Answers
Answer
P1 = 0.466 atm
Explanation
Given:
Initial volume (V1) = 154.3 L
Final volume (V2) = 50.0 L
Initial Temperature (T1) = 27 °C = 300 K
Final Temperature (T2) = 40.0 °C = 313 K
Initial pressure (P1) = ?
Final pressure (P2) = 1.5 atm
Required: Initial pressure (P1)
Solution
To solve this problem, we will use combined gas law:
\(\frac{P_1V_1}{T_1}\text{ = }\frac{P_2V_2}{T_2}\)Re-arrange:
P1 = (P2V2T1)/T2V1
P1 = (1.5 x 50.0 x 300)/(313 x 154.3)
P1 = 0.466 atm
Related Questions
2. Why can animal waste be toxic?
O A. The ammonia in manure smells like rotten gas.
O B. The methane in manure is converted to particulate matter
OC. The hydrogen sulfide in manure can cause respiratory failure
O D. Carbon dioxide accumulates on top of liquid manure and is highly flammable
Answers
Considering it is the only one that can be considered toxic”
Pewter is a solidified solution of tin and lead or tin and zinc. In both cases, tin is the main component. Which metal would you classify as the solute in each type of pewter?
Answers
How many grams of calcium chloride would be dissolved in 1.0L of a 0.10m solution of calcium chloride?
Answers
Answer:
2365 g
Explanation:
Determine the molecular formula for the following compounds from the experimental data:80.0% carbon, 20% hydrogen, and a molecular mass of 30.0 amu
Answers
Answer
The molecular formula for the compound is C₂H₆Explanation
Given:
% composition: 80.0% carbon, 20% hydrogen.
Molecular mass = 30.0 amu
What to find:
The molecular formula for the compound.
Step-by-step solution:
Assume we have 100 grams total of the substance.
Grams C: 80.0/100 x 100 g = 80.0 g
Grams H: 20/100 x 100 = 20 g
The next step is to convert the number of grams of each element into moles as shown below using the molar masses of H = 1.00784 g/mol and C = 12.011 g/mol.
Note: Mole = Mass/Molar mass.
Moles C = 80.0g/12.011 g/mol = 6.66 mol
Moles H = 20g/1.00784 g/mol = 19.84 mol
Now, let's divide the number of moles of each element by the smallest number obtained:
C = 6.66/6.66 = 1
H = 19.84/6.66 = 3
The empirical formula for the compound is CH₃
Using the molecular mass of 30.0 amu, the molecular formula is calculated as follows:
(Empirical mass)n = (Molecular mass)
(CH₃)n = 30.0
(12.011 + 3 x 1.00784)n = 30
(12.011 + 3.02352)n = 30
(15.03452)n = 30
Divide both sides by 15.03452
n = 2
Moleculare formula = (CH₃)₂ = C₂H₆
Therefore, the molecular formula for the compound is C₂H₆
Which organelles surround the cell? Select two options.
Answers
Answer:
The answer is cytoplasm and the nucleus
Explanation:
Answer:
nucleus
Explanation:
Vitamin D is produced in the skin when 7-dehydrocholesterol reacts with UVB rays (ultraviolet B) having wavelengths between
270 nm and 300 nm. What is the energy range Emin < E< Emax of the UVB photons?
Answers
The energy range expected is 6.6 × 10^-19 J < E < 7.33 × 10^-19 J
The energy of the photon is given by;
E = hc/λ
E = energy of the photon
h = Plank's constant
c = speed of light
λ = wavelength of light
For the upper boundary range;
E = ?
h = 6.6 × 10^-34 Js
c = 3 × 10^8 m/s
λ = 270 × 10^-9
E = 6.6 × 10^-34 Js × 3 × 10^8 m/s / 270 × 10^-9
E = 7.33 × 10^-19 J
For the lower range;
E = ?
h = 6.6 × 10^-34 Js
c = 3 × 10^8 m/s
λ =300 × 10^-9
E = 6.6 × 10^-34 Js × 3 × 10^8 m/s / 300 × 10^-9
E = 6.6 × 10^-19 J
Hence, the energy range 6.6 × 10^-19 J < E < 7.33 × 10^-19 J
Learn more: https://brainly.com/question/24857760
The rate equation for a reaction between substances C and D is:
rate = k[C]^2 [D]^2
The initial rate is found to be 7.5 x 10^-3mol dm^-3s^-1when the initial concentration of
C is of 0.25 mol dm^-3and the initial concentration of D is 0.50 mol dm^-3.
