa/m³ and c = -23 kPa/ (m³)². Calculate the work done during this process by implementing integrations. [4 marks] e) A 1000-W iron with a mass of 0.4155 kg has a specific heat, cp = 875 J/kg°C. Initially, the iron is in thermal equilibrium with the ambient air at 22°C. Determine the minimum time needed for the plate temperature to reach 200°C. [2 marks]
Answers
a) No, the energy required to heat air from 295 to 305 K is not the same as the energy required to heat it from 345 to 355 K.
b) Enthalpy is the total heat content of a system at constant pressure, including the internal energy and the product of pressure and volume.
c) No, it is not possible to compress an ideal gas isothermally in an adiabatic piston-cylinder device because an isothermal process requires constant temperature, while an adiabatic process implies no heat transfer and can result in temperature changes.
d) The work done during the process can be calculated by integrating the given pressure-volume relation, P=a+bV+cV², over the initial and final volumes.
e) The minimum time needed for the plate temperature to reach 200°C can be determined by calculating the heat transfer using the equation Q = mcΔT and then dividing it by the power of the iron, t = Q / P.
a) No, the energy required to heat air from 295 to 305 K is not the same as the energy required to heat it from 345 to 355 K. The energy required to heat a substance is directly proportional to the change in temperature, so a greater temperature difference will require more energy.
b) Enthalpy (H) is a thermodynamic property that represents the total heat content of a system at constant pressure. It takes into account the internal energy (U) of the system plus the product of pressure (P) and volume (V).
c) No, it is not possible to compress an ideal gas isothermally in an adiabatic piston-cylinder device. Isothermal compression implies that the temperature of the gas remains constant during the compression process. In an adiabatic process, there is no heat exchange with the surroundings, which means that the temperature of the gas will change during compression or expansion.
d) The work done during the process can be calculated by integrating the expression for pressure with respect to volume. The work done (W) is given by:
W = ∫(P dV) = ∫(a + bV + cV²) dV
By integrating the given expression, the work done during the process can be determined.
e) To determine the minimum time needed for the plate temperature to reach 200°C, we need to consider the heat transfer equation:
Q = mcΔT
where Q is the heat transferred, m is the mass of the iron, c is the specific heat capacity of iron, and ΔT is the temperature difference.
Using the given values and rearranging the equation, we can solve for the time (t):
t = Q / P
where P is the power of the iron.
By substituting the known values, the minimum time required for the plate temperature to reach 200°C can be calculated.
For more such questions on heat transfer
https://brainly.com/question/16055406
#SPJ4
Cl2 +
NaBr
what’s this answer?!
Answers
A. The pendulum would accelerate to the left
B. The pendulum would accelerate downward
C. The pendulum would accelerate to the right
D. The pendulum would accelerate upward
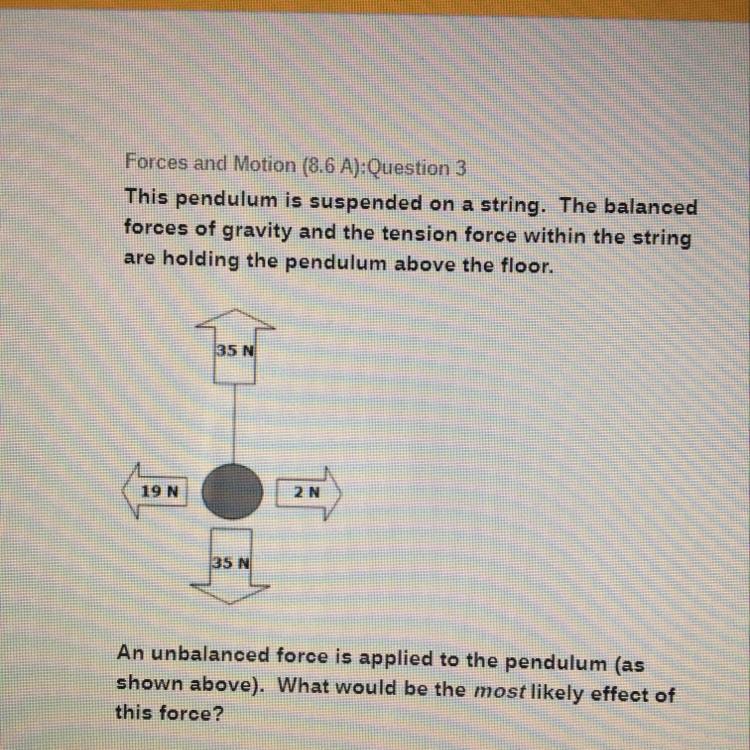
Answers
Answer:
The pendulum would accelerate to the left
Explanation:
hope this helps
5) If an atom has a positive charge what is it called?
