HA and HB are two strong monobasic acids. 25.0cm3 of 6.0mol/dm3 HA is mixed with 45.0cm3 of 3.0mol/dm3 HB.
What is the H+ (aq) concentration in mol/dm3 in the resulting solution ?
A 1.9
B 2.1
C 4.1
D 4.5
If anyone could explain how to reach an answer that would be so helpful !!
Answers
Answer:
The H+ (aq) concentration of the resulting solution is 4.1 mol/dm³
(Option C)
Explanation:
Given;
concentration of HA, \(C_A\) = 6.0mol/dm³
volume of HA, \(V_A\) = 25.0cm³, = 0.025dm³
Concentration of HB, \(C_B\) = 3.0mol/dm³
volume of HB, \(V_B\) = 45.0cm³ = 0.045dm³
To determine the H+ (aq) concentration in mol/dm³ in the resulting solution, we apply concentration formula;
\(C_iVi = C_fV_f\)
where;
\(C_i\) is initial concentration
\(V_i\) is initial volume
\(C_f\) is final concentration of the solution
\(V_f\) is final volume of the solution
\(C_iV_i = C_fV_f\\\\Based \ on \ this\ question, we \ can \ apply\ the \ formula\ as;\\\\C_A_iV_A_i + C_B_iV_B_i = C_fV_f\\\\C_A_iV_A_i + C_B_iV_B_i = C_f(V_A_i\ +V_B_i)\\\\6*0.025 \ + 3*0.045 = C_f(0.025 + 0.045)\\\\0.285 = C_f(0.07)\\\\C_f = \frac{0.285}{0.07} = 4.07 = 4.1 \ mol/dm^3\)
Therefore, the H+ (aq) concentration of the resulting solution is 4.1 mol/dm³
Related Questions
One third of a bottle of water fills one sixth of a fish tank how much water is needed for the whole tank?
Answers
Answer: 1/2
Explanation:
It’s really all in the question so if 1/3 = 1/6
1/3 x 3 = 3 ( mind you were dealing with isolation)
So after you do that 3 x 1/6 = 1/2
(This maybe wrong but like I’m in middle school so what do expect like what am I doing on brainless a 3 am )
What type of solution is formed when 20g of NaCl is heated to 80 degrees?
Answers
Answer:
As temperature increases, its solubility increases as well. Notice, however, that it does not increase significantly. In fact, you can expect to be able to dissolve no more than 40 g of sodium chloride per 100 g of water at 80∘C
Explanation:
HOPE THIS HELPS !
no.of elements in KMnO4
Answers
Answer:
3 three
Explanation:
potassium (K), manganese (Mn), and oxygen (O).
Answer:
3 different elements (7 in total as there are 4 oxygens (The answer is either one of these))
Explanation:
A chemical reaction performed inside a bomb calorimeter causes the temperature of the water to rise
by 32.5 o
C. How many Joules of energy were released by the reaction? The calorimeter contains 250.0
mL of water; the specific heat of water is 4.182 J/g.oC.
Answers
In the hypothetical situation, a chemical reaction inside a bomb calorimeter causes the water inside it to heat up to 32.5 °C. Many computations are needed to figure out how much energy the process releases.
First, the density of water (1 g/mL) is used to convert the volume of water (250.0 mL) to its mass, so that the mass is 250.0 g.
The formula energy = mass of water * specific heat of water *temperature change is then used to determine the energy released. In general, the specific heat of water is 4.182 J/g°C.
Using known values to fill in the blanks in the equation, we calculate the energy released as approximately 34,001.25 joules.
The amount of energy released during a chemical reaction can be calculated. This shows how important it is to understand the specific heat capacity of substances such as water when estimating the energy changes brought about by reactions.
Learn more about energy, here:
https://brainly.com/question/30672691
#SPJ1
Explain how the rate of diffusion of a gas is related to its molar mass.
Carbon dioxide gas (CO2) effuses 3. 2 times faster than an unknown gas. Determine the molar mass of the unknown gas. Show your work or explain your answer, giving specific values used to determine the answer
Answers
The molar mass of the unknown gas is approximately 4.28 g/mol.
