Give reasons that might help explain the following observations:
a) A student was unsuccessful to prepare a Grignard reagent from 4-bromocyclohexanol
b) Benzenesulfonic acid does not undergo Friedel-Crafts alkylation
c) (2S, 3R)- 2,3-Dibromobutane has a specific rotation, [a]D, 0⁰
d) A student tries to prepare an ether via the Williamson ether synthesis from ethanol and t-butyl bromide, but obtains an alkene instead.
e) Doubling the concentration of a nucleophile has no effect on the rate of a substitution reaction
Answers
Answer:
Transcribed image text: QUESTION 1 [11] Give reasons that might help to explain the following observations: a) A student was unsuccessful to prepare a Grignard reagent from 4-bromocyclohexanol. b) Benzenesulfonic acid does not undergo Friedel-Crafts alkylation. c) (2S, 3R)-2,3-Dibromobutane has a specific rotation, [a]D, of 0°. d) A student tries to prepare an ether via the Williamson ether synthesis from ethanol and t-butyl bromide, but obtains an alkene instead. e) Doubling the concentration of a nucleophile has no effect on the rate of a substitution reaction.
The Benzenesulfonic acid does not undergo Friedel-Crafts alkylation because of the deactivation of the compound by the carboxylic group.
What is the Grignard reagent?The Grignard reagent is a compound that contains alkyl magnesium halide.
a) The student will be unsuccessful to prepare a Grignard reagent from 4-bromocyclohexanol because of the -OH group that reacts with the Grignard reagent when formed.
b) The Benzenesulfonic acid does not undergo Friedel-Crafts alkylation because of the deactivation of the compound by the carboxylic group.
c) The compound (2S, 3R)- 2,3-Dibromobutane has a specific rotation, [a]D, 0⁰ because it is a meso compound.
d) This is because, the tertiary alkyl halide is more prone to elimination reaction giving the alkene.
e) This is because, the reaction may be occurring by an SN1 mechanism and the rate determining step is the formation of the carbocation.
Learn more about substitution reaction:https://brainly.com/question/16811879
#SPJ6
Related Questions
6. What is the specific heat capacity of a substance if 2.41x10* J are needed to
change the temperature of 105.0 g of it from 25.0°C to 75.0°C?
Answers
Answer: 4.59 x 10^-3 J/(g x degrees Celsius) = Cs
Explanation:
Use the equation q = m x Cs x delta T to find the specific heat capacity of the substance.
q = heat in joules
m = mass of substance in grams
Cs = specific heat capacity in J/(g x degrees Celsius)
delta T = change in temperature in degrees Celsius
Step 1: Identify all quantities given in the problem.
q = 2.41 x 10 J
Note: Not sure if this number was supposed to have an exponent or not. I used what was posted by the person asking the question.
m = 105.0 g
Cs = variable we are trying to find
delta T = Temp. final – Temp. initial = 75.0 degrees Celsius – 25.0 degrees Celsius = 50.0 degrees Celsius
Step 2: Place all known quantities into the equation.
q = m x Cs x delta T
2.41 x 10 J = 105.0 g x Cs x 50.0 degrees Celsius
Step 3: Isolate the unknown variable, in this case Cs.
2.41 x 10 J = (105.0 g x Cs x 50.0 degrees Celsius) / (105.0 g x 50.0 degrees Celsius)
When you divide to isolate Cs, the units of grams and degrees Celsius cancel on the right side of the equation.
2.41 x 10 J / (105.0 g x 50.0 degrees Celsius) = Cs
4.59 x 10^-3 J/(g x degrees Celsius) = Cs
Patra drops her toy from the balcony. The toy hits the ground in: 2.5 seconds. What is the velocity of
the toy the instant before it hits the ground?
