From the table of densities found in this experiment, iron has a density of 7. 85 g/cm3 and water has a density of 1. 0 g/ml. Iron is denser than water and will sink when placed in water. Explain how ships made from iron stay afloat using density principles.
Answers
The water displaced by the boat is greater than the weight of the ship, resulting in a density less than that of the water in which it is floating. Hence, ships made of iron stay afloat using density principles. Hence, it is clear that the ships made of iron are kept afloat with the help of the water displacement principle.
According to the density theory, a ship's total weight divided by its volume is referred to as its density. If a ship has a lower density than water, it will float. When the weight of the displaced water equals the weight of the ship, the ship's density is equal to that of the water it is floating in. If the weight of the displaced water is less than the weight of the ship, the ship's density is higher than that of the water it is floating in. As a result, the ship sinks. For example, a ship made of iron with a density of 7.85 g/cm³ would sink if placed in water because its density is greater than water's density of 1.0 g/ml. However, this is not the case because ships are designed to displace a huge amount of water as they float. The boat's shape and size are built to take up a lot of space and allow for water displacement to keep the boat afloat. Therefore, the water displaced by the boat is greater than the weight of the ship, resulting in a density less than that of the water in which it is floating. Hence, ships made of iron stay afloat using density principles. Hence, it is clear that the ships made of iron are kept afloat with the help of water displacement principle.
To Know more about water displacement principle visit:
brainly.com/question/4421565
#SPJ11
Related Questions
You are heating a piece of glass and now want to pick it up. you should?
Answers
Answer:
pick it up with tongs
Explanation:
if the mercury in a barometer raises 21.0 centimeters due to a change in ambient pressure, what is the corresponding change in pressure in atm?
Answers
The corresponding change in pressure in atm is 0.276 atm.
How does pressure work?Physical pressure applied to an object. Per unit area, a perpendicular force is delivered to the area of the objects. Pascals are a unit of pressure (Pa).
Briefing :
The change in pressure in the mercury barometer is given by ΔP = ρgh where
ρ = density of mercury = 13600 kg/m³,
g = acceleration due to gravity = 9.8 m/s² and
h = height of mercury = 21 cm = 0.21 m
So, ΔP = ρgh
= 13600 kg/m³ × 9.8 m/s² × 0.21 m
= 27988.8 kg/m-s²
= 27988.8 N/m²
= 27988.8 Pa
The change in pressure in atm
Since 1 atm = 101325 Pa
27988.8 Pa = 27988.8 Pa × 1 atm/101325 Pa
= 0.276 atm
So, the corresponding change in pressure in atm is 0.276 atm.
To know more about pressure visit :
https://brainly.com/question/12152879
#SPJ4
Given the reaction fe o2 right arrow. fe2(3 )o3(2-), what happens to the oxidation states of these elements? fe o
Answers
The oxidation state of iron changes from 0 to +3, and of oxygen changes from 0 to -2.
What is oxidation state?Oxidation state of any atom will tells about the number of exchangeable electrons.
Given chemical reaction is:
Fe + O₂ → Fe₂O₃
Initially in the reactant form, oxidation state of iron (Fe) atom and oxygen (O₂) molecule is zero as they are in elemental form. Oxidation state of 2 iron atoms in the product (Fe₂O₃) is +3 as it is combined with the 3 atoms of oxygen whose oxidation state is -2.
Hence, oxidation state of Fe and oxygen changes as we going from reactants to product side.
To know more about oxidation state, visit the below link:
https://brainly.com/question/8990767
Answer:
B and A
Explanation:
The nucleus of a hellum atom Is Identical to:
A.a gamma particle
B.an alpha particle
C.a beta particle
D.all of the above
Answers
Answer:
B
Explanation:
Its nucleus is identical to an alpha particle, and consists of two protons and two neutrons. Alpha decay of heavy elements in the Earth's crust is the source of most naturally occurring helium-4 on Earth, produced after the planet cooled and solidified.
Answer: B. An Alpha particles
As helium is consist of 2 protons and 2 neutrons
Alpha particles, also consist of 2 protons and 2 neutrons bound together into a particle that's why identical to a helium-4 nucleus.
Deduce the starting materials that would yield the cyclopropane derivative in the presence of a strong base.
Answers
The starting materials that would yield the cyclopropane derivative in the presence of a strong base is alkyl halide and alkene in presence of KOH.