Answers
Answer:
An atom that has a negative or positive charge it is called an ion.
Answer:
It''s called a Cation
Explanation:
NEED HELP!! What types of waves are transmitted from the H-E-L-P device ?
Answers
Answer:
What do you mean need more details
Explanation:
Since a help device is a a communication gadget, we know that the help device will make use of radio waves.
What is a help device?A help device refers to a device that can be used for emergency communication. They are basically communication gadgets.
We must note that our communication gadgets such as television, radio, cell phone etc all make use of radio waves. Hence a help device would make use of radio waves.
Learn more about radio waves: https://brainly.com/question/3393755?
3 H2SO4 + 2 Al(OH)3 → \ Al2(SO4)3 + 6 H2O
3
Use the balanced equation above to answer the questions that follow.
If a student puts 25 grams of H2SO4 and 23 grams of Al(OH)3, how many grams of Al2(SO4)3
would you be able to produce?
1. Which reactant is limiting the reaction?
(show your work below)
Answers
The limiting reactant in this reaction is H₂SO₄.
To determine the limiting reactant, we compare the amount of product that can be formed from each reactant. We will calculate the number of moles of Al₂(SO₄)₃ that can be produced from both reactants and compare their values.
Given:
Mass of H₂SO₄ = 25 grams
Molar mass of H₂SO₄ = 98.09 g/mol
Molar ratio between H₂SO₄ and Al₂(SO₄)₃ = 3:1
Number of moles of H₂SO₄ = mass / molar mass
= 25 g / 98.09 g/mol
= 0.255 mol
Number of moles of Al₂(SO₄)₃ formed from H₂SO₄ = 0.255 mol / 3 = 0.085 mol
Given:
Mass of Al(OH)₃ = 23 grams
Molar mass of Al(OH)₃ = 78.00 g/mol
Molar ratio between Al(OH)₃ and Al₂(SO₄)₃ = 2:1
Number of moles of Al(OH)₃ = mass / molar mass
= 23 g / 78.00 g/mol
= 0.295 mol
Number of moles of Al₂(SO₄)₃ formed from Al(OH)₃ = 0.295 mol / 2 = 0.148 mol
Comparing the moles of Al₂(SO₄)₃ formed from each reactant, we find that the H₂SO₄ produces a lower amount (0.085 mol) compared to Al(OH)₃ (0.148 mol). Therefore, the H₂SO₄ is the limiting reactant in this reaction.
To calculate the mass of Al₂(SO₄)₃ produced, we can use the molar mass of Al₂(SO₄)₃:
Molar mass of Al₂(SO₄)₃ = 342.15 g/mol
Mass of Al₂(SO₄)₃ = number of moles * molar mass
= 0.085 mol * 342.15 g/mol
≈ 29.1 grams
Therefore, you would be able to produce approximately 29.1 grams of Al₂(SO₄)₃ using 25 grams of H₂SO₄.
To learn more about limiting reactant, here
https://brainly.com/question/32459503
#SPJ4
Can someone please explain to me how to find the molar mass of hydrated sodium thiosulfate. I put the formula in above if you need it.

