H3PO4 has three acidic protons with the following Ka values:
Ka1 = 7.1 x 10^â3
Ka2 = 6.3 x 10^â8
Ka3 = 4.5 x 10^â13
If you have this acid in a solution with a pH=1.5 what is the predominate form of the compound in solution?
Answers
H3PO4 is the chemical formula for phosphoric acid.
It has three ionizable hydrogen atoms and is therefore a triprotic acid. It releases hydrogen ions in the aqueous environment, leading to the development of hydrogen ions (H+) or hydronium ions (H3O+), and negatively charged ions (H2PO4-, HPO42- and PO43-). This compound has three acidic protons with the following Ka values:
Ka1 = 7.1 x 10^-3Ka2 = 6.3 x 10^-8Ka3 = 4.5 x 10^-13
If H3PO4 is in a solution with a pH of 1.5, it indicates that the solution is highly acidic, therefore H3PO4 will fully dissociate. In other words, in solution, it will be present as its conjugate base and three protons. In this case, the predominate form of the compound in solution will be H2PO4-.
To know more about H3PO4 refer to:
https://brainly.com/question/3506521
#SPJ11
Related Questions
molecules can be dissolved? Why?
Answers
Answer:
Dissolving is when the solute breaks up from a larger crystal of molecules into much smaller groups or individual molecules. This break up is caused by coming into contact with the solvent. In the case of salt water, the water molecules break off salt molecules from the larger crystal lattice.
Explanation:
Answer:
Gases can dissolve in water. The dissolving of a gas in water depends on the interaction between the molecules of the gas and the water molecules. The amount of gas that can be dissolved in water depends on the temperature of the water. More gas can dissolve in cold water than in hot water.
Explanation:
What mass of hclo4 should be present in 0. 600 l of solution to obtain a solution with each of the following ph values?.
Answers
The mass of HClO₄ required to produce the desired pH in a 0.600 L solution can be calculated using the Henderson-Hasselbalch equation and the molecular weight of HClO₄.
The Henderson-Hasselbalch equation relates the pH of a solution to the acid dissociation constant (pKa) and the ratio of the concentrations of the acid (HA) and its conjugate base (A-). For HClO₄, pKa is approximately -7.2. Therefore, [A-]/[HA] can be solved for using the rearranged Henderson-Hasselbalch equation: [A-]/[HA] = 10^(pH - pKa).
For example, to obtain a solution with pH = 1.0, [A-]/[HA] = 10^(1.0 - (-7.2)) = 7.94 x 10^8. Assuming that the total volume of the solution is 0.600 L, and that the desired concentration of ClO4- is equal to the concentration of HClO₄, which is equal to [HA], then [HA] = 0.600 L / (1 + 7.94 x 10^8) = 7.54 x 10^-10 M. The mass of HClO₄ required to produce this concentration can be calculated using the molecular weight of HClO₄: 7.54 x 10^-10 mol/L x 100.46 g/mol = 7.57 x 10^-8 g, or 0.0757 mg.
Learn more about conjugate base here:
https://brainly.com/question/28545620
#SPJ11
Molarity of Kool Aid solutions can be calculated by comparing the concentrations of Kool Aid powder and sugar added to a given volume of water. The molar mass of Kool Aid will be the same as that of sugar for our purpose. The molecular formula for sugar is C12H22O11- Your objective for this lab will be to calculate the molarity of Kool Aid desired based on package directions. You will then be provided two concentrated Kool Aid solutions. You will use dilution calculations to determine the amount of water and concentrated solution you will need in order to prepare 65 mL of the desired molarity.
Calculate the molarity of Kool Aid desired based on the following information from the package directions.
1 package Kool Aid powder = 4. 25 grams 1 cup sugar = 192. 00 grams
2. 00 quarts of water (1. 06 quarts = 1 liter)
Answers
The amount of concentrated solution needed is (0.286 M)(65 mL) / C M, and the amount of water needed is 65 mL minus the volume of the concentrated solution.