Calculate the value of the rate constant, k, at this temperature and deduce its units.
*Important Asap please *
Answers
Answer:
\(rate = k[C] {}^{2} [D] {}^{2} \\ 7.5 \times {10}^{ - 3} = k {(0.25)}^{2} {(0.50)}^{2} \\ k = \frac{7.5 \times {10}^{ - 3} }{ {(0.25)}^{2} {(0.50)}^{2} } \\ k = 0.48 \: {mol}^{ - 3} {dm}^{9} {s}^{ - 1} \)
Which of the following examples from everyday life are made possible or explained possible or explained by modern chemistry
Answers
Answer: Where is the folowing?
Explanation:
Which solids are insoluble in water.
Answers
Some types of solids that are insoluble in water are:
Metals. (most of them)Non-Metallic ElementsMetal OxidesSome Non-Metallic ElementsMetal Carbonates (most of them)Metal Sulfides (most of them)Salts (some of them)Which solids are insoluble in water?Many solids are insoluble in water, meaning they do not dissolve in water to a significant extent. Here are some examples of common solids that are generally insoluble in water:
Metals: Most metals, such as gold, silver, platinum, and copper, are insoluble in water.
Non-Metallic Elements: Many non-metallic elements, such as carbon (in the form of graphite or diamond), sulfur, phosphorus, and iodine, are insoluble in water.
Metal Oxides: Some metal oxides, particularly those of less reactive metals, are insoluble in water. Examples include aluminum oxide (Al2O3), iron(III) oxide (Fe2O3), and lead(II) oxide (PbO).
Metal Carbonates: Most metal carbonates are insoluble in water. Examples include calcium carbonate (CaCO3), lead(II) carbonate (PbCO3), and copper(II) carbonate (CuCO3).
Metal Sulfides: Many metal sulfides are insoluble in water. Examples include lead(II) sulfide (PbS), silver sulfide (Ag2S), and mercury(II) sulfide (HgS).
Insoluble Salts: Certain salts have limited solubility in water. Examples include silver chloride (AgCl), lead(II) iodide (PbI2), and calcium sulfate (CaSO4).
It's important to note that while these solids are generally insoluble in water, they may exhibit some solubility to a small extent. The solubility of a solid in water can vary depending on factors such as temperature, pressure, and the presence of other solutes.
Learn more about solubility:
https://brainly.com/question/23946616
#SPJ1
PLS ANSWER I WILL MARK U BRAINLIEST AND GIVE U THANK U
A student learns that salts like potassium chloride and sodium chloride are introduced in a weekly conducting solution. What option explains the chemical reactions that salts undergo and affect the conductivity of the solution?
a.Salts react chemically with the water particles and decrease the conductivity of the solution
b. Salts help to make the solution non-conducting by reacting with the particles of the solution
c. Salts mix with the particles of the weak conducting solution and helps to produce oxygen from the solution
d. Salts undergoes a chemical reaction with the particles of the solution and increase its conductivity
Answers
Answer:
d. Salts undergoes a chemical reaction with the particles of the solution and increase its conductivity
Explanation:
Salt compounds like potassium chloride (KCl) and sodium chloride (NaCl) mentioned in this question have the ability to disintegrate into their respective ions or charged atoms when dissolved in water. For example;
NaCl (aq) → Na+ + Cl-
However, these ions (Na+ and Cl-) are responsible for the ELECTRICAL CONDUCTIVITY of a solution. Hence, a salt compound when placed in an aqueous solution will form ions, which will conduct electricity. Hence, salinity of a solution increases its conductivity.