What is molar mass?
The molar mass is the mass of a substance in grams that is equal to one mole of the substance. The mole is a unit of measurement in chemistry that represents the number of entities in a substance, such as atoms, molecules, or ions. One mole of any substance contains Avogadro's number of entities, which is approximately 6.022 x 10^23.
The rate of diffusion of a gas is inversely proportional to its square root of molar mass. This relationship is known as Graham's Law of Diffusion.
Given that CO2 diffuses 3.2 times faster than the unknown gas, we can set up the following equation:
(Diffusion rate of CO2) / (Diffusion rate of unknown gas) = 3.2
(1/√(molar mass of CO2)) / (1/√(molar mass of unknown gas)) = 3.2
Squaring both sides:
(1/molar mass of CO2) / (1/molar mass of unknown gas) = 3.2^2 = 10.24
Therefore,
(1/molar mass of unknown gas) = (1/molar mass of CO2) / 10.24
Given that the molar mass of CO2 is 44.01 g/mol,
(1/molar mass of unknown gas) = (1/44.01) / 10.24
Solving for molar mass of the unknown gas:
molar mass of unknown gas = 44.01 / 10.24 = 4.28 g/mol
So, the molar mass of the unknown gas is approximately 4.28 g/mol.
To learn more about molar mass:
https://brainly.com/question/21334167
#SPJ4
A sample of oxygen was collected in a tube over water. In the tube, there is a mixture of oxygen gas and gaseous water (steam). The total pressure of the gas mixture is 760. Torr. If the pressure of gaseous water is 23. 8 torr, what is the pressure of the pure oxygen?.
Answers
The ideal gas law exhibits the relationship between the pressure and the volume of the gas. The pressure of the pure oxygen in the sample will be 736.2 torrs.
A theoretical gas called an ideal gas is one that has lots of point particles flying around arbitrarily and not being affected by other particles. The ideal gas notion is advantageous because it complies with the ideal gas law, a condensed equation of state, and is amenable to statistical mechanics analysis. A theoretical gas called an ideal gas is one that has many of randomly moving particles but doesn't have any interparticle interactions. The converse is true for a real gas; it takes up space and its molecules interact. As a result, PV is always equal to nRT.
Learn more about ideal gas here:
https://brainly.com/question/28257995
#SPJ4
28. Which of the following reactions at equilibrium would NOT be affected by volume
changes at constant temperature?
a. 2 CO(g) + O2(e) < > 2 CO2(g)
b. 2 NO2(g) → N2048)
c. 2 NO(g) + 3 F2(g) → 2 F3NO(e)
d. O3(e) + NO(g) + > NOzle) +
e. None of the above.
Ozig)
Answers
Answer:
Explanation:
is the
Name the following covalent molecules:
SeF
Answers
Answer:
do you mean DeF?
Explanation:
If you have 8.5 moles of water (H2O), how many grams of water do you have?
Answers
Answer: i think it is 144.16
Explanation:
enough of a monoprotic acid is dissolved in water to produce a 1.74 m solution. the ph of the resulting solution is 2.83 . calculate the ka for the acid.
Answers
The Ka for the monoprotic acid is 10^-4.60. This solution shows that the concentration of the acid, the pH of the solution, and the Ka of the acid are all interrelated and can be used to solve for each other.
To solve this problem, we first need to understand the relationship between the pH, the concentration of the acid, and the Ka of the acid. We can use the formula for Ka, which is Ka = [H+][A-]/[HA], where [H+] is the concentration of hydrogen ions, [A-] is the concentration of the conjugate base, and [HA] is the concentration of the acid.
We know that the pH of the solution is 2.83, which means that the concentration of hydrogen ions is 10^-2.83 M. We also know that the concentration of the acid is 1.74 M, which means that the concentration of the conjugate base is negligible in comparison. Therefore, we can assume that [HA] = 1.74 M and [H+] = 10^-2.83 M.