To calculate the molarity of Kool Aid desired, we need to determine the number of moles of Kool Aid powder and sugar in the package. Since the molecular formula for sugar is C12H22O11, we can calculate its molar mass as follows:
Molar mass of C12H22O11 = (12 * 12.01) + (22 * 1.01) + (11 * 16.00)
= 144.12 + 22.22 + 176.00
= 342.34 g/mol
Given that the package contains 4.25 grams of Kool Aid powder, we can calculate the number of moles of Kool Aid powder using its molar mass:
Number of moles of Kool Aid powder = Mass / Molar mass
= 4.25 g / 342.34 g/mol
≈ 0.0124 mol
Similarly, for the sugar, which has a molar mass of 342.34 g/mol, we can calculate the number of moles of sugar using its mass:
Number of moles of sugar = Mass / Molar mass
= 192.00 g / 342.34 g/mol
≈ 0.5612 mol
Now, to calculate the molarity of the desired Kool Aid solution, we need to determine the volume of water. Given that 1.06 quarts is equal to 1 liter, and we have 2.00 quarts of water, we can convert it to liters as follows:
Volume of water = 2.00 quarts * (1.06 liters / 1 quart)
= 2.12 liters
To find the molarity, we use the formula:
Molarity (M) = Number of moles / Volume (in liters)
Molarity of Kool Aid desired = (0.0124 mol + 0.5612 mol) / 2.12 L
≈ 0.286 M
To prepare 65 mL of the desired molarity, we can use dilution calculations. We need to determine the volume of concentrated solution and the volume of water needed.
Let's assume the concentration of the concentrated Kool Aid solution is C M. Using the dilution formula:
(C1)(V1) = (C2)(V2)where C1 is the initial concentration, V1 is the initial volume, C2 is the final concentration, and V2 is the final volume.
Given that C1 = C M and V1 = V mL, and we want to prepare a final volume of 65 mL (V2 = 65 mL) with a final concentration of 0.286 M (C2 = 0.286 M), we can rearrange the formula to solve for the volume of the concentrated solution:
(C M)(V mL) = (0.286 M)(65 mL)
V mL = (0.286 M)(65 mL) / C M
So, the amount of concentrated solution needed is (0.286 M)(65 mL) / C M, and the amount of water needed is 65 mL minus the volume of the concentrated solution.
For more such questions on concentrated visit:
https://brainly.com/question/28564792
#SPJ8
what turns colour in an acid and base
Answers
Answer:
Chemists use a solution called Universal Indicator to identify acids and bases. ... The Universal Indicator Color Guide shows that Universal Indicator turns red when it is added to a strong acid, it turns purple when it is added to a strong base, and it turns a yellowish-green when it is added to a neutral solution.
Explanation:
Answer:
Phenolphthalein
Explanation:
An atom is the simplest particle of an element that retains all the properties of that element.
True
False
Answers
In normal air, the partial pressure of oxygen is 0.18 atm, the partial pressure of nitrogen is 0.77 atm, and the partial pressure of carbon dioxide is 0.05 atm. Scuba divers often use tanks with an enhanced mixture of only oxygen and nitrogen. If the total pressure in the scuba tank is 3.33 atm and the partial pressure of oxygen is 1.20 atm, what is the partial pressure of nitrogen?
____ atm
Answers
Answer:
First, we can find the partial pressure of carbon dioxide in the scuba tank by subtracting the sum of partial pressures of oxygen and nitrogen from the total pressure:
partial pressure of CO2 = total pressure - partial pressure of O2 - partial pressure of N2
partial pressure of CO2 = 3.33 atm - 1.20 atm - (unknown partial pressure of N2)
partial pressure of CO2 = 2.13 atm - (unknown partial pressure of N2)
Since the enhanced mixture only contains oxygen and nitrogen, the sum of their partial pressures should equal the total pressure:
partial pressure of O2 + partial pressure of N2 = total pressure
1.20 atm + partial pressure of N2 = 3.33 atm
partial pressure of N2 = 3.33 atm - 1.20 atm
partial pressure of N2 = 2.13 atm
Now we can substitute this value into the equation we found for the partial pressure of CO2:
partial pressure of CO2 = 2.13 atm - partial pressure of N2
partial pressure of CO2 = 2.13 atm - 0.77 atm
partial pressure of CO2 = 1.36 atm
Therefore, the partial pressure of nitrogen in the scuba tank is 0.77 atm.