According to this question, salts undergoes a chemical reaction to with the particles of the solution to form ions that increase its conductivity.
Co-60 is used medically for radiation therapy as implants and as an external source of radiation exposure. The half-life of
Co-60 is 5, 272 years. How much of a 2.000 mg sample will remain after 21, 088 years? You must show your work to receive
credit.
. Show the equation needed
b. Show a picture of you solving for the unknown.
c. Show the final answer
Answers
Co-60 is used medically for radiation therapy as implants and as an external source of radiation exposure the half-life of Co-60 is 5, 272 years 2.000 mg sample will remain after 21, 088 years is 0.0625 gm left
Radiation therapy is the cancer treatment that uses high doses of radiation to kill cancer cell and shrink tumor
Here given data is Co-60 is 5, 272 years and we have to find the number of half lives in 21, 088 years = ?
21, 088/5, 272 = 4 half lives
(1/2)⁵ = 1/32 nd of the original will be left
1/32×2.000mg = 0.0625 gm left
Know more about half life
https://brainly.com/question/9963723
#SPJ1
help asap ..
using the stock name method when naming a translation metal ion that can have more than one common ionic charge, the numerical value of the charge is indicated by a ____ .
a: arabic number following the name .
b: superscript after the name .
c: roman numeral following the name .
d: prefix .
Answers
Below is a reaction of calcium chloride solution (CaCl2) and potassium carbonate (K2CO3). Draw the reactant
with the appropriate amount of K2CO3, particles in the empty box. The product calcium carbonate (CaCO3)
forms a precipitate. Draw the appropriate example of the precipitate and the ions that remain in the solution.
Add two water molecules around each ion in the solution.
Answers
The chemical reaction is shown by \(CaCl_{2} (aq) + K_{2} CO_{3} (aq) ----- > CaCO_{3}(s) + 2KCl(aq)\). The solvation of the KCl is shown in the image attached.
What is the reaction taking place?We know that a reaction is taking place when there is a change in the arrangement of the atoms that surround the reactants and then the products are formed. It is clear that there is a cleavage of the bods that surround the reactants and there is a recombination of these atoms as the products are formed. This recombination of the atoms now takes place in a different way so that we can be able to get new substances at the end of the reaction.
In this case we are looking at the kind of reaction that occurs between potassium carbonate and calcium chloride. One of the reactants is going to separate out of the solution and we would call it the precipitate. The other product is solvated by the water molecules.
Learn more about reaction:https://brainly.com/question/28984750
#SPJ1

How many neutrons does this atom have?
04
O 6
O 10
O 14
plzzzz i need help i will give 50 points
Answers
Answer:
c maybe if right plz mark brainliest
Explanation:
Answer:
14
Explanation:
it 14 there are 14 neutrons
Which of the following are NOT considered NPO's?
Answers
Answer: (d) Private Enterprises
Explanation:
Non-Profit Organizations (NPOs) are those run without the intent to make profit. They are instead founded and run in order to provide a public benefit or contribute to a social cause such as education or sports.
Private enterprises do not fall under this distinction as they are set up and run by people to make profit for the shareholders. They therefore do not get to be classified as Non Profits.
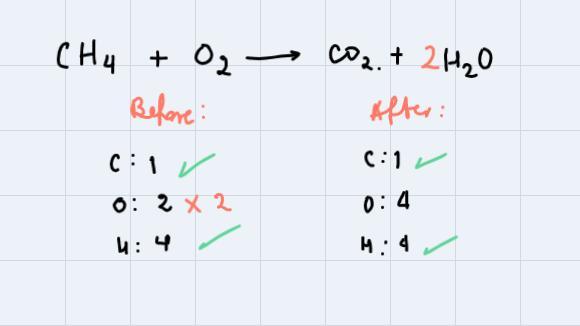
What are the coefficients when the equation below is balanced?___ CH4 + ___ O2 = ___ CO2 + ___ H2O1,2,3,41,3,4,12,2,1,21,2,1,2
Answers
To balance a chemical equation we need to make sure that the number of atoms of each element before and after the reaction occurs is the same.