Plugging these values into the Ka formula, we get:
Ka = (10^-2.83 M)(x)/1.74 M
where x is the concentration of the conjugate base, which we can assume to be negligible. Solving for Ka, we get:
Ka = 10^-4.60
Therefore, the Ka for the monoprotic acid is 10^-4.60. This solution shows that the concentration of the acid, the pH of the solution, and the Ka of the acid are all interrelated and can be used to solve for each other.
To know more about monoprotic acid visit :
https://brainly.com/question/31732916
#SPJ11
(True or false) The blood carries hormones to target tissues around the body so the hormones can control the activity of those cells
Answers
Answer:
True
Explanation:
Answer:
True
Explanation:
Hormones are chemical messengers secreted into blood or extracellular fluid by one cell that affect the functioning of other cells. Most hormones circulate in blood, coming into contact with essentially all cells. However, a given hormone usually affects only a limited number of cells, which are called target cells.
What behavioral adaptation helps zebras protect themselves against predators?
a group of zebras in a plain
Having a tail
Having thick fur
Looking like each other
Traveling in herds
Answers
Case Study-1 The reactor is now ready to build concentration for the start-up. The system is under nitrogen pressure of 10 bar pressure and a temperature of 88 °C. The target concentration is expected to be reached within three hours. Calculate 1. Mass Flow rate for each component 2. Nitrogen final molar concentration and its partial pressure You can use ideal gas equation (PV = nRT) to calculate mass flow rate where value of R = 8.314 J/mol.K TARGET CONCENTRATION Ethylene Partial pressure H2/C2 C4/C2 Isopentane Reactor Pressure Volume 6.7 bara 0.12 mole 0.3 mole 10% mole. 22 Bar A 790,000 Litre
Answers
The Mass flow rate of ethylene, hydrogen, and isopentane is 391.07 kg/h, 12,640 kg/h, and 70.12 kg/h respectively. The Nitrogen final molar concentration is 0.36 Molar and its partial pressure is 0.12 bar.
Given Parameters:
Reactor Pressure (P) = 22 bar a
Volume (V) = 790,000 Litre
Temperature (T) = 88 °C
Molar Percentage of C2H4 = 0.12
Molar Percentage of H2 = 0.3
Molar Percentage of C4H10 = 10% mole
1. Mass Flow rate for each component:
Mole fraction = Mole of Component / Total moles of all component
= 1/Sum of mole fraction
PV = nRTn = PV/RTm = n
Molecular weightMole fraction of ethylene, Xc2h4 = 0.12
Mole fraction of hydrogen, Xh2 = 0.3
Mole fraction of Isopentane, XC5H12 = 0.1
Mole fraction of Nitrogen, XN2 = 1 - (Xc2h4 + Xh2 + XC5H12) = 1 - (0.12 + 0.3 + 0.1) = 0.48
Gas Constant, R = 8.314 J/mol. K
Temperature, T = 88 + 273 = 361 K
Pressure, P = 22 + 1.013 = 23.013 bar
Mass flow rate of ethylene:m(c2h4) = PVnRT/MW(c2h4)m(c2h4)
= 23.013 × 0.12 × 790000/8.314 × 361/28.05m(c2h4)
= 391.07 kg/h
The mass flow rate of hydrogen:
m(h2) = PVnRT/MW(h2)m(h2)
= 23.013 × 0.3 × 790000/8.314 × 361/2.016m(h2)
= 12,640 kg/h
The mass flow rate of Isopentane:
m(c5h12) = PVnRT/MW(c5h12)m(c5h12)
= 23.013 × 0.1 × 790000/8.314 × 361/72.15m(c5h12)
= 70.12 kg/h
2. Nitrogen final molar concentration and its partial pressure:
Given:
Nitrogen pressure, Pn2 = 10 bar
Nitrogen is under Nitrogen Pressure, P = 23.013 - 10 = 13.013 bar
Nitrogen Molar concentration,
nN2 = Pn2VRTnN2
= Pn2VRT/RTnN2
= Pn2/P × 0.48nN2
= 10/(13.013) × 0.48nN2
= 0.36 Molar
Nitrogen partial pressure,
Pn2 = nN2RT/VPn2 = 0.36 × 8.314 × 361/790000
Pn2 = 0.12 bar
Hence, The Mass flow rate of ethylene, hydrogen, and isopentane is 391.07 kg/h, 12,640 kg/h, and 70.12 kg/h respectively. The Nitrogen final molar concentration is 0.36 Molar and its partial pressure is 0.12 bar.