Explanation:
silver metal reacts with nitric acid according to the equation: 3ag(s) 4hno3(aq) 3agno3 (aq) no(g) 2h2o(l) what volume of 1.15 m hno3(aq) is required to react with 0.784 g of silver?
Answers
To react with 0.784 g of silver, you will need 0.00843 L or 8.4 mL of 1.15 M HNO₃(aq).
To solve this problem, we need to use stoichiometry and dimensional analysis. First, we need to convert the mass of silver given in grams to moles by dividing it by its molar mass:
0.784 g Ag / 107.87 g/mol Ag = 0.00725 mol Ag
According to the balanced chemical equation, 4 moles of HNO₃ react with 3 moles of Ag. So we can set up a proportion:
4 mol HNO₃ / 3 mol Ag = x mol HNO₃ / 0.00725 mol Ag
Solving for x, we get:
x = 4/3 * 0.00725 mol HNO₃ = 0.00967 mol HNO₃
Now we can use the molarity of the nitric acid solution given to calculate the volume of solution needed:
Molarity = moles of solute/liters of solution
1.15 M = 0.00967 mol HNO₃ / V liters HNO₃
Solving for V, we get:
V = 0.00967 mol HNO₃ / 1.15 M = 0.0084 L HNO₃
Finally, we can convert the volume from liters to milliliters:
0.0084 L HNO₃ * 1000 mL/L = 8.4 mL HNO₃
Therefore, 8.4 mL of 1.15 M HNO₃ solution is required to react with 0.784 g of silver.
Learn more about silver at https://brainly.com/question/18503331
#SPJ11
Use the periodic table to compare the sizes of ionic radii with the corresponding atomic radii or other ionic radii. Choose from smaller and larger in the blanks below: A sodium cation is than a sodium atom. A phosphorus anion is than a phosphorus atom. A magnesium ion is than a sodium ion. A chlorine ion is than a phosphorus ion. A potassium ion is than a sodium ion.
Answers
Answer:smaller larger smaller smaller larger
Explanation:
Just took the test on edge
Answer:
smaller
larger
smaller
smaller
larger
Explanation:
on edge
arrange the compounds from lowest boiling point to highest boiling point.
Answers
Answer:
ion- ion> H - bonding > dipole > dipole> london dispersion
Calculate the molarity of a solution that contains 85.0 g of Zn(C2H3O2)2 in 250. mL of solution (don't forget to convert mL to L first). Round to the nearest hundredth.
Answers
Answer:
[Zn(C₂H₃O₂)₂] = 1.85M (3 sig-figs accurate to 0.01 Molar)
Explanation:
Concentration is defined as amount of solute in a specified volume of solution. That is, Concentration = mass of solute/volume of solution (solution = solute + solvent). When concentration is in terms of Molar values the essential relationship is Molarity(M) = moles solute / volume of solution in liters.
For this problem => first, convert mass of Zn(C₂H₃O₂)₂ into moles and then the volume of solution into liters. Take ratio of moles/volume of solution (L).
=> Molar Concentration = moles of Zn(C₂H₃O₂)₂ / Liters of solution
moles of Zn(C₂H₃O₂)₂ = 85.0 grams Zn(C₂H₃O₂)₂ / formula weight of Zn(C₂H₃O₂)₂ = 85.0g/183.5g·mole⁻¹ = 0.463 mole Zn(C₂H₃O₂)₂.
Volume of solution in Liters = 250ml / 1000ml/L = 0.250 liters
Molarity of Zn(C₂H₃O₂)₂ solution = 0.463 mole Zn(C₂H₃O₂)₂ /0.250 liters of solution = 1.85 Molar in Zn(C₂H₃O₂)₂
_____________
Note: The symbiology convention for 'molar solution concentration' is to place brackets around the molecular formula. That is, [Zn(C₂H₃O₂)₂] = 1.85M
the threshold frequency for gold is 1.20x 1015 hz. if the total energy emitted by a sample of gold is 12.2 kj, calculate the number of atoms present in the sample? (assume each atom emits one photon)
Answers
Answer: Number of atoms: 1.82 x 10^21
Explanation:
To calculate the number of atoms present in the sample of gold, we can use the equation:
E = Nhf
Where:
E is the total energy emitted by the sample,
N is the number of atoms,
h is the Planck's constant (approximately 6.626 x 10^-34 J·s),
f is the frequency of each photon.