In this case, we're asked to balance the equation:
As you can see, to balance Hydrogen we could multiply the coefficient of the products where Hydrogen is, by 2. Then, counting again:
Finally, we need to balance oxygen. For this, we could multiply the coefficient of O2 by 2 so we can have the same number of Oxygen atoms before and after the reaction occurs. Therefore, the balanced reaction is:
And, the coefficients when the equation is balanced, are:
__1_ CH4 + _2__ O2 = __1_ CO2 + __2_ H2O.
So, the correct answer is 1,2,1,2.


Please Help me ASAP - Next 90 minutes!! With my Homework Assignment!
100 Points + Brainliest if correct! Both questions please!!!!

Answers
Answer:
see below
Explanation:
1) here we are given that one mole of propane releases 2221kJ of energy.
now use unitary method as ,
to burn 1 mol (44g) propane 2221kJ of energy is required
to burn 1g of propane 2221kJ/44g of energy is required
therefore to burn 25g of propane 2221/44*25kJ of energy is required= 1261.93kJ ≈ 1262 kJ
Hence 1262kJ of energy is required to burn 25g propane.
2) amount of heat absorbed= 25kJ
amount of work done = 12J
change in energy= (25-12)kJ = 13kJ
Hence the value of ∆E is 13kJ .
and we are done!
Nedd help! Will mark brainliest!! Only answer if you know you're right!!
Q. Bjorn and Ingrid live in a country with cold winters. They live in identical houses but Bjorn heats his house with coal while Ingrid heats her house with wood. they both fill up their food stores for winters. who will need a larger store? explain your answer
Answers
Answer:
Bjorn will need a large store because he is using coal which will be finish after used comparing Ingrid he is using wood whose coal can be used after burning it...
Explanation:
hope u understand and it's helpful
When molecules begin to react, the rate of the forward reaction is faster than the rate of the reverse reaction. true or false?
Answers
When molecules begin to react, then the rate of the forward reaction is faster than the rate of reverse reaction: true
Why forward reaction is faster than reverse?When molecules begin to react, rate of the forward reaction is faster than reverse reaction. As reactants are consumed and products accumulated, the rate of the forward reaction decreases and rate of the reverse reaction increases.
Greater is the activation energy, slower is the reaction. Therefore, reverse reaction, for the exothermic reaction, has a greater activation energy than the forward reaction. Therefore, the reverse reaction proceeds at a slower rate than the forward reaction.
To know more about forward and reverse reaction, refer
https://brainly.com/question/11365485
#SPJ4
1. How do meteorologists indicate different types of weather fronts on a weather map?
Answers
Which element is more electronegative than nitrogen (N)?
The Periodic Table
A. Phosphorus (P)
B. Fluorine (F)
C. Lithium (Li)
O D. Helium (He)
SUBM
Answers
You are a forensic scientist who has been asked to test two blood samples. You know that one sample is suspected of containing barbiturates and the other contains no drugs; however, you cannot tell the two samples apart. Describe in one - two paragraphs how you would use the concept of pH to determine which sample contains barbiturates. Explain your reasoning
Answers
Answer:
I will find the different between the samples
Which of these is an example of a physical property?
A. Iron combines with oxygen to rust.
B. Potassium reacts in water to form a base.
C. Sodium metal is soft and malleable.
D. Sodium ignites when placed in water.
Answers
What classifies a substance as an element?
What classifies a substance as a compound?