Learn more about concentration here:
https://brainly.com/question/17206790
#SPJ11
How can you tell the difference between two clear liquids
Answers
Answer:
To identify a pure liquid substance using the physical properties of solubility, density, and boiling point. The physical properties of a pure substance can be measured without changing the composition of the substance.
Explanation:
Which compound contains both sigma and pi bonds
Answers
If a balloon is rubbed on your hair, it gains electrons contain polar and becomes negatively charged. When these charges come close to a stream of water, the stream of water bends. Use what you know about polar molecules to explain why the bending occurs.
Answers
Answer:
The bending occurs because the water has positively charged electrons which do not want to be with negatively charged electrons on the balloon.
Explanation:
How do spores help the survival of spore-bearing plant?
Answers
Answer:
When weather conditions are ideal, some ferns, algae, moss,and even fungi, release spores into the air, often carried by the wind, by insects or birds until they land. Spores contain both male and female reproductive organs, which allows these plants to replicate themselves in a form of cloning.
Explanation:
Which of these weak bases is the weakest electrolyte in aqueous solution? ethyl amine, Kb = 4.3 x 10-4 O aniline, Kp = 4.0 x 10-10 O hydrazine, Kp = 8.5 x 10-7 O trimethyl amine, Kb = 6.5 x 10-5
Answers
Among the given weak bases, aniline is the weakest electrolyte in an aqueous solution.What is an electrolyte?An electrolyte is a substance that conducts electricity in an aqueous solution or in a molten state.
In water, they break up into ions and conduct electricity. Electrolytes may be categorized into two types: strong and weak electrolytes. Strong electrolytes dissociate completely into ions in aqueous solution, whereas weak electrolytes only partially dissociate into ions and exist in equilibrium with undissociated molecules. In the given weak bases, aniline is the weakest electrolyte.
Here's how to solve the problem: Aniline has a Kp of 4.0 × 10-10, which is the smallest value of Kp among all the given weak bases. Therefore, aniline is the weakest electrolyte in an aqueous solution.
Read more about electrolyte here;https://brainly.com/question/17089766
#SPJ11
2. Describe the changes in bonding and hybridization of the carbon atoms that take place during the polymerization of styrene to form polystyrene.
Answers
During the polymerization of styrene to form polystyrene, the bonding and hybridization of the carbon atoms undergo significant changes.
In styrene, the carbon atoms are originally sp2 hybridized and form a conjugated system of double bonds in the aromatic ring. However, during the polymerization process, the double bonds in styrene undergo addition polymerization.As a result, the carbon atoms in polystyrene undergo a transformation in hybridization from sp2 to sp3. The double bonds break, and new sigma bonds are formed between the carbon atoms, leading to the formation of a long chain of repeating units.
This change in hybridization allows the carbon atoms in polystyrene to form stronger and more stable sigma bonds with neighboring carbon atoms or other substituents. Consequently, the structure of polystyrene becomes a three-dimensional network of carbon-carbon bonds throughout the polymer chainThe transition from an aromatic, conjugated system in styrene to a saturated, three-dimensional polymer structure in polystyrene is vital in the polymerization process, providing the desired properties and structural integrity to the resulting polymer.
To know about more hybridization,sp2 hybridized,carbon-carbon bonds,sigma bonds visit:
https://brainly.com/question/29020053
https://brainly.com/question/31610604
https://brainly.com/question/29663260
https://brainly.com/question/31659836
#SPJ11
PPPPPPPPPPPPPPPLLLLLLLLLLLLLLLLLEEEEEEEEEEEEEEEEEESSSSSSSSSSSSEEEEEE
HHHHHHHHHHHHHHHHHHHHHHHEEEEEEEEEEEEEEEEELLLPPPPPPPPPPPPPPPPPPMMMMMMMMMMEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEEE!