We are given the total energy emitted by the sample as 12.2 kJ, which we need to convert to joules:
12.2 kJ = 12.2 x 10^3 J
The frequency of each photon is related to the threshold frequency by the equation:
f = threshold frequency
Substituting the given values:
f = 1.20 x 10^15 Hz
Now we can rearrange the equation E = Nhf to solve for N:
N = E / (hf)
Substituting the values:
N = (12.2 x 10^3 J) / ((6.626 x 10^-34 J·s) * (1.20 x 10^15 Hz))
Performing the calculation:
N ≈ 1.82 x 10^21
Therefore, the number of atoms present in the sample is approximately 1.82 x 10^21.
A runner can run at a speed of 4km/h in 2 hours. What distance will she
cover in that time? Remember to put the correct units too. *

Answers
Answer:
Distance cover = 8 km
Explanation:
Given:
Speed of runner = 4 km/h
Time taken = 2 hour
Find:
Distance cover
Computation:
Distance = Speed x Time
Distance cover = Speed of runner x Time taken
Distance cover = 4 x 2
Distance cover = 8 km
What does it mean if a light wave is TRANSMITTED?*
1 point
Transmission means light waves bending when it enters a different medium
Transmission means light waves bouncing off something
Transmission means light waves move energy from one place to another
Transmission means light waves are taken in or dispersed within a medium
Answers
Answer:
Transmission means light waves bending when it enters a different medium
Explanation:
Carbon cycle – What are the main reservoirs
of the carbon cycle? Where do the inorganic and organic carbon
cycles interact? What are the major differences and similarities
between the inorganic and organic carbon?
Answers
The main reservoirs of the carbon cycle are the atmosphere, oceans, land (including vegetation and soils), and fossil fuels. In these reservoirs, carbon exists in both inorganic and organic forms.
The inorganic carbon cycle involves the exchange of carbon dioxide (CO2) between the atmosphere and oceans through processes like photosynthesis and respiration.
Organic carbon, on the other hand, is found in living organisms, dead organic matter, and soil organic matter. It is cycled through processes such as decomposition and consumption by organisms. The interactions between the inorganic and organic carbon cycles occur primarily in the biosphere, where photosynthesis converts inorganic carbon into organic carbon compounds. While inorganic carbon is primarily in the form of CO2, organic carbon is present in complex organic molecules. Both forms of carbon play crucial roles in energy transfer, nutrient cycling, and climate regulation.
Learn more about Carbon Cycle
brainly.com/question/13729951
#SPJ11
Classify the following compounds as an Arrhenius acid or an Arrhenius base.
H2S ____________
RbOH____________
Mg(OH)2 ____________
H3PO4____________
Answers
Arrhenius :
acid: a compound that releases H⁺ ions in water
base: a compound that releases OH⁻ ions in water
H2S Arrhenius acid
RbOH Arrhenius base.
Mg(OH)2 Arrhenius base.