Answers
An element is a substance that cannot be broken down by chemical means. Elements are extremely particular compounds that serve as the foundation for all life and matter (well other than the stuff smaller than atoms). It can contain one atom or trillions of them for anything to be an element, however atoms of different types cannot be combined in. That is to say, every atom has a set number of protons, ranging from 1 to 118. You can be positive that the substance you have is hydrogen if there is just one proton present. Mercury is what you get if you have 80 protons. Atoms of pure hydrogen only contain one proton. As most people are aware, if you add oxygen to it, it turns into water, which is no longer an element but a compound. Nevertheless, the building blocks are the elements. Every single object you can see is composed of elements, whether there are many of them, as there are in the human body, or only a few, as there are in salt.
A compound is a substance with a definite composition (with some leeway there, there are 'non-stoichiometric' compounds), that is composed of 2 or more elements.
Further explanation:
A compound in chemistry is a material that is created by mixing two or more distinct chemical elements in such a way that the atoms of the various elements are kept together by strong chemical bonds. These bonds form as a result of the sharing or exchange of electron s among the atoms. A molecule is the smallest, unbreakable unit of a substance.
A mixture is not a compound since there is no bonding between the atoms of the constituent substances in a mixture. In certain cases, mixing dissimilar elements causes chemical reactions that result in the formation of bonds between the atoms and the molecules of a compound. Other possibilities allow mixing distinct components without causing a reaction, preserving the separate identities of the elements. When elements are combined, reactions can happen quickly or slowly (for example, when iron is exposed to oxygen) (as when lithium is exposed to oxygen). There are times when an element is introduced to a chemical, a reaction takes place, creating new compounds (as when pure elemental sodium is immersed in liquid water).
A compound frequently looks and acts quite different from any of the constituent parts. Think about hydrogen (H) and oxygen, for instance (O). At standard atmospheric pressure and room temperature, both of these substances are gases. However, they combine to form the well-known material known as water, which is a liquid at room temperature and at normal atmospheric pressure and whose molecules each contain two hydrogen atoms and one oxygen atom (H2O).
Few elements' atoms readily combine with those of other elements to produce compounds. These gases—helium, neon, argon, krypton, xenon, and radon—are referred to as noble or inert gases. Compounds made of certain elements can be formed easily with other elements. Examples include fluorine, chlorine, and oxygen.
2) The adjective compound refers to something that is made up of several different components. Examples of this usage include compound eyes, which are found in a variety of insects, compound microscopes, which are high-power magnifying devices made up of multiple lenses, compound sentences, which are organized collections of smaller sentences that form a single integrated perceptual environment, and compound documents.
For a chemical reaction in a closed system, mass Connor be _____ or _______. We can say that throughout the reaction mass is ______.
Answers
Answer:
conserved or created/destroyed. We can say that throughout the reaction mass is conserved.
For a chemical reaction in a closed system, mass Connot be or destroyed . We can say that throughout the reaction mass is conserved .
During a chemical reaction, atoms are rearranged and bonded together in new combinations, forming different substances. However, the total number of atoms remains the same. This means that the total mass of the reactants must be equal to the total mass of the products.
The law of conservation of mass is based on the principle that atoms are neither created nor destroyed during a chemical reaction. Instead, they are rearranged and redistributed into different chemical species.
It is important to note that while mass is conserved, the substances involved in the reaction may undergo changes in physical state (solid, liquid, gas) or experience changes in energy, such as the release or absorption of heat. These changes do not affect the total mass of the system.
In summary, for a chemical reaction in a closed system, mass cannot be created or destroyed. The law of conservation of mass states that the total mass of the reactants is equal to the total mass of the products, and throughout the reaction, mass is conserved.
This principle is a fundamental concept in chemistry and plays a crucial role in understanding and balancing chemical equations.
For more such questions on chemical reaction visit:
https://brainly.com/question/25769000
#SPJ8
what is the double bond of XeF4
Answers
What can you deduce about the molecular composition of the reactants in a chemical reaction with the following atomic masses?
Reactants: (12 + 1 + 1 + 1 + 1) + (24 +16) = 56 u
Answers
Stoichiometric amounts relate to the proportional amounts of reactants and products in a balanced chemical equation.
What transpires during a chemical reaction to the molecules of the reactants?