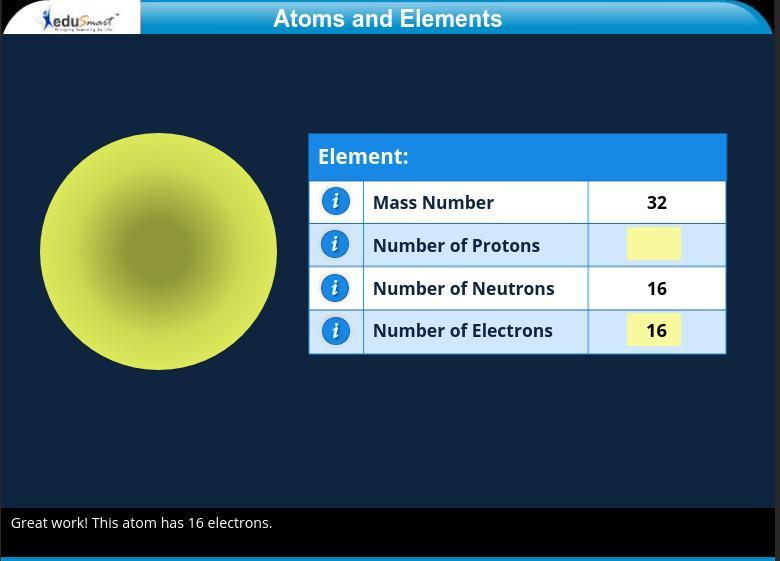
Answers
Explanation: you subtract the neutrons from the mass number so 32-16=16
Answer:
Explanation:
The answer is 16
6.(a) State any two ways by which water gets polluted.
(b) Name the components present in water gas.
Answers
ABG results are: pH-7.5, PaCO2 32, HCO3 23. What acid/base imbalance does the nurse determine that this client has developed?
Answers
Based on the ABG results provided, the nurse can determine that the client has developed respiratory alkalosis. This is indicated by the elevated pH of 7.5 and the decreased PaCO2 of 32. Respiratory alkalosis occurs when there is a hyperventilation that causes the carbon dioxide level in the blood to decrease, leading to an increase in pH.
The HCO3 level of 23 is within normal range, indicating that metabolic compensation has not occurred yet. Possible causes of respiratory alkalosis include anxiety, pain, fever, hypoxia, or overuse of mechanical ventilation.
The nurse should identify the underlying cause and provide appropriate interventions to correct the acid-base imbalance and prevent further complications. These may include reducing anxiety, providing supplemental oxygen, or adjusting mechanical ventilation settings.
Close monitoring of the client's ABG results is essential to ensure effective management of their condition.
Based on the provided ABG results (pH-7.5, PaCO2 32, HCO3 23), the nurse can determine that the client has developed respiratory alkalosis. This is because the pH level is above the normal range of 7.35-7.45, indicating alkalosis, while the PaCO2 level is below the normal range of 35-45 mmHg, suggesting a respiratory cause. The HCO3 level remains within the normal range of 22-26 mEq/L, which further supports that the primary issue is respiratory rather than metabolic.
To know more about ABG results. please visit.....
brainly.com/question/30867802
#SPJ11
Question 25b please.
Will give branliest!!!

Answers
25 (b) 106 g of Na2CO3 is needed to produce 22.4 dm³ of CO2 at STP in this reaction.
What is meant by molar volume?Volume occupied by one mole of a substance at a given temperature and pressure is known as molar volume.
Balanced equation shows that 1 mole of Na2CO3 produces 1 mole of CO2. Therefore, the number of moles of CO2 produced in 22.4 dm^3 of gas at STP is:
n = V/VM = 22.4/22.4 = 1 mol
So we need 1 mole of Na2CO3 to produce this amount of CO2.
Molar mass of Na2CO3 is:
2Na + C + 3O = (2x23) + 12 + (3x16) = 106 g/mol
Therefore, mass of Na2CO3 needed is:
As mass = n x molar mass = 1 mol x 106 g/mol = 106 g
So, 106 g of Na2CO3 is needed to produce 22.4 dm³ of CO2 at STP in this reaction.