H3PO4 Arrhenius acid
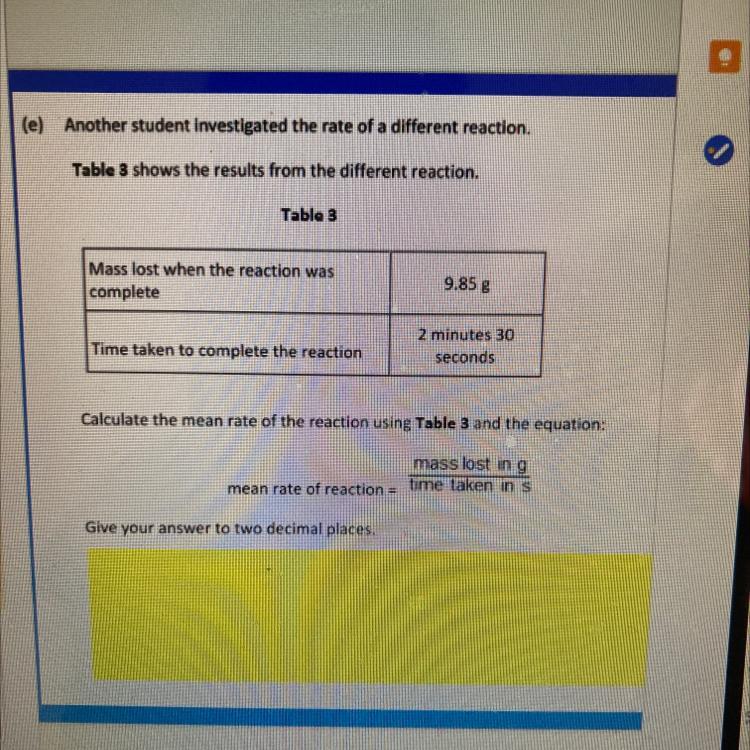
(e) Another student investigated the rate of a different reaction
Table 3 shows the results from the different reaction
Headings that you a
will appear here.
Table 3
Mass lost when the reaction was
complete
9.85 g
Time taken to complete the reaction
2 minutes 30
seconds
Calculate the mean rate of the reaction using Table 3 and the equation:
mass lost in g
mean rate of reaction time taken in s
Give your answer to two decimal places.
Answers
Answer:
0.07 g/s.
Explanation:
From the question given above, the following data were obtained:
Mass lost = 9.85 g
Time taken = 2 min 30 s
Mean rate =?
Next, we shall convert 2 min 30 s to seconds (s). This can be obtained as follow:
1 min = 60 s
Thus,
2 min = 2 × 60 = 120 s
Therefore,
2 min 30 s = 120 s + 30 s = 150 s
Finally, we shall determine the mean rate of the reaction. This can be obtained as illustrated below:
Mass lost = 9.85 g
Time taken = 150 s
Mean rate =?
Mean rate = mass lost / time taken
Mean rate = 9.85 / 150
Mean rate = 0.07 g/s
Therefore, the mean rate of the reaction is 0.07 g/s
For the reaction 2H2 + O2 - 2H20, how many grams of water are produced from 3.00 mol of hydrogen?
Answers
explanation:
Which statement best describes why radioactive atoms can be used to date materials?
A.
Nonradioactive atoms in rocks eventually turn into radioactive atoms.
B.
The Sun interacting with atoms in a rock causes new atoms to form over time.
C.
The ratio of radioactive atoms to other kinds of atoms changes over time.
D.
The number of radioactive atoms in a substance increases over many years.
Answers
The ratio of radioactive atoms to other kinds of atoms changes over time. Hence, option C is correct.
What is radioactive decay?In the process of radioactive decay the unstable atom nuclei of the parent nucleus break down and split so as to release the daughter atoms or nuclei along with the release of energy in the form of heat. Thus as the decay process proceeds the concentration of the parent atom decreases but the concentration of the daughter atom increases.
Radioactive atoms can be used to date materials because the radioactive decay occurs at a known rate, and the density of fission tracks for the amount of uranium within a mineral grain can be used to determine its age.
Hence, option C is correct.
learn more about radioactive decay here:
https://brainly.com/question/13673451
#SPJ5
which type of bonds and solids are characteristics of organic compounds? 1) ionic bonds and ionic solid3) covalent bonds and ionic solids 2) ionic bonds and molecular solids4) covalent bonds and molecular solids
Answers
Organic compounds are characterized by covalent bonds and molecular solids. Therefore, the correct option is 3.
Covalent bonds are formed when atoms share electrons to achieve a stable electron configuration. In organic compounds, carbon atoms are usually bonded to other carbon atoms and to hydrogen atoms, forming a variety of functional groups. These functional groups give organic compounds their unique properties and reactivity.