What is strong base?Strong base is defined as a substance with the capacity to take a proton out of an extremely weak acid. Water can totally dissolve strong bases. Their pH is often between 12 and 14, which is extraordinarily high.
Donor and acceptor group substituted cyclopropane derivatives are particularly well suited for synthetic applications because the electronic effects of these substituents ensure that the cyclopropanes are activated and that the products after ring cleavage are highly versatile.
Thus, the starting materials that would yield the cyclopropane derivative in the presence of a strong base is alkyl halide and alkene in presence of KOH.
To learn more about strong base, refer to the link below:
https://brainly.com/question/16749233
#SPJ1
If 36g of Al react with 34L of Oz, how many moles of Al2O3 are produced?
4Al +302 --> 2Al2O3
145 moles
0.667 moles
0.165 moles
101 moles
Answers
Answer:
.........................................................................................................................................................................................................................................................................................................................................................................................................................................................
Explanation:
If 36g of Al react with 34L of Oz, 0.667 moles of Al2O3 are produced. The correct option is b, 0.667 moles
What are moles?The mole is a SI unit of measurement that is used to calculate the quantity of any substance.
\(\rm 4Al +3O_2 -- > 2Al_2O_3\)
Step1- calculate the moles of aluminum
The mass of Al is 36 g
The molar mass of Al is 27
\(\rm Number\;of \;moles= \dfrac{mass}{molar\;mass}\\\rm Number\;of \;moles\;of\;Al = \dfrac{36}{27} = 1.33\;mol\)
Step2- calculate the moles of oxygen
The mass of O₂ is 34 L
\(\rm 1 L = \dfrac{1}{22.4 }\)
\(\rm Number\;of \;moles\;O_2= \dfrac{34}{22.4} = 1.51\)
The limiting reagent is 1.33 MOL. By the rule of stoichiometry, four moles of Al produced 2 moles of Al.
\(\rm \dfrac{1.33}{2} = 0.665 \;moles\)
Thus, the correct option is b. 0.667 moles.
Learn more about moles
https://brainly.com/question/26416088
#SPJ2
Os subníveis mais energéticos de um dado átomo são: ...4s2 3d10 4p5 a) indique o seu número atomico b) quantos electrões de valência apresenta esse átomo c) a que família pertence?
Answers
Answer:
A. 35
B. 7
C. halogênios
Explanation:
Aqui, para responder a essa pergunta, precisaremos conhecer o elemento particular em questão.
..... 4s ^ 2 3d ^ 10 4p ^ 5 significa que está a cinco elétrons da configuração eletrônica do último elemento na primeira camada dos metais pesados.
O último elemento da 1ª série do elemento de transição é o zinco, portanto, como está a apenas 5 elementos de distância, o átomo de que estamos falando é o átomo de Bromo de Bromo.
A. O zinco tem um número atômico 30 e como o bromo está a 5 elementos de distância, seu número atômico é 35
B. Uma vez que pertence ao grupo halogênio, tem 7 elétrons de valência como o resto da família
C. Pertence à família dos halogênios
tính nồng độ của các ion trong dung dịch CA (OH)2 0.7M
Answers
Answer:
what are you saying ma guy?
Explanation:
How are the steps you went through when discovering a new species similar to the steps of the scientific method? Were any steps skipped?
Answers
New species form by speciation, in which an ancestral population splits into two or more genetically distinct descendant populations.
What are the steps when discovering a new species?
By taking bits of a single gene, scientists are using DNA barcoding to identify new species. If a portable hand-held scanning device can be developed, one ecologist says, it could “do for biodiversity what the printing press did for literacy. These skills involve observing natural phenomena, identifying different species of organisms, classifying them into categories, and mapping the data for conservation and management in the future. Scientists identify species by examining physical characteristics.
So we can conclude that: New species form by speciation, in which an ancestral population splits into two or more genetically distinct descendant populations.
Learn more about New Species here: https://brainly.com/question/12683358
#SPJ1
what is the electron group geometry and hybridisation state-of-the carboxyl carbon and an ester linkage
Answers
In a carboxylic acid, the carbon atom in the carbonyl group (C=O) is typically sp2 hybridized and forms three sigma bonds with neighboring atoms, including two sigma bonds with two oxygen atoms and one sigma bond with a hydrogen or another carbon atom. The fourth valence electron of the carbon atom is located in a p orbital, which is perpendicular to the plane formed by the three sigma bonds.
In terms of electron group geometry, the carboxyl carbon is located at the center of a trigonal planar arrangement of electron groups, which consists of the three sigma bonds. Therefore, the electron group geometry of the carboxyl carbon is trigonal planar. In an ester linkage, the carbonyl carbon is also typically sp2 hybridized and forms three sigma bonds with neighboring atoms, including two sigma bonds with two oxygen atoms and one sigma bond with another carbon atom. The fourth valence electron of the carbon atom is located in a p orbital, which is perpendicular to the plane formed by the three sigma bonds. In terms of electron group geometry, the carbonyl carbon in an ester linkage is also located at the center of a trigonal planar arrangement of electron groups, which consists of the three sigma bonds. Therefore, the electron group geometry of the carbonyl carbon in an ester linkage is also trigonal planar.
To know more about sp2 hybridization click here:
brainly.com/question/17012569
#SPJ11
The electron group geometry and hybridization state-of-the carboxyl carbon and an ester linkage is trigonal planar.
What is trigonal planar?A trigonal planar compound consists of a central atom connected to three atoms arranged in a triangular pattern around the central atom.
Also, a trigonal planar is a molecular geometry model with one atom at the center and three atoms at the corners of an equilateral triangle, called peripheral atoms, all in one plane.
If we consider aldehydes and ketones, the geometry around the carbon atom in the carbonyl group is trigonal planar; the carbon atom exhibits sp2 hybridization.
Thus, the electron group geometry and hybridization state-of-the carboxyl carbon and an ester linkage is trigonal planar.
Learn more about trigonal planar here: https://brainly.com/question/29589466
#SPJ4
Which of the following solutions is matched with its correct intermolecular force between solute and solvent?
A. PH3 and F2:Dispersion
B. PH3 and NH3:Dipole-dipole
C. CH2F2 and CH20: Hydrogen Bonding
D. CH2F2and PH3: dipole-induced dipole
Answers
The difference in electronegativity between their constituent atoms create a polar molecule, leading to dipole-dipole interactions between the solute and solvent. Thus, answer B: \(PH_{3}\) and \(NH_{3}\) is correct.
In a solution, the solute is the substance that gets dissolved, while the solvent is the substance that does the dissolving. Intermolecular forces are the forces between molecules that hold them together in a solution.
For option A, PH3 and F2 are both nonpolar molecules, so the interaction between them would be dispersion forces, not dipole-dipole forces.Option B, PH3 and NH3, is the correct match. Both molecules are polar due to the difference in electronegativity between their constituent atoms, leading to dipole-dipole interactions between the solute and solvent.Option C, \(CH_{2} F_{2}\) and \(CH_{2}O\) involves two polar molecules, but hydrogen bonding is not possible here as hydrogen is not directly bonded to a highly electronegative atom (such as oxygen, nitrogen, or fluorine) in both molecules.Lastly, option D, \(CH_{2}F_{2}\) and \(PH_{3}\), involves a polar molecule \(CH_{2}F_{2}\) and a nonpolar molecule \(PH_{3}\). This would lead to dipole-induced dipole interactions, not dipole-dipole interactions.
Learn more about electronegativity here:
https://brainly.com/question/17762711
#SPJ11
Which property of matter is conserved in chemical reactions and shown by balanced equations?
A. mass
B. volume
C. density
D. shape
Answers
Answer:
Mass is your answer
Explanation:
A sample of solid candle wax is put into a pot on the stove. Heat energy is transferred to the wax at a constant rate by the burner on the stove. Match the segments on the graph to the correct description of what is happening to the wax.
A-B
B-C
C-D
D-E
E-F
(match them)
melting
gasseous wax heating
solid wax heating
boiling
liquid wax heating

Answers
Explanation:
The temperature increases as each phase is heating. The temperature remains constant as each phase is changing to the next. The phases associated with increasing temperature are solid, liquid, gas — in that order.
A-B solid wax heating
B-C melting
C-D liquid wax heating
D-E boiling
E-F gaseous wax heating
How does antifreeze rely on colligative properties to work?
A. It causes the vapor pressure to increase and the increased outward pressure of the gasoline vapors prevents it from solidifying into ice.
B. It causes freezing point elevation which raises the temperature of the gasoline without letting it freeze.
C. It causes freezing point depression which requires the gasoline to reach a much lower temperature before freezing.
D. It causing boiling point elevation, which raises the temperature of the gasoline without letting it boil so that it can resist the cold.
WILL GIVE BRAINLIEST FOR CORRECT!!!
Answers
It causes freezing point depression which requires the gasoline to reach a much lower temperature before freezing. Hence, option C is correct.
What is the freezing point?Whenever a solute is added to a solution/solvent, it leads to depression in the freezing point of that solution/solvent. Depression in the freezing point of a solution on the addition of a solute is a colligative property.
A colligative property is a physical property which depends upon the number of solutes added not on the nature of solutes which means it does not matter whether we are adding 1000 particles of sugar or salt in water, the depression in the freezing point will occur by the same °C. Also, the more a solute is added the more will be the depression at the freezing point.
The formula for depression at the freezing point is mentioned as under:
Δ T = K x m
where Δ T = freezing point depression;
K = cryoscopic constant;
m = molality of the solution.
For example, The freezing point of water is 0 °C but as soon as we add a 92 gm solute like NaCl (common salt) in 1000 gm of water, its freezing point will be lower to −3.72 °C.
Hence, option C is correct.
Learn more about freezing point here:
https://brainly.com/question/12940710
#SPJ5
A hot metal plate at 150°C has been placed in air at room temperature. Which event would most likely take place over
the next few minutes?
O Molecules in both the metal and the surrounding air will start moving at lower speeds.
O Molecules in both the metal and the surrounding air will start moving at higher speeds.
O The air molecules that are surrounding the metal will slow down, and the molecules in the metal will speed up.
O The air molecules that are surrounding the metal will speed up, and the molecules in the metal will slow down.
Answers
Answer:
It would be the third answer.
Explanation:
This is because over the next few minutes, the heat would radiate away from the atoms in the metal plate and the atoms around the plate would absorb this radiation.
Please, can anyone confirm that my answers are correct?
Will give brainiest to first correct answer! (No links pls)




Answers
Arrange the following order of increasing intermolecular forces: methane (ch4), propane (c3h8), butane (c4h10).
Answers
Order of increasing intermolecular forces will be CH4 < C3H8 < C4H10.
What is meant by intermolecular forces ?The electromagnetic forces of attraction or repulsion that operate between atoms and other types of nearby particles, such as atoms or ions, are examples of intermolecular forces (IMFs), also known as secondary forces. In comparison to intramolecular forces, which keep a molecule together, intermolecular forces are weak. For instance, the forces between adjacent molecules are substantially less than the covalent bond, which involves exchanging electron pairs between atoms.Both sets of forces are crucial components of the force fields that molecular mechanics frequently employs.Macroscopic observations that show the existence and operation of forces at the molecular level serve as the starting point for the study of intermolecular forces.Learn more about intermolecular forces refer to :
https://brainly.com/question/2193457
#SPJ4
Cl2 +2e- —> 2Cl-
if you’re adding 2 electrons to the Cl2, why is it still Cl- and not just Cl?
Answers
When two electrons are added to chlorine has, Cl2, each atom of Cl will receive one electron. Hence, the atoms becomes ions having a negative charge, Cl-
IonsIons are atoms or group of atoms possessing an electrical charge which may be positive or negative.
Negatively charged ions are formed when atoms accept or gain electrons. The negative charge is because there are more electrons than protons in the atom.
Negatively charged ions are usually formed by non-metals. For example, chlorine gas accepts two electrons to become 2 Cl-
Cl2 + 2e- ----> 2 Cl-
Therefore, when two electrons are added to chlorine has, Cl2, each atom of Cl will receive one electron. Hence, the atoms becomes ions having a negative charge, Cl-
Learn more about negative ions at: https://brainly.com/question/1046561
A silver-colored metal is placed in a blue solution. After a few minutes, a red coating forms
on the metal and the solution turns clear. Which best describes the products of this
reaction?
Answers
The reaction when silver metal is coated with red color after the reaction is known as displacement reaction.
The silver colored metal is coated with red color after reaction so the coating is a single element which is displaced by the silver colored reactant and the silver colored metal will form a compound because it displaces the red colored metal. So the products are a single metal and a compound and the reaction is displacement reaction.
Thus, the reaction describes a displacement reaction.
To learn more about metal check the link below:
https://brainly.com/question/25597694
#SPJ4
What is this help ASAP
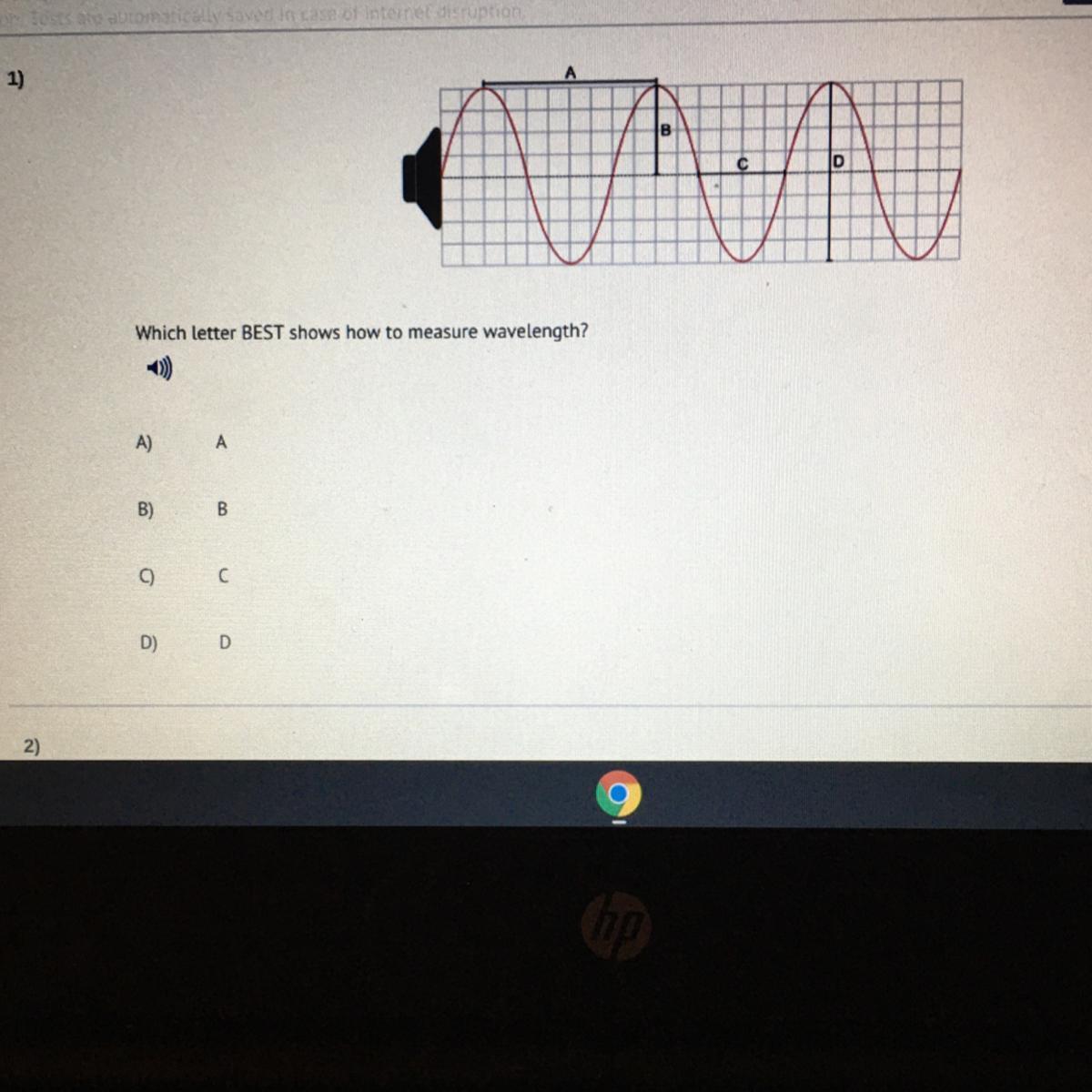
Answers
Answer:
A, im gonna say. Hopefully this helps!
Explanation:
Length is horizontal, and A is horizontal too.
2 NH3 + 3 CuO → 3 Cu + N2 + 3 H2O
In the above equation, how many grams of N2 can be made when 5.3 moles of CuO are consumed?
Round your answer to the nearest tenth. If you answer is a whole number like 4, report the answer as 4.0
Use the following molar masses. If you do not use these masses, the computer will mark your answer incorrect.:
Element Molar Mass
Hydrogen 1
Nitrogen 14
Copper 63.5
Oxygen 16
Question 2
S + 6 HNO3 → H2SO4 + 6 NO2 + 2 H2O
In the above equation, how many grams of water can be made when 19.5 moles of HNO3 are consumed?
Round your answer to the nearest tenth. If you answer is a whole number like 4, report the answer as 4.0
Use the following molar masses. If you do not use these masses, the computer will mark your answer incorrect.:
Element Molar Mass
Hydrogen 1
Nitrogen 14
Sulfur 32
Oxygen 16
Question 3
3 Cu + 8HNO3 → 3 Cu(NO3)2 + 2 NO + 4 H2O
In the above equation, how many grams of water can be made when 15.4 moles of HNO3 are consumed?
Round your answer to the nearest tenth. If you answer is a whole number like 4, report the answer as 4.0
Use the following molar masses. If you do not use these masses, the computer will mark your answer incorrect.:
Element Molar Mass
Hydrogen 1
Nitrogen 14
Copper 63.5
Oxygen 16
Question 4
For the reaction C + 2H2 → CH4, how many grams of carbon are required to produce 5.7 moles of methane, CH4 ?
Round your answer to the nearest tenth. If you answer is a whole number like 4, report the answer as 4.0
Use the following molar masses. If you do not use these masses, the computer will mark your answer incorrect.:
Element Molar Mass
Hydrogen 1
Carbon 12
Answers
Answer:
Explanation:
Question 1:
The balanced chemical equation is:
2 NH3 + 3 CuO → 3 Cu + N2 + 3 H2O
The molar ratio between CuO and N2 is 3:1, which means that for every 3 moles of CuO consumed, 1 mole of N2 is produced.
To find how many grams of N2 can be produced from 5.3 moles of CuO, we need to first calculate how many moles of N2 can be produced:
Moles of CuO = 5.3 mol CuO
Moles of N2 = Moles of CuO / 3 (from the molar ratio)
Moles of N2 = 5.3 mol CuO / 3 = 1.77 mol N2
Now we can use the molar mass of N2 to calculate the mass:
Molar mass of N2 = 14 g/mol
Mass of N2 = Moles of N2 x Molar mass of N2
Mass of N2 = 1.77 mol x 14 g/mol = 24.78 g
Rounded to the nearest tenth, the answer is 24.8 g of N2.
Therefore, 24.8 grams of N2 can be made when 5.3 moles of CuO are consumed.
Question 2:
The balanced chemical equation is:
S + 6 HNO3 → H2SO4 + 6 NO2 + 2 H2O
The molar ratio between HNO3 and H2O is 6:2, which means that for every 6 moles of HNO3 consumed, 2 moles of H2O are produced.
To find how many grams of H2O can be produced from 19.5 moles of HNO3, we need to first calculate how many moles of H2O can be produced:
Moles of HNO3 = 19.5 mol HNO3
Moles of H2O = Moles of HNO3 x 2/6 (from the molar ratio)
Moles of H2O = 19.5 mol HNO3 x 2/6 = 6.5 mol H2O
Now we can use the molar mass of H2O to calculate the mass:
Molar mass of H2O = 18 g/mol
Mass of H2O = Moles of H2O x Molar mass of H2O
Mass of H2O = 6.5 mol x 18 g/mol = 117 g
Rounded to the nearest tenth, the answer is 117.0 g of H2O.
Therefore, 117.0 grams of H2O can be made when 19.5 moles of HNO3 are consumed.
Question 3:
The balanced chemical equation is:
3 Cu + 8HNO3 → 3 Cu(NO3)2 + 2 NO + 4 H2O
The molar ratio between HNO3 and H2O is 8:4, which means that for every 8 moles of HNO3 consumed, 4 moles of H2O are produced.
To find how many grams of H2O can be produced from 15.4 moles of HNO3, we need to first calculate how many moles of H2O can be produced:
Moles of HNO3 = 15.4 mol HNO3
Moles of H2O = Moles of HNO3 x 4/8 (from the molar ratio)
Moles of H2O = 15.4 mol
The clean-room in a computer industry requires perfect filtration efficiency to the incoming air; i.e. penetration factor P = 0. The ventilation rate is maintained at λ = 3 h¹. Consider the manufacture is located in an area with rather constant outdoor particle number concentration 0 = 12000 cm³ of a certain particle size, which has deposition rate 2 = 1 h¹¹. Assume that the indoor particle number concentration, C, satisfies the mass-balance equation dC -= P2O-(2+2)C to answer the following questions: dt a. Show that the indoor concentration can be mathematically described by C(t)= Ce+", where Co is the initial indoor particle number concentration at t=0? b. Assume at t=0 the indoor particle number concentration was Co=5000 cm³, then how many hours would it take to reduce this concentration into C/2?
Answers
a. substituting in the expression of C(t) obtained in part a, we get,2500 = 12000/ (1 + 12000/ 5000 - 1) * e^(-2*3*t) we get,t = 1/ (6 * log (2)) * log (5/3)≈ 0.276 h Therefore, it would take approximately 0.276 hours to reduce this concentration into C/2.
The differential equation for the indoor concentration of the given computer industry can be given as follows: dC/dt = P (0- C) - 2C²The above differential equation can be solved by the method of separating the variables as follows: dC/ (P (0- C) - 2C²) = dtIntegrating both sides, we get,-1/ [2P log (C/ (C- P0))] + (P0/ [P (C- P0)]) - (1/ (2C)) = t + c where c is the constant of integration. After simplification, the above equation can be expressed as:C(t) = P0/ (1 + (P0/ Co - 1) e^(-2Pt))The initial particle concentration Co is the value of C at t = 0. Hence, Ce = P0/ (1 + P0/ Co - 1) which can be simplified as Ce = Co/ (1 + P0/ Co - 1) = Co/P0b. Given that Co = 5000 cm³ and C/2 = 5000/2 = 2500 cm³,
to know more about equation, visit
https://brainly.com/question/29174899
#SPJ11
20. Which of the following are pure substances?
I. Concrete
11. Wood
Table salt
IV. Milk
V. Copper
a. I only
b. II and IV
IV and V
d. III and V
III, IV, and V
e.lll, lV , and B
Answers
An earthquake is the shaking and trembling that results from the movement of rock beneath Earth's surface. Earthquakes most commonly occur near tectonic plate boundaries.
Why is this most likely the case?
Answers
Answer:
Explanation: Because they do not all move in the same direction, plates often directly collide or move laterally along each other, a tectonic environment that makes earthquakes frequent.
Name the following compounds:

Answers
The IUPAC nomenclature is based on a systematic approach that involves identifying and prioritizing functional groups, substituents, and other structural features of the compound. The names of the given compounds are:
2-methyl, 2-hexene4-ethyl, 3,5-dimethyl, nonane4-methyl, 2-heptyne5-propyl decaneSpecific priority rules are used to determine the parent chain (main carbon backbone) in organic compounds, the selection of functional groups, and the numbering of carbon atoms. Prefixes and suffixes are employed to indicate substituents, functional groups, and other structural elements present in the compound.
Learn more about IUPAC nomenclature, here:
https://brainly.com/question/14379357
#SPJ1
PLEASE HELP WILL GIVE BRAINLIEST!!!!
Which of the following is the best definition of a chemical property?
A. The solubility of salt.
B. The ability of gunpowder to explode.
C. The ability of something to undergo a change or reaction.
D. Something that can be observed or measured without changing the identity of the substance.
Answers
Answer:
The B. Is perfect match of a definition of chemical property.
Explanation:
The ability of gunpowder to explode.
The ability of gunpowder to explode is the best definition of a chemical property.
What is chemical property?The change of one type of matter into another type of matter and inability to change into the previous forms is called a chemical property. Examples of chemical properties include reactivity and heat of combustion.
So we can conclude that the ability of gunpowder to explode is the best definition of a chemical property.
Learn more about property here: https://brainly.com/question/1538726
#SPJ5
Calculate the molecular weight of a gas with a density of 1.524 g/L at STP. a. 16 g/mol b. 28 g/mol c. 32 g/mol d. 44 g/mol
Answers
None of the given options are correct. This calculation suggests that the molecular weight of the gas is much larger than the options provided. It is possible that there was an error in the measurement of the density or the problem was intended to be more complex.
To calculate the molecular weight of a gas with a given density at STP, we need to use the Ideal Gas Law equation PV=nRT and rearrange it to solve for the molecular weight. At STP, the pressure (P) is 1 atm and the temperature (T) is 273.15 K.
First, we need to find the molar volume of the gas at STP, which is 22.4 L/mol. Then, we can use the density (d) and the molar volume (V) to calculate the number of moles (n) using the formula n = d/V.
n = 1.524 g/L / 22.4 L/mol = 0.068 mol
Next, we can use the formula n = m/M, where m is the mass of the gas and M is the molecular weight we want to find. Since we know the density and the molar volume, we can find the mass of the gas using the formula m = dV.
m = 1.524 g/L x 22.4 L/mol = 34.13 g/mol
Now we can plug in the values for n and m in the formula n = m/M and solve for M.
0.068 mol = 34.13 g/mol / M
M = 34.13 g/mol / 0.068 mol = 501.9 g/mol
To know more about molecular weight
https://brainly.com/question/837939
#SPJ11
Isotopes are the atoms of the same element which have same ______ but different _____.A. atomic numberB.mass number
Answers
Isotopes are the atoms of the same element which have the same atomic number but different mass numbers.
Isotopes are variants of a particular chemical element that have the same number of protons but different numbers of neutrons. For example, carbon-12, carbon-13, and carbon-14 are three isotopes of the element carbon that all have the same number of protons but differing numbers of neutrons in their atomic nuclei. Since isotopes have the same number of protons, they also have the same atomic number, but because they have different numbers of neutrons, they differ in mass number.
In summary, isotopes are the atoms of the same element which have the same atomic number but different mass numbers.
To know more about Atomic number refer here :
https://brainly.com/question/29793337
#SPJ11
7.70 mol of a monatomic ideal gas, kept at the constant pressure 1.62E+5 Pa, absorbs 3870 J of heat. If the change in internal energy is zero and this process occurs with a change in temperature 24.2 °C, How much did the volume of the gas change during this process?
Answers
The volume of the gas changed by approximately 0.280 m³ during the process.
To find the change in volume of the gas during the process, we can use the equation:
ΔQ = nCvΔT
where: ΔQ is the heat absorbed (3870 J),
n is the number of moles of the gas (7.70 mol),
Cv is the molar heat capacity at constant volume,
ΔT is the change in temperature (24.2 °C = 24.2 K).
Since the change in internal energy is zero (ΔU = 0), we know that ΔU = ΔQ + ΔW, where ΔW is the work done by the gas. In this case, since the process is at constant pressure, we can write ΔW = PΔV, where P is the pressure (1.62E+5 Pa) and ΔV is the change in volume.
Now, using the ideal gas law, we can express ΔV in terms of ΔT:
ΔV = (nRΔT) / P
where R is the ideal gas constant (8.314 J/(mol·K)).
Substituting the given values into the equations:
ΔQ = nCvΔT
3870 J = 7.70 mol × Cv × 24.2 K
From the equation ΔV = (nRΔT) / P, we have:
ΔV = (7.70 mol × 8.314 J/(mol·K) × 24.2 K) / (1.62E+5 Pa)
Simplifying the equations and performing the calculations:
ΔQ = nCvΔT
3870 J = 7.70 mol × Cv × 24.2 K
Cv ≈ 2.00 J/(mol·K) (calculated from the above equation)
ΔV = (7.70 mol × 8.314 J/(mol·K) × 24.2 K) / (1.62E+5 Pa)
ΔV ≈ 0.280 m³
Therefore, the volume of the gas changed by approximately 0.280 m³ during this process.
Read more on internal energy here: https://brainly.com/question/29546504
#SPJ11
The compound, P4S10 is used in the manufacture of safety matches. What is its name? 1. phosphorus sulfide 2. phosphoric sulfide 3. phosphorus decasulfide 4. tetraphosphorus decasulfide 5. phosphorus sulfite
Answers
4: tetraphosphorus decasulfide. This compound is used in the manufacture of safety matches because it is a highly reactive substance that ignites when rubbed against a rough surface.
An explanation for why this compound is used in safety matches is that it contains both phosphorus and sulfur, which are two highly reactive elements that can generate a lot of heat and light when they react with oxygen.
When the match is struck against the rough surface, the friction and heat generated cause the tetraphosphorus decasulfide to react with the oxygen in the air, producing a flame that ignites the matchstick.
In summary, tetraphosphorus decasulfide is the compound used in the manufacture of safety matches because of its highly reactive nature and ability to generate heat and light when it reacts with oxygen.
Learn more about oxygen click here:
https://brainly.com/question/26073928
#SPJ11