To know more about molar volume, refer
https://brainly.com/question/11676583
#SPJ1
1. If there is a switch it needs to be turned on. If not, the circuit will be
A. Open
B. Closed
C. Frozen
D. All of the above
Answers
Answer:
The Circuit will be OPEN since the current source is disconnected.
So if its not turned on... The circuit remains Open
Answer
Option A.
write a mechanism that describes the formation of the two more important products
Answers
Answer:
The two or more important products are :-
1. 3 methlcycodexene
2. 1 methlylcohexene(2pt)
1. what is different between each tube when you set up this tube dilution test?
Answers
Here are the typical differences between each tube in a tube dilution test is; Dilution Factor, Concentration, Volume, and Test Conditions.
In a tube dilution test, different dilutions of a substance or sample are prepared in tubes to determine the effect of the substance on a test organism or reaction.
Each tube contains a different dilution of the substance. The dilution factor represents the ratio of the volume of the original substance to the volume of the diluted substance in each tube. As you move from one tube to another, the dilution factor increases, resulting in a progressively lower concentration of the substance.
The concentration of the substance decreases as you move from tube to tube. The initial tube usually contains the highest concentration of the substance, while subsequent tubes contain progressively lower concentrations.
The volume of the substance being tested varies from tube to tube. Generally, the initial tube contains the highest volume of the undiluted substance, and subsequent tubes contain decreasing volumes due to dilution with a diluent (such as a buffer or solvent).
Each tube may be subjected to different test conditions or parameters, depending on the specific purpose of the tube dilution test.
To know more about dilutions here
https://brainly.com/question/13072017
#SPJ4
MAX TEN MINUTES PLZZZZZ HURRY
Essential Question:
How does the transfer of thermal energy become minimized or maximized?
explain you hypothisith
Answers
Answer:
when it reduces heat.
Explanation:
Thermal energy is transferred to material,the motion of its particles speeds up and it's temperature differences.Insulating materials are bad conductors and so this reduces the heat loss by conduction.The material also prevents air circulating inside the cavity,therefore reducing heat loss by convection.Heat loss through the roof can be reduced by laying loft insulation.
PLEASE ANSWER THIS QUICK 50 POINTS :) RIGHT ANSWERS ONLY

Answers
Following the formula they gave you need to do
i × kb × mass
1 × 2.65 × 2
= 5.3
Change in temperature should be 5.3°C
a chemist designs a galvanic cell that uses these two half-reactions:half-reactionstandard reduction potential(g)(l)(aq)(aq)(g)(aq)answer the following questions about this cell. n2 4h2o 4e n2h4 4oh
Answers
The galvanic cell described in the question consists of two half-reactions: N2 + 4H2O + 4e- ⟶ N2H4 + 4OH-.
To analyze this cell, we can examine the standard reduction potentials of the half-reactions involved and determine the overall cell potential, as well as the direction of electron flow and the species undergoing reduction and oxidation.
Explanation:
To evaluate the galvanic cell, we need to consider the standard reduction potentials of the half-reactions involved:
N2 + 4H2O + 4e- ⟶ N2H4 + 4OH- (reduction)
The standard reduction potential for this half-reaction is not provided in the question. The standard reduction potential is a measure of the tendency of a species to gain electrons and undergo reduction. With the given information, we cannot determine the standard reduction potential and, consequently, the standard cell potential.
In a galvanic cell, the species undergoing reduction occurs at the cathode (positive electrode), while the species undergoing oxidation occurs at the anode (negative electrode). Without knowing the standard reduction potential, we cannot determine the direction of electron flow or which species is undergoing oxidation or reduction in this specific cell.
In summary, without the standard reduction potential for the given half-reaction, we cannot determine the standard cell potential or the direction of electron flow in the galvanic cell.
Learn more about galvanic cell here :
brainly.com/question/33558906
#SPJ11
the model below shoes an atom of an element. what is the atomic number of this atom?
A- 6
B- 8
C- 9
D- 16