Molecular solids are formed when molecules are held together by intermolecular forces, such as van der Waals forces, hydrogen bonds, and dipole-dipole interactions. Organic compounds typically have low melting and boiling points due to the weak intermolecular forces between the molecules. However, there are exceptions to this general rule, such as polymers, which can have high melting and boiling points due to their long, chain-like structures.
In contrast, ionic bonds and ionic solids are characteristic of inorganic compounds, which typically involve the transfer of electrons between atoms to form ions. Ionic solids are held together by strong electrostatic forces between ions of opposite charges, resulting in high melting and boiling points. In summary, covalent bonds and molecular solids are the characteristic bonds and solids of organic compounds.
To know more about covalent bonds visit:
https://brainly.com/question/19382448
#SPJ11
need help with this question plsssss

Answers
Answer:
234
Explanation:
Thomas sits half way down a grassy slope. What force stopped him slamming down
Answers
i would Luke to ??

Answers
Answer:
1 answer
Explanation:
Can somebody plz help answer all these true and false questions CORRECT!!
WILL MARK BRAINLIEST :D

Answers
Answer:
1-T
2-F
3-T
4-T
Explanation:
big brain
2) 2KClO3 --> 2KCl + 3O2
a) How many moles of O2 are produced from 19 moles of KClO3?
b) How many kilograms of KClO3 would decompose to form 62 moles of KCl?
c) How many grams of O2 are required to react with 39 grams of KCl?
show work
Answers
\(2 \text{ KClO}_3 \to 2 \text{ KCl}+3\text{ O}_2\)
a)
\(2 \text{ mols of KClO}_3 \equiv 3 \text{ mols of O}_2\)
\(19 \text{ mols of KClO}_3 \equiv 3\cdot 9,5 \text{ mols of O}_2\)
\(\boxed{19 \text{ mols of KClO}_3 \equiv 28,5 \text{ mols of O}_2}\)
b)
\(2 \text{ mols of KClO}_3 \equiv 2 \text{ mols of KCl}\)
\(62 \text{ mol of KClO}_3 \equiv 62 \text{ mol of KCl}\)
Using the atomic mass given in the periodic table:
\(62\cdot(39+35,5+16\cdot3) \text{ g of KClO}_3 \equiv 62 \text{ mol of KCl}\)
\(62\cdot122,5 \text{ g of KClO}_3 \equiv 62 \text{ mol of KCl}\)
\(7595 \text{ g of KClO}_3 \equiv 62 \text{ mol of KCl}\)
\(\boxed{7,595 \text{ kg of KClO}_3 \equiv 62 \text{ mol of KCl}}\)
c)
\(2 \text{ KCl}+3\text{ O}_2\to 2 \text{ KClO}_3\)
\(3 \text{ mols of O}_2 \equiv 2 \text{ mols of KCl}\)
Using the atomic mass given in the periodic table:
\(3\cdot(2\cdot 16) \text{ g of O}_2 \equiv 2\cdot(39+35,5) \text{ g of KCl}\)
\(96\text{ g of O}_2 \equiv 149\text{ g of KCl}\)
\(\dfrac{39}{149}\cdot 96\text{ g of O}_2 \equiv \dfrac{39}{149}\cdot 149\text{ g of KCl}\)
\(\boxed{25,13\text{ g of O}_2 \equiv 39\text{ g of KCl}}\)
This result is an aproximation.
What do these floors represent?
Answers
Explanation:
What type of floor. You have uploaded no image
calculate how many grams of agarose you will need to prepare a 1% agarose gel with 30 ml total volume.
Answers
0.3 agarose is needed to prepare a 1 % agarose gel with 30 ml total volume .
We know that , a 1 % agarose gel in 100 ml can be prepared by adding 1 g of agarose in 100 ml of water.(solvent).
1 % agarose gel in 100 ml of solvent = 1 gram of agarose
Therefore , 1 % agarose gel in 30 ml can be prepared from ,
1 % agarose gel in 30 ml of solvent = \(\frac{1}{100}\) × 30 gram of agarose
= \(\frac{30}{100}\) g of agarose
= 0.3 g of agarose
Therefore , the amount of agarose required to prepare 1 % agarose gel with 30 ml total volume is 0.3 g
Learn more about weight percent ,
https://brainly.com/question/32200537?