Only atoms from the reactants can wind up in the products of a chemical reaction. No atoms are annihilated or made into new ones. To create the products, the reactants come into contact with one another, the bonds between their atoms are broken, and the atoms then rearrange and establish new bonds.
The frequency of collisions between the two reactants will grow as the reactant concentration rises. There are times when collisions don't cause a response (atoms misaligned or insufficient energy, etc.). More collisions and reaction possibilities result from higher concentrations.
To learn more about atoms use:
https://brainly.com/question/6258301
#SPJ1
The volume of a gas is directly proportional to its temperature (in Kelvin) at constant
pressure. What does this mean?
As one increases, the other will increase at the same rate. The graph will show a
straight line.
As one increases, the other will decrease at the same rate. The graph will show a
straight line.
As one increases, the other will decrease at the same rate. The graph will show a
inverse line.
As one increases, the other will increase at the same rate. The graph will show an
inverse line.
Answers
Answer: A
Explanation:
As one increases the other goes in a straight line. It is called a direct proportionality. Forms a linear graph.
Iron reacts with chlorine to form iron(III) chloride.
2Fe + 3Cl2 → 2FeCl3
What mass (in grams) of chlorine gas is needed to react with 251 grams of iron?
Select one:
a.
71 grams
b.
392 grams
c.
479 grams
d.
622 grams
Answers
The mass (in grams) of chlorine gas is needed to react with 251 grams of iron is 479 grams. Option C.
To determine the mass of chlorine gas needed to react with 251 grams of iron, we need to use the stoichiometry of the balanced chemical equation:
2Fe + 3Cl2 → 2FeCl3
From the balanced equation, we can see that 2 moles of iron (Fe) react with 3 moles of chlorine gas (Cl2) to produce 2 moles of iron(III) chloride (FeCl3).
To calculate the mass of chlorine gas, we can follow these steps:
Step 1: Convert the given mass of iron (Fe) to moles.
Using the molar mass of iron (Fe), which is approximately 55.85 g/mol, we can calculate the number of moles of iron:
moles of Fe = mass of Fe / molar mass of Fe
moles of Fe = 251 g / 55.85 g/mol
moles of Fe ≈ 4.5 mol (rounded to one decimal place)
Step 2: Use the mole ratio from the balanced equation to find the moles of chlorine gas (Cl2) needed.
From the balanced equation, we know that 2 moles of Fe react with 3 moles of Cl2. Therefore, the moles of Cl2 can be calculated as:
moles of Cl2 = (moles of Fe / 2) * 3
moles of Cl2 = (4.5 mol / 2) * 3
moles of Cl2 ≈ 6.75 mol (rounded to two decimal places)
Step 3: Convert the moles of chlorine gas to grams.
Using the molar mass of chlorine gas (Cl2), which is approximately 70.90 g/mol, we can calculate the mass of chlorine gas:
mass of Cl2 = moles of Cl2 * molar mass of Cl2
mass of Cl2 = 6.75 mol * 70.90 g/mol
mass of Cl2 ≈ 479 grams (rounded to the nearest whole number) Option C is correct.
For more such question on mass. visit :
https://brainly.com/question/19385703
#SPJ8
Sat 4 write about six uses of salt
Answers
Salt is an essential mineral that has many uses in various industries and in everyday life. Here are six uses of salt:
Food seasoning: Salt is commonly used to add flavor to food and is found in many types of cuisine around the world.
Food preservation: Salt has antimicrobial properties that can help preserve food and prevent spoilage. This is why it has been used for centuries to preserve meat, fish, and other perishable items.
Water treatment: Salt is used in the water treatment process to soften hard water and remove impurities.
Chemical manufacturing: Salt is used as a raw material in the production of chemicals such as chlorine, sodium hydroxide, and sodium carbonate.
De-icing: Salt is often used to melt ice and snow on roads and sidewalks during the winter months.
Health and beauty: Salt is used in various health and beauty products, including bath salts, body scrubs, and toothpaste. It is also used in some medicinal treatments, such as saline solutions for nasal irrigation.