Answers
Answer:
Is that all in the Image take a bigger pic
Explanation:
Answer:
Name: Sodium Thiosulfate Pentahydrate.
Formula: Na2S2O3.5H2O.
Molar Mass: 248.1841.
Explanation:
Hope this helps :D
and feel free to click that thanks button
According to Dalton's Law of Partial Pressures, the pressure of oxygen in dry air would be

Answers
The pressure of the oxygen in the air is 0.21 atm. The partial pressure of a gas is the contribution that gas makes to the total pressure when the gas is part of a mixture.
An 886 mL sample of Neon gas is at 752 torr and 299 K. What will be the new volume if, with the pressure and amount of gas held constant the temperature is increased to 371 K?
Answers
Answer:
\(V_2=1099.35mL\)
Explanation:
Hello there!
In this case, according to the given information, it is possible to infer that as both the amount of the gas and the pressure remains the same, we can solve this problem via the Charles' law a directly proportional relationship between the volume and temperature:
\(\frac{V_2}{T_2} =\frac{V_1}{T_1}\)
Thus, by solving for the final volume, V2, we obtain:
\(V_2 =\frac{V_1T_2}{T_1} \\\\V_2 =\frac{886mL*371K}{299K}\\\\V_2=1099.35mL\)
Best regards!
Which of the following is the correct name for N2S5?
Answers
Convert 3.02 x 1024 molecules of carbon (C) to moles.
Answers
Answer:
5.02 mol C
Explanation:
Step 1: Define
Avagadro's Number - 6.02 × 10²³ atoms, molecules, formula units, etc.
Step 2: Use Dimensional Analysis
\(3.02(10)^{24} \hspace{3} molecules \hspace{3} C(\frac{1 \hspace{3} mol \hspace{3} C}{6.02(10)^{23} \hspace{3} molecules \hspace{3} C} )\) = 5.01661 mol C
Step 3: Simplify
We have 3 sig figs.
5.01661 mol C ≈ 5.02 mol C
In the given the following chemical reaction identify the substance oxidized,the substance reduced,the oxidizing agent and reducing agent
CuO+H2--->Cu+H2O
Answers
The CuO is reduced and acts as the oxidizing agent, while H2 is oxidized and serves as the reducing agent in this chemical reaction.
n the given chemical reaction, CuO + H2 -> Cu + H2O, copper(II) oxide (CuO) is reduced to copper (Cu), while hydrogen gas (H2) is oxidized to water (H2O).
The substance oxidized: H2 (hydrogen gas) is oxidized. It loses electrons and undergoes an increase in oxidation state from 0 to +1 in water.
The substance reduced: CuO (copper(II) oxide) is reduced. It gains electrons and undergoes a decrease in oxidation state from +2 to 0 in copper metal.
The oxidizing agent: CuO acts as the oxidizing agent since it accepts electrons from hydrogen gas during the reaction, causing the hydrogen to be oxidized.
The reducing agent: H2 acts as the reducing agent since it donates electrons to copper(II) oxide, causing the reduction of copper(II) oxide to copper metal.
For more such questions on oxidizing
https://brainly.com/question/14041413
#SPJ11
Raoul ate a meal of oats before seeing his doctor. Oats are made of mostly starch molecules. Raoul feels tired, so his doctor gave him a test and found that raoul’s cells contained glucose molecules, but they did not contain enough oxygen molecules. Does this explain why raoul feels tired?.
Answers
No, his digestive system is breaking down starch molecules, but his respiratory system may not be working properly.
Oat starch which is mainly stored in the oat endosperm is the most abundant carbohydrate component of oats, and its content varies between 40-50% depending on the variety and growing conditions. Sayer and White 2011. Starch after extraction of oat amylose content lipids was determined by Gudmundson and Eliasson.