To prepare a 1% agarose gel with a total volume of 30 ml, you will need 0.3 grams of agarose.
Explanation:To calculate how many grams of agarose you will need to prepare a 1% agarose gel with a 30 ml total volume, you can use the formula:
Agarose mass (g) = 1% x Total volume (ml)
Plugging in the values, the calculation becomes:
Agarose mass (g) = 1% x 30 ml = 0.01 x 30 = 0.3 g
Therefore, you will need 0.3 grams of agarose to prepare the 1% agarose gel with a total volume of 30 ml.
Learn more about Agarose gel preparation here:https://brainly.com/question/32135543
#SPJ2
Balance the equation NH3 + O2 −→ N2 + H2O Given 3.53 mol of the reactant NH3, determine the corresponding amount of O2. Answer in units of mol.
Answers
A chemical equation is said to be balanced when the number of atoms of each element on both sides of the equation are the same.
According to this question, a reaction occured between ammonia gas and oxygen gas to produce nitrogen gas and water.
Based on the equation, 4 moles of ammonia requires 3 moles of oxygen gas to react.
This means that 3.53 moles of ammonia will require 3.53 × ¾ = 2.65 moles of oxygen gas.
Learn more about balanced equation at: https://brainly.com/question/7181548
#SPJ1
Question 1 (multiple choice worth 5 points)
(06.01 mc)
why do skeletal muscles work in pairs?
one tightens and the other relaxes to make the bones move.
o multiple muscles have to tighten to make joints move.
o they cancel each other out.
o bones are resistant to movement.
Answers
One tightens and the other relaxes to make the bones move. Hence, option A is correct.
What are muscles?A muscle is a group of muscle tissues which contract together to produce a force. A muscle consists of fibres of muscle cells surrounded by protective tissue, bundled together with many more fibres, all surrounded by thick protective tissue.
Muscles work in pairs, when one muscle shortens the opposite muscle lengthens e.g. the biceps shortens to bend the elbow at the same time its opposite muscle, the triceps, lengthens to allow the movement to occur. Good working between pairs of muscles allows for smooth controlled movement.
One tightens and the other relaxes to make the bones move. Hence, option A is correct.
Learn more about the muscles here:
https://brainly.com/question/9883108
#SPJ1
The apparatus in Image A above is used to separate a mixture of ethyl alcohol and water. The mixture is heated in the flask on the left and the ethyl alcohol drips into the flask on the right after condensing in the cooling tube in the middle. Which method was used to separate the mixture?
a. chromatography
b .filtration
c .sorting
d distillation
Answers
The method used to separate a mixture of ethyl alcohol and water is distillation. Thus option (d) is true.
What is distillation?Distillation is defined as a process involving the conversion of liquid into vapor that is subsequently condensed back into liquid form.
It is a widely used method for separating the mixture based on difference in conditions required to change the phase of components.
Some of the types of distillation are:
Simple distillation Steam distillation Fractional distillation Vacuum distillationZone distillation etc. and many more are the type of distillation.Thus, the method used to separate a mixture of ethyl alcohol and water is distillation. Thus option (d) is true.
To learn more about distillation, refer to the link below:
https://brainly.com/question/13345735
#SPJ1
Your question is incomplete, but most probably your complete question was
The apparatus in Image A above is used to separate a mixture of ethyl alcohol and water. The mixture is heated in the flask on the left and the ethyl alcohol drips into the flask on the right after condensing in the cooling tube in the middle. Which method was used to separate the mixture?
a. chromatography
b .filtration
c .sorting
d distillation

What does this dialogue reveal about Paul’s feelings toward Ole Grey?
He has a deep fear of the horse, so he is cautious in his approach.
He is worried that the horse will not listen to him during the race.
He has a deep respect for the horse, so he treats him with care.
He is more concerned with winning than with the horse’s well-being.
Answers
Answer:
He has a deep respect for the horse,so he treats him with care
Answer:
It's C
Explanation:
Good luck on the test.