Answers
Answer:
this is physics but
5 miles pr hour
Explanation:
Which of the following cities has both the lowest water vapor content AND highest relative humidity? Group of answer choices City C: Temperature = 60°F, Dew Point Temperature = 20°F City A: Temperature = 20°F, Dew Point Temperature = 15°F City B: Temperature = 100°F, Dew Point Temperature = 80°F
Answers
Answer:
TEMPRATURE = 60° F, DEW POINT TEMERATURE =20° F
Explanation:
Mg + HCl → MgCl2 + H2
How many liters of hydrogen gas are produced at 298 K and 0.940 atm if 4.00 moles of hydrochloric acid react with an excess of magnesium metal?
Answers
52.05 liters of hydrogen gas are produced at 298 K and 0.940 atm if 4.00 moles of hydrochloric acid react with an excess of magnesium metal.
What is Ideal Gas Law ?The ideal gas law states that the pressure of a gas is directly proportional to the volume and temperature of the gas.
It is expressed as
PV = nRT
where,
P is pressure
V is volume in liters
n is number of moles of gas
R is Ideal gas constant
T is Temperature in kelvin
What is Balanced Chemical equation ?The balanced chemical equation is the equation in which the number of atoms on the reactant side is equal to the number of atoms on the product side in an equation.
Now first we have to write the balanced chemical equation
Mg + 2HCl → MgCl₂ + H₂
Here we can se that 2 mole of hydrochloric acid produces 1 mole of hydrogen gas.
So 4 moles of hydrochloric gas will give \(\frac{1}{2} \times 4\) moles of hydrogen gas that is 2 moles of hydrogen gas.
Now put the values in above equation, we get
PV = nRT
0.940 atm × V = 2 × 0.0821 L atm / K mol × 298
V = \(\frac{2 \times 0.0821\ \text{L atm / K mol} \times 298}{0.940\ \text{atm}}\)
V = 52.05 liter
Thus from the above conclusion we can say that 52.05 liters of hydrogen gas are produced at 298 K and 0.940 atm if 4.00 moles of hydrochloric acid react with an excess of magnesium metal.
Learn more about the Ideal gas law here: https://brainly.com/question/25290815
#SPJ2
i need help with the question below

Answers
How many moles in 206.91 g Na
Answers
Answer:
The answer is 9.000090040048176
Which of the following best describes the type of particle found in the cloud around an atom’s nucleus?
It has a negative charge and much less mass than a neutron
It has a negative charge and about the same mass as a neutron.
It has a negative charge and about the same mass as a neutron.
It has a positive charge and about the same mass as a neutron.
Answers
A 1.85-mole sample of H₂O2 weighs
(A) 33.3 amu
(B) 35.9 g
C) 62.9 g
(D) 1.85 g
E 33.3 g
Answers
Considering the definition of molar mass, the correct answer is option c): the mass of 1.85 moles H₂O₂ is 62.9 grams.
Definition of molar massThe molar mass of substance is a property defined as the amount of mass that a substance contains in one mole.
The molar mass of a compound is the sum of the molar mass of the elements that form it (whose value is found in the periodic table) multiplied by the number of times they appear in the compound.
Molar mass of H₂O₂In this case, you know the molar mass of the elements is:
O= 16 g/moleH= 1 g/moleSo, the molar mass of the compound H₂O₂ is calculated as:
H₂O₂= 2× 1 g/mole + 2× 16 g/mole
Solving:
H₂O₂= 34 g/mole
Mass of 1.85 moles H₂O₂You can apply the following rule of three: If by definition of molar mass 1 mole of the compound contains 34 grams, 1.85 moles of the compound contains how much mass?
mass= (1.85 moles× 34 grams)÷ 1 mole
mass= 62.9 grams
Finally, the mass of 1.85 moles H₂O₂ is 62.9 grams.
Learn more about molar mass:
brainly.com/question/5216907
#SPJ1
As the food burned,
energy was
nergy. Thus, a
thermal
transformed intos
chemical
form of Select
nuclear
$ converted to a
form of
Select
energy.
Check
Answers
Answer:
I don't get it is it even a question?
A reaction in which A,B, and C react to form products is zero order in A, one-half order in B and second order in C.(b) What is the overall order of the reaction?(c) By what factor does the reaction rate change if A is doubled (and the other reactant concentrations are held constant)? Express your answer numerically.(d) By what factor does the reaction rate change if B is doubled (and the other reactant concentrations are held constant)? Express your answer numerically.(e) By what factors does the reaction rate change if C is doubled (and the other reactant concentrations are held constant)? Express your answer numerically.(f) By what factor does the reaction rate change if the concentrations of all three reactants are doubled? Express your answer numerically.
Answers
Products rate law from data rate = k [A]0 [B]1/2 [C]2 b) overall order = 2.5 overall order = 0 + 1/2 + 2 = 5/2 = 2.5
If A is doubled and the concentrations of the other reactants remain constant, how does this affect the rate of the reaction?The reaction is described by this general equation in kinetics: A and B are the reactants, and R is the result of the reaction. aA + bB ---> cR The stoichiometric coefficients of the two reactants and the product are represented by the terms a, b, and c, respectively. The following equation describes the reaction rate for elementary reactions:
When a second-order reaction is doubled, what happens?Second Request Response with Various Reactants This implies that when the centralization of reactant An is multiplied, the pace of the response will twofold, and quadrupling the convergence of reactant in a different examination will fourfold the rate.
To know more about reactants visit :-
https://brainly.com/question/17096236
#SPJ4
Discuss the following statement:
"Small changes in the chemical nature of polysaccharides results in significant differences in biological function"
Answers
Answer:
Explanation:
Small changes in the chemical nature of polysaccharides can make a big difference in how they work in our bodies. Polysaccharides are complex carbohydrates found in things like fiber and medicines. Even tiny changes in their structure can affect how they are digested, how they interact with cells, and their overall impact on our health. Scientists can use these changes to create materials with specific properties or develop new treatments. So, even small tweaks in polysaccharides can have a significant impact on how they function in our bodies.
Clarence grew three bearded iris plants. He put one in direct sunlight, one in
the shade, and one in the dark. The plant in the sun grew the most quickly and
looked the healthiest. Clarence concluded that bearded iris plants grow best
in the sun.
How would you evaluate Clarence's conclusion?
A. His conclusion is valid because his experiment included a control.
B. His conclusion is flawed because it is based on the appearance of the plants
C. His conclusion is flawed because he did not perform enough trails
D. His conclusion is bailed because he tested only one variable
Answers
Which of the following is the best definition of a physical change?
A. A change in a substance where a new substance is formed
B. A change in a substance in which mass is conserved
C. A change in a substance inwhich bonds are broken
D. A change in a substance with no new substances being formed
Answers
D. A change in a substance with no new substances being formed
Explanation:There are 2 ways a substance can change, physical changes and chemical changes. In all changes, physical or chemical, mass is always conserved.
Physical vs. Chemical
The main difference between a physical and chemical change is the substance at the end.
Physical changes do not form new substancesChemical changes do form new substancesNew substances are only created by chemical changes. Additionally, only chemical changes break apart bonds. Physical changes maintain the same compounds and elements, so bonds are not broken.
Examples of Physical Changes
Physical changes do not change the actual substance, but they can change the form of the substance.
MeltingBoilingFreezingCrumblingCuttingBreakingAll of these change the state of matter or shape of the substance, but not the chemical makeup.
Air quality is a measure of how clean or polluted the air is.
Answers
The given statement stating that air quality is a measure of how clean or polluted the air in an ecosystem is, hence the statement is true.
What is an ecosystem?Ecosystem is defined as a system which consists of all living organisms and the physical components with which the living beings interact. The abiotic and biotic components are linked to each other through nutrient cycles and flow of energy.
Energy enters the system through the process of photosynthesis .Animals play an important role in transfer of energy as they feed on each other.As a result of this transfer of matter and energy takes place through the system .
Learn more about ecosystem,here:
https://brainly.com/question/1673533
#SPJ1
What is the main difference between producers and consumers?
A. Producers make their own energy, while consumers must eat other things for energy.
B. Producers absorb energy from other organisms, while consumers get their energy from the sun.
C. Producers absorb energy from other organisms, while consumers must eat other organisms for energy.
D. Producers get their energy from the sun, while consumers absorb nutrients obtained from decomposing dead organisms.
HELP MEEE PLEASE!
Answers
Answer:
The answer is A.
Explanation:
Producers make their own energy, whereas consumers eat other things for energy.
The "Ring of Responsibility" requires a next to water bodies. O 50-foot 43 3-foot O 5-foot O 15- to 25-foot untreated buffer zone
Answers
The "Ring of Responsibility" requires a 15- to 25-foot untreated buffer zone next to water bodies.
What is buffer zone?A buffer zone is an area of land that separates two or more countries, states, or territories, and is often demilitarized. The purpose of a buffer zone is to provide a space for negotiations and to reduce the possibility of conflict and war. Buffer zones can also be used to protect sensitive natural resources or habitats, such as areas of wilderness or wildlife. Buffer zones can be permanent or temporary, and can range in size from a few miles to hundreds of miles. In addition to physical barriers, buffer zones can also include economic, political, and social measures to reduce tensions between two or more parties. Buffer zones are an important tool in international relations, as they can help to prevent armed conflict and promote peaceful resolution of disputes.
This buffer zone is intended to prevent pollutants from entering the water body and protect it from potential environmental damage.
To learn more about buffer zone
https://brainly.com/question/30899142
#SPJ1
How would describe the characteristics of the urine
Answers
Urine has the color of pale yellow to deep amber. It is odorless and has a pH of 4.5-8.0
Please Help me solve for B
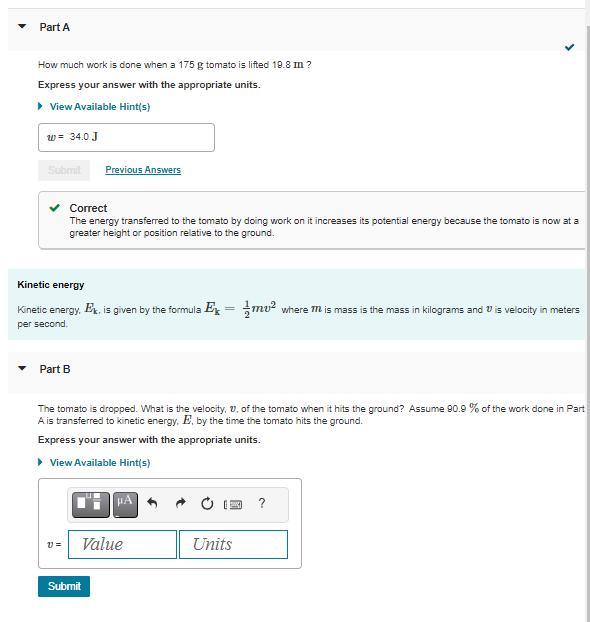
Answers
The velocity of the tomato when it hits the ground is approximately 13.49 meters per second.
The potential energy of the tomato is at the height of 10 meters. When the tomato hits the ground, most of the potential energy is E1 = 0.909*mgh.
By the conservation of energy principle, the kinetic energy \(E_1\) is equal to the kinetic energy \(E_2\) of the tomato just before it hits the ground.
The kinetic energy \(E_2\) is given by\(1/2mv^2\), where v is the velocity of the tomato just before it hits the ground. Equating \(E_1\) and \(E_2\) solving for v, we get:
\(v = \sqrt{(20.909gh)\)
Substituting the values of \(g = 9.81 m/s^2\)and h = 10 m, we get:
v = \(\sqrt{(20.9099.81*10)}\) = 13.49 m/s
To know more about potential energy, here
brainly.com/question/24284560
#SPJ1
--The complete Question is, Suppose a tomato is dropped from a height of 10 meters. If 90.9% of the work done on the tomato is converted to kinetic energy by the time it hits the ground, what is the velocity (in meters per second) of the tomato when it hits the ground? --
Draw a second resonance structure for the following ion (be sure to include the charges and all lone pairs)... + ..N=N=N <-------------->.. ..
Answers
Answer:
Explanation:
Resonance structure occurs in an organic compound that undergoes resonance effects. This resonance effect is sometimes called the mesomeric effect helps to increases the stability of organic compounds that have alternating single bonds and double bonds.
The second resonance structure diagram for the ion given in the question can be found in the attached diagram below.

Which statements are true about catalysts

Answers
The true statements about catalysts are the statement 1,2 and 3.
1. Catalysts increase the rate of reaction: Catalysts facilitate chemical reactions by providing an alternative reaction pathway with lower activation energy. They enhance the rate of the reaction without being consumed in the process.
2. Catalysts behave as reactants in the reaction mixture: Catalysts participate in the reaction by interacting with the reactants. They form temporary bonds with the reactant molecules, leading to the formation of an intermediate complex that ultimately results in the desired products.
3. Catalysts decrease the activation energy of a reaction: Catalysts lower the energy barrier required for a reaction to occur by providing an alternative pathway with a lower activation energy. This enables the reactants to overcome the energy barrier more easily, thus increasing the reaction rate.
4. Catalysts show no physical change at the end of the reaction: Catalysts are not consumed or permanently altered in the reaction. They remain chemically unchanged and are available to participate in subsequent reaction cycles.
The statement "Catalysts are required in large concentrations in a reaction" is false. Catalysts work effectively even in small concentrations, as their role is to facilitate the reaction rather than being directly involved in the stoichiometry of the reaction.
For more questions on catalysts, click on:
https://brainly.com/question/12507566
#SPJ8
How many grams (mass) are in 1.7 moles of Nickel II oxide NiO?
Answers
Answer:
126.99g
Explanation:
NiO has a molar mass of 74.7 g/mol. This means that one mole of nickel (II) oxide has a mass of 74.7 grams. We can use stoichiometry/dimensional analysis to figure out how many grams are in 1.7 moles.
\(1.7mol NiO (\frac{74.7grams}{1 mole} ) = 126.99g\)
Answer: mass m = n M = 1.7 mol × 74.69 g/mol
Explanation:
Molar mass M(NiO) = (58.69+16) g/mol
If you burn 32.0 g of methane, how many kilojoules of heat will be released? (4 pts) CH4(g) + 2 O2(g) CO2 (g) + H2O(g) H = -890 kJ/mole
Answers
The question requires us to calculate the amount of heat released in the methane combunstion reaction when 32 g of methane are burned.
The first step in this question is balance the reaction:
\(\text{CH}_{4(g)}+2O_{2(g)}\rightarrow CO_{2(g)}+2H_2O_{(g)}\)Now that we have the balanced reaction, we need to calculate the molar mass of methane in order to have the amount of moles that will be burned. To calculate the molar mass, we conisder the atomic mass of carbon (12 u) and hydrogen (1 u) and the number of these atoms in the molecule:
molar mass (CH4) = (1 * 12) + (4 * 1) = 16 g/mol
Next, we can calculate the number of moles of methane contained in 32g of this compound:
16 g CH4 ----- 1 mol CH4
32 g CH4 ----- x
Solving for x, we have that there are 2 moles of methane and thus this is the amount that will be burned in this reaction.
Finally, knowing how many moles of methane will burn, we can calculate the amount of energy released from the molar enthalpy given:
1 mol CH4 ---------- 890 kJ of energy
2 mol CH4 ---------- y
Then, solving for y, we have that 1780 kJ of heat will be released when 32 g of methane are burned.
What has a higher boiling point C2H4O or CH3OH
Answers
Answer:
Explanation:
i wish i can help
Which of the following would be an example of a compound? *
5 poir
oxygen
carbon dioxide
sand
O carbon
Answers
Answer:
carbon dioxide
Explanation:
An example of a compound from the given choices is carbon dioxide.
A compound is any substance composed of two or more atoms /elements joined together in a definite grouping .
The properties of a compound are different and distinct from the elements that makes them. From the choices, carbon dioxide is made up of two elements which are carbon and oxygen. oxygen and carbon are elements.Sand is a mixture.How do I do this ? What was AMU again?

Answers
Answer
n=4
Procedure
Data
Sulfur has an atomic mass of 32.065 u
Fluorine has an atomic mass of 18.9984 u
To solve this problem we need to find the number of atoms of fluorine, this can be done using an equation as follows
\(108.01=32.065+18.9984x\)Solving for x we have
\(x=\frac{108.01-32.065}{18.9984}=3.99\approx4\)Hence as we can't have a decimal atom we consider 4 atoms of fluorine.
which 2 criteria are the most important of engineers to consider when developing a procsses to produce
Answers
Two key criteria that engineers must prioritize are efficiency and safety. By emphasizing efficiency and safety during process development, engineers can create robust and reliable processes that not only maximize productivity but also prioritize the well-being of personnel and the environment.
When developing a process, engineers need to consider several important criteria. Two key criteria that engineers must prioritize are efficiency and safety.
Efficiency is crucial in process development to ensure optimal use of resources, time, and energy. Engineers strive to design processes that maximize productivity, minimize waste, and reduce costs. This involves optimizing reaction conditions, streamlining workflow, and implementing automation where possible. Efficiency considerations also extend to energy consumption, raw material utilization, and overall process sustainability.
Safety is another critical aspect that engineers must prioritize. They need to identify and mitigate potential hazards associated with the process, ensuring the safety of both personnel and the environment. This involves conducting thorough risk assessments, implementing safety protocols, and designing equipment and systems with safety features. Engineers must also consider the safe handling and storage of materials, as well as potential risks during transportation and disposal.
By emphasizing efficiency and safety during process development, engineers can create robust and reliable processes that not only maximize productivity but also prioritize the well-being of personnel and the environment.
For more question on environment
https://brainly.com/question/1186120
#SPJ8
Periodic table the first row of elements fits in the period blank after the element blank the second row of elements fits in period blank
Answers
Answer:
The first row fits in period 2
The second row fits in period 3
Explanation:
Elements arranged vertically in the periodic table in groups that share similar chemical properties.
Elements are also organized horizontally in rows or periods
The length of each period is determined by the number of electrons that can occupy the sublevels being filled in that period.
Help as soon as possible

Answers
The system from the description must be an open system. Option C
What is an open system?
An open system is one that communicates with and is influenced by its surroundings. An open system interacts with its environment by exchanging matter, energy, and information, as opposed to a closed system, which is self-contained and runs autonomously.
The idea of open systems highlights how interconnected and reliant on its environment a system is. For the analysis of system behavior, adaptability, and responses to external forces, understanding these interconnections is essential.
Learn more about open system:https://brainly.com/question/29977128
#SPJ1
5. A Brønsted-Lowry acid
A. is any species that donates a proton.
B. is any species that accepts a proton.
C. has a lower pH than vinegar.
D. is limited to aqueous solutions.
Answers
Answer:
A. is any species that donates a proton.Explanation:
A Bronsted-Lowry acid is a chemical species that donates one or more hydrogen ions in a reaction.
In contrast, a Bronsted-Lowry base accepts hydrogen ions.
Avogadro's number, 6.022 x 1023, represents
Answers
Answer: One mole
Explanation: One mole of a substance is equal to 6.022 × 10²³ units of that substance (such as atoms, molecules, or ions). The number 6.022 × 10²³ is known as Avogadro's number or Avogadro's constant.