Whole grain oats contain large amounts of valuable nutrients such as protein starch unsaturated fatty acids, and dietary fiber in soluble and insoluble fractions. Starch is composed of two glucose polymers amylopectin, and amylose which together form insoluble semi-crystalline starch granules. Both polymers are composed of conjugated glucan chains connected by branch points, but their structure and biosynthesis differ.
Learn more about Raoul feeling tired here:-https://brainly.com/question/28751479
#SPJ1
help i could use all the help and will be posting more questions later

Answers
you need to add 9L of water to 10mL of the solution of HCl with a pH of 3 to change the pH of 6.
First, calculate the amount of HCl present in 10mL of the solution with a pH of 3:
10mL x 0.1M = 1 mmol HCl
Then, calculate the amount of HCl required to raise the pH to 6:
1 mmol HCl x (10^3 - 10^6) = 9 mmol HCl
Finally, calculate the amount of water required to add 9 m mol of HCl to the solution:
9 mmol HCl x (1L/1000 mmol HCl) = 9L water
Hence, you need to add 9L of water to 10mL of the solution of HCl with a pH of 3 to change the pH of 6.
What is ph?
pH is a measure of acidity and alkalinity. It is measured on a scale of 0 to 14, with 0 being the most acidic and 14 being the most basic (alkaline). A pH of 7 is neutral.
Therefore, you need to add 9L of water to 10mL of the solution of HCl with a pH of 3 to change the pH of 6.
To learn more about ph
Here: https://brainly.com/question/12609985
#SPJ1
____Na + ____Cl2 ---> ____NaCl
Answers

Green plants use light from the Sun to drive photosynthesis, a chemical reaction in which liquid water and carbon dioxide gas form aqueous glucose C6H12O6 and oxygen O2 gas. Calculate the moles of water needed to produce 0.500mol of glucose. Be sure your answer has a unit symbol, if necessary, and round it to 3 significant digits.
Answers
In order to find the answer we need to set the equation up first, so the reaction for photosynthesis is:
6 CO2 + 6 H2O + energy -> C6H12O6 + 6 O2
Now by checking the molar ratio between water and glucose we see that for every 6 moles of H2O we will end up having 1 mol of C6H12O6, so we have a 6:1 molar ratio, now in order to produce 0.500 we will find out by doing the following calculation:
6 H2O = 1 C6H12O6
x H2O = 0.500 C6H12O6
x = 3.00 moles of H2O are needed to produce 0.500 moles of glucose
which reagents can be used to convert an aldehyde to a carboxylic acid
Answers
To convert an aldehyde to a carboxylic acid, oxidation of the aldehyde functional group is required.
There are several reagents that can be used for this conversion:
1. Strong Oxidizing Agents:
- Potassium permanganate (KMnO4): In the presence of acidic conditions, KMnO4 can oxidize aldehydes to carboxylic acids.
- Chromic acid (H2CrO4): It is a strong oxidizing agent that can convert aldehydes to carboxylic acids.
2. Tollens' Reagent:
Tollens' reagent, also known as silver mirror reagent, is a solution of silver nitrate (AgNO3) and ammonia (NH3) in water. It can oxidize aldehydes to carboxylic acids under mild conditions. It produces a silver mirror on the inner surface of the reaction vessel.
3. Jones Reagent:
Jones reagent consists of a solution of chromium trioxide (CrO3) in diluted sulfuric acid (H2SO4). It is a strong oxidizing agent that can convert aldehydes to carboxylic acids.
These are some commonly used reagents to convert aldehydes to carboxylic acids through oxidation. The choice of reagent may depend on factors such as reaction conditions, desired selectivity, and other functional groups present in the molecule.
To know more about aldehyde visit;
brainly.com/question/30459994
#SPJ11
Which of the following is in French
The floor is pink
A dog barks
A cup with steam coming from it will be hot to touch
Answers
Answer:
A dog barks
Explanation: