Hello! Can you please help me do this question? I have to pick two but I’m not really sure what to pick..

Answers
Answer:
B and D
Explanation:
it is B and D because in no gravity if you change the size of the ball it does not change anything. and if you swing fast it does not change anything but the speed. if you change the angle it is just the same modle but different angle
Answer:
D, and E
Explanation:
they may not be correct but the answers seem correct. please tell me If I'm wrong.
Related Questions
Cordell bought new tires for his bicycle. As he rode his bike on the hot street, the temperature of the air in the tires increased. If the volume of the air stayed the same, what happened to the pressure inside the tires?
A. It decreased. B. It increased. C. It stayed the same. D. It was inversely proportional to the temperature
Answers
Answer: The answer is B. The pressure inside the tires increased.
Explanation:
The relationship between the pressure, volume, and temperature of a gas is described by the ideal gas law, which is usually written as:
\($$PV = nRT$$\)
where:
- \(\(P\)\) is the pressure,
- \(\(V\)\) is the volume,
- \(\(n\)\) is the number of moles of gas,
- \(\(R\)\) is the ideal gas constant, and
- \(\(T\)\) is the temperature (in Kelvin).
In this case, the volume \(\(V\)\) and the number of moles \(\(n\)\) of air in the tires stay the same. The temperature \(\(T\)\) is increasing. Therefore, for the equation to remain balanced, the pressure \(\(P\)\) must also increase.
So, the answer is B. The pressure inside the tires increased.
After comparing the 20% cranberry juice absorption spectrum to the 20% apple juice absorption spectrum, do you think that apple juice contributes to the color of cranberry-apple juice at the λmax you selected? why or why not?.
Answers
Color of apple and cranberry juice is yellow and red respectively as the two colors of juices are different each color has it's own λ max according to Beer's law and hence apple juice contributes to color of cranberry-apple juice .
What is Beer's law?The Beer's law states that the absorbance is directly proportional to the concentration of a solution that is,A∝C .Most substances follow Beer's law from low to moderate temperature ranges only.It is not followed well in case of saturation effects which are present in highly concentrated samples.
Due to the direct relation between absorbance and concentration , absorbance is preferred over transmittance for recording the spectra.
Learn more about Beer's law,here:
https://brainly.com/question/12185222
#SPJ1
PLSS I NEED HELP
WILL GIVE A LOT

Answers
For the reactions shown;
a) MgCl2 + H2SO4 → MgSO4 + 2 HCl
c) 6K + Al2O3 → 3K2O + 2Al
d) 2H2O ----> 2H2 + O2
e) 2C4H10 + 13O2 → 8CO2 + 10H2O
f) will not proceed
g) Will proceed
h) Will proceed
i) Will not proceed
j) Will not proceed
What is a balanced chemical reaction?
A balanced chemical reaction is a chemical equation that represents equal numbers of atoms of each element involved in the reaction, ensuring that the same amount of matter is present before and after the reaction takes place. The coefficients in the equation indicate the relative proportions of reactants and products.
In the case of the replacement reactions, we know that the substance that is higher in the electrochemical series will replace the substance that is lower in the series as shown.
Learn more about reaction:https://brainly.com/question/28984750
#SPJ1
I have to re ask this but pls help me I don't have a lot of time
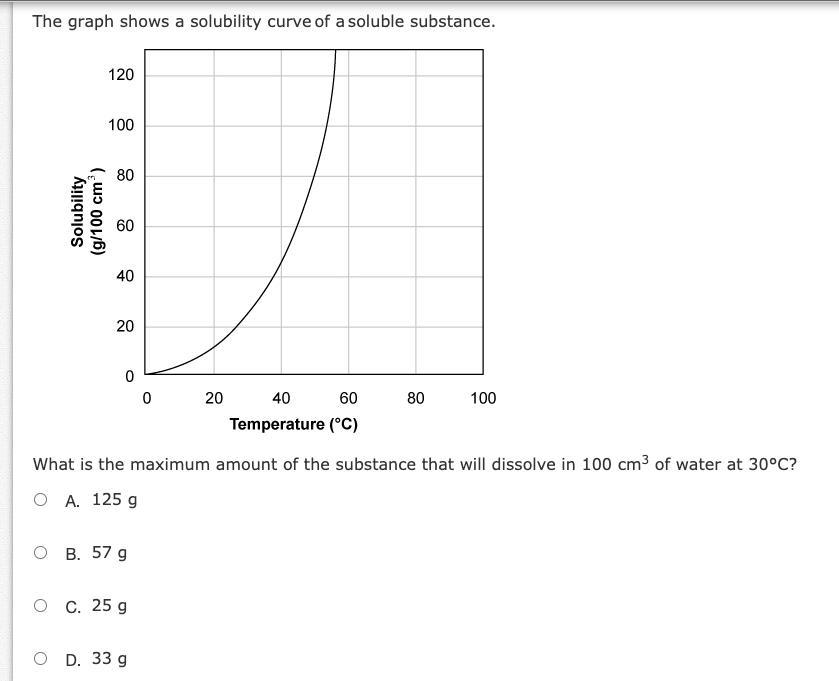
Answers
Answer:a
Explanation:
Answer:
Hello there!
If i'm doing this correctly it is A (125 g).
Hope that helped, if I wasn't correct feel free to tell me.
Have a great day!
how many moles of H2O will be required to make 17.6 moles of O2
Answers
Answer:
1 mole
Explanation:
A mole of water molecules contains 2 moles of hydrogen atoms and 1 mole of oxygen atoms.
Explain biomass combustion and energy recovery using grate
furnace or fluidized bed systems
Answers
Biomass combustion is referred to as a process in which organic materials are burnt and their remains are used to produce energy.
The process of combustion is very simple it refers to the burning of biomass which include wood, farm waste, and crops which are further used to produce or generate energy in the form of electricity and also heat, it can be termed as renewable energy that utilized the energy of biomass to produce another form of energy.
The Grate furnace method is one of the common methods used for biomass combustion and comprises several steps for the recovery of energy.
The first step consists of drying up the biomass by removing all the moisture using heat. The next step includes the production of flames and heat by combusting hydrogen present in it. After that, the remaining solid waste will undergo combustion in the presence of oxygen.
The last step includes the disposal of ash which gets accumulated due to incombustible materials like sand.
Learn more about combustion
https://brainly.com/question/23992512
A 30.0-ml sample of 0.165 M propanoic acid is titrated with 0.300 M KOH.
1. Calculate the{\rm pH}at 0{\rm mL}of added base
2. Calculate the{\rm pH}at 5{\rm mL}of added base.
3. Calculate the{\rm pH}at 10{\rm mL}of added base.
4. Calculate the{\rm pH}at the equivalence point.
5. Calculate the{\rm pH}at one-half of the equivalence point.
6. Calculate the{\rm pH}at 20{\rm mL}of added base.
7. Calculate the{\rm pH}at 25{\rm mL}of added base.
Answers
The pH of 0mL of added base is 1.92, the pH of 5 ml added base is 4.39, the pH at 10ml of added base is 4.87, the pH at the equivalence point is, the pH at one-half of the equivalence point is 4.87, the pH at 20 of added base is 9.04, the pH at 25 of added base is 4.76.
At 0 mL of added base, the solution is just propanoic acid, so the pH can be calculated using the expression for the acid dissociation constant, Ka:
Ka = [H+][C3H5O2-]/[C3H5O2H].
Setting up an ICE table and solving for [H+], we get:
Ka = 1.3 x 10^-5
[H+] = sqrt(Ka*[C3H5O2H]) = 0.012 M
pH = -log[H+] = 1.92
At 5 mL of added base, propanoic acid has been partially neutralized, so we need to calculate the concentration of both the acid and its conjugate base to determine the pH. Using the mole-to-mole ratio of propanoic acid to KOH, we can calculate that 0.015 moles of propanoic acid have been neutralized, leaving 0.015 moles of propanoic acid and 0.015 moles of C3H5O2- in the solution. The total volume is 35 mL (30 mL of acid + 5 mL of KOH). Using the Henderson-Hasselbalch equation, we get:
pH = pKa + log([C3H5O2-]/[C3H5O2H])
pKa = -log(Ka) = 4.89
[C3H5O2-]/[C3H5O2H] = 0.015/0.15 = 0.1
pH = 4.89 + log(0.1) = 4.39
At 10 mL of added base, the same process can be repeated with 0.03 moles of propanoic acid neutralized and 0.03 moles of C3H5O2- in the solution. The total volume is 40 mL.
[C3H5O2-]/[C3H5O2H] = 0.03/0.12 = 0.25
pH = 4.89 + log(0.25) = 4.12
At the equivalence point, the moles of KOH added are equal to the moles of PA originally present. This means that all the PA has been converted to KPA. The equation for the reaction between KPA and water (H2O) is:
KPA + H2O ⇌ PA + KOH
The salt KPA is a strong electrolyte and completely dissociates in water, so the solution at the equivalence point contains PA and KOH in equal amounts, and the {\rm pH} is equal to the pKa of propanoic acid, which is 4.87.
At one-half of the equivalence point, half of the moles of PA have been neutralized by KOH. This means that the concentration of PA is equal to the concentration of KPA, and the solution contains a buffer composed of PA and KPA. The pH of a buffer is given by the Henderson-Hasselbalch equation:
pH = pKa + log([A-]/[HA])
where A- is the conjugate base (in this case KPA) and HA is the acid (in this case PA). At one-half of the equivalence point, the concentrations of KPA and PA are equal, so [A-]/[HA] = 1, and the pH is equal to the pKa, which is 4.87.
At 20 mL of added base, we can calculate the moles of PA remaining by multiplying the initial concentration by the volume of acid that has not yet reacted
moles of PA = 0.165 M × (30.0 mL – 20.0 mL)/1000 mL = 0.00165 moles
The moles of KOH added are
moles of KOH = 0.300 M × 20.0 mL/1000 mL = 0.006 moles
Since the moles of KOH are greater than the moles of PA, we are in the region beyond the equivalence point, and the excess KOH will react with water to form OH- ions. The OH- ions will react with the PA that remains to form KPA, and the solution will be a buffer composed of KPA and PA. To calculate the pH, we can use the Henderson-Hasselbalch equation with the concentrations of KPA and PA at this point
pH = pKa + log([A-]/[HA])
At this point, the concentration of KPA is
[KPA] = moles of KOH added/total volume = 0.006 moles/(30.0 mL/1000 mL) = 0.2 M
The concentration of PA is
[PA] = moles of PA remaining/total volume = 0.00165 moles/(30.0 mL/1000 mL) = 0.055 M
Substituting these values into the Henderson-Hasselbalch equation gives
pH = 4.87 + log(0.2/0.055) = 9.04
Propanoic acid is a weak acid, so its reaction with KOH produces a buffer solution. The balanced chemical equation for the reaction is:
CH3CH2COOH + KOH → CH3CH2COOK + H2O
At the equivalence point, all of the propanoic acid is neutralized and converted to the salt, CH3CH2COOK. The volume of KOH needed to reach the equivalence point can be calculated as follows:
moles of propanoic acid = 0.165 M x 0.030 L = 0.00495 mol
moles of KOH needed = 0.00495 mol
volume of KOH needed = 0.00495 mol / 0.300 M = 0.0165 L = 16.5 mL
So, at 25 mL of added base, the reaction is not yet at the equivalence point. To calculate the pH at this point, we need to use the Henderson-Hasselbalch equation for a buffer solution:
pH = pKa + log([A-]/[HA])
The pKa of propanoic acid is 4.87. We can assume that at 25 mL of added base, most of the propanoic acid has been converted to propanoate ion, so we can use its concentration in the calculation:
[HA] = 0.165 - moles of propanoic acid neutralized by KOH
[A-] = moles of KOH added / total volume of solution
moles of propanoic acid neutralized by KOH = 0.300 M x 0.025 L = 0.0075 mol
[HA] = 0.165 - 0.0075 mol / 0.0555 L = 0.137 M
[A-] = 0.0075 mol / 0.0555 L = 0.135 M
pH = 4.87 + log(0.135/0.137) = 4.76
Therefore, the pH at 25 mL of added base is 4.76.
To know more about pH:
https://brainly.com/question/15289741
#SPJ4
50.0 ml sample of 0.108 m h2so4 is diluted to 250.0 ml. what is its new molarity?
Answers
The new molarity is 0.0216 M
What is molarity?The quantity of solute molecules per liter of solution is known as a solute's molarity or molar concentration. Sometimes the chemical formula of the solute is enclosed in square brackets to indicate the molar concentration of the solute. We may translate between the volume of the solution and the moles (or mass) of the solute using molar concentration.
Given :Volume of sample used = 50 mL
molarity of H2SO4 used = 0.108 M
final volume of the solution = 250 mL
formula used :M1 x V1 = M2 x V2
where ,
M1 = molarity before dilution
V1 = initial volume
M2 = molarity of final solution
V2 = volume of the final solution
Solution:M2 = M1 x V1 / V2
= > M2 = 0.108 x 50 / 250 = 0.0216
Therefore, the molarity of the final solution is 0.0216 M
To learn more about molarity:
https://brainly.com/question/8732513
#SPJ4
which two gases are primarily responsible for the greenhouse effect because of their ability to absorb infrared energy?
Answers
The two primary gases responsible for the
greenhouse effect
are carbon dioxide (CO2) and water vapor (H2O). They absorb infrared energy, which is a type of energy that is emitted from the Earth's surface, and trap it in the atmosphere.
This energy can't escape, which causes the atmosphere to warm up, resulting in the greenhouse effect.
The greenhouse effect is a natural process that helps to keep the Earth's temperature relatively stable, which is important for life.
The amount of CO2 and H2O in the atmosphere are regulated by natural processes, such as respiration and
photosynthesis
,
but human activities, such as burning fossil fuels and deforestation, have caused these levels to increase significantly over the past few decades.
This has resulted in a further increase in the temperature of the atmosphere, leading to climate change.
CO2 absorbs more infrared energy than other gases, but H2O also plays an important role in the greenhouse effect.
H2O exists in the atmosphere in both vapor and liquid forms, and is able to absorb and trap heat energy more effectively than CO2.
H2O also has the ability to reflect incoming sunlight, which further helps to keep the temperature of the atmosphere warm.
CO2 and H2O are the two primary gases responsible for the greenhouse effect because of their ability to absorb infrared energy and trap heat in the atmosphere.
These two gases are essential for regulating the temperature of the Earth and maintaining the climate.
Human activities have caused their levels to increase, resulting in a further increase in the temperature of the atmosphere and leading to climate change.
to know more about
greenhouse effect
refer here:
https://brainly.com/question/13706708#
#SPJ11
Rank the given compounds based on their relative Brensted acidities. strongest Bronsted acid,weakest Bronsted acid H-CH_3, H-OH, H-I, H-F, H-NH_2
Answers
The compounds ranked based on their relative Bronsted acidities from strongest to weakest are as follows:
1. H-I (Hydrogen iodide)
2. H-CH3 (Methyl radical)
3. H-OH (Hydroxide ion)
4. H-NH2 (Ammonia)
5. H-F (Hydrogen fluoride)
Bronsted acidities can be determined by analyzing the stability of the corresponding conjugate bases. A stronger acid will have a more stable conjugate base. Here is the explanation for the ranking:
1. H-I: Hydrogen iodide (HI) is a strong acid because iodide ion (I-) is a stable conjugate base. Iodide ion is large and can effectively disperse negative charge, leading to stability.
2. H-CH3: Methyl radical (CH3) is weaker than HI but stronger than the remaining compounds. It is a stable radical and has resonance structures that stabilize its conjugate base.
3. H-OH: Hydroxide ion (OH-) is less acidic than HI and CH3. It forms a stable conjugate base, but it is not as stable as iodide ion or the methyl radical.
4. H-NH2: Ammonia (NH3) is weaker than the previous compounds. The lone pair on the nitrogen atom can be donated to accept a proton, making NH2- a relatively unstable conjugate base.
5. H-F: Hydrogen fluoride (HF) is the weakest acid among the given compounds. The fluoride ion (F-) is a relatively strong base, and its conjugate acid, HF, is a weaker acid compared to the others.
The ranking of the given compounds based on their relative Bronsted acidities, from strongest to weakest, is H-I, H-CH3, H-OH, H-NH2, and H-F. This ranking is determined by analyzing the stability of their respective conjugate bases, with stronger acids having more stable conjugate bases.
Learn more about the Bronsted acidities visit:
https://brainly.com/question/15516010
#SPJ11
The melting point of a solid is 24.9OC. As heat is added to melt the solid, what happens to the particles?
The motion of the particles increases.
The particles move closer together.
The motion of particles decreases.
The particles move farther apart.

Answers
Explanation:
As heat is added to melt the solid, the motion of the particles increases. This is because the heat energy increases the kinetic energy of the particles, causing them to vibrate more and move faster. Eventually, the increased motion overcomes the forces holding the particles together in a solid lattice structure, and the solid melts into a liquid.
Interpretation:
1. What is the origin time of the earthquake (at what time did the earthquake occur)?
2. Which seismograph recorded the earliest P-wave arrival? The latest?
3. What does the difference described in #2 suggest about the relative locations of each seismograph?
4. Where was the epicenter (which State and/or Country?) of this earthquake located?
5. Use your Plate Tectonics map to determine what type of plate boundary is located here..
6. Which plates are found along this boundary?
7. Describe what might be happening here to cause earthquakes at this location. BE SPECIFICI
8. Your circles may not have intersected precisely at one point. Other than error in your measurements, what are
the possible reasons for this? (be specific!)
M
CHALLENGE
Question
W
You have been using the P- and S-wave travel time curve to determine the
distance to epicenter. We have asked you to use this curve for every
earthquake you study. Explain why this curve might not be appropriate in
all situations, and justify your answer.
Answers
Answer:
Match the celestial bodies with their descriptions.
meteorite
asteroid
meteor
comet
an object from space burning up as
it enters the atmosphere of a planet
arrowRight
a small body in space, usually composed
of rock or metal
arrowRight
an object from space that falls to the
surface of a planet without completely
burning up
arrowRight
a body made mainly of ice and dust
that leaves a trail of dust known as
a tail as it approaches the Sun
arrowRight
Explanation:
What is the volume of 12.7 g of a solid that has a density of 9.6 g/cm^3?
Answers
Answer:
\(\boxed {\tt 1.32291667 \ cm^3}\)
Explanation:
Volume can be found by by dividing the mass by the density.
\(v=\frac{m}{d}\)
The mass of the solid is 12.7 grams.
The density of the solid is 9.6 grams per cubic centimeter.
\(m= 12.7 \ g \\d= 9.6 \ g/cm^3\)
Substitute the values into the formula.
\(v=\frac{12.7 \ g}{9.6 \ g/cm^3}\)
Divide. Note that the grams, or g, will cancel out.
\(v=\frac{12.7}{9.6 \ cm^3}\)
\(v= 1.32291667 \ cm^3\)
The volume of the solid is 1.32291667 cubic centimeters.
If it’s volume you’re trying to find it will be v=m/d but if it’s mass then it’s m= v * d
Which list consists of elements that have the most similar chemical properties?
a) Cu, Zn, Fe
b) K, Ca, Br
c) Mg, Al, Si
d) Cs, Na, K
Answers
Answer:
D. Cs, Na, K
Explanation:
Elements in the same column of the Periodic Table have similar chemical properties.
Answer:
d) Cs, Na, K
Explanation:
The vertical columns on the periodic table are called groups or families because of their similar chemical behavior. Cs, Na, and K are all in the same vertical column (which is called Group IA or the alkali metals).
Emma wants to go swimming the first day the pool opens in May, but she is worried it will be too cold since summer only just started. She only likes to swim in the water if the pool is at least 82°F. The thermometer in the pool reads 24°C. Is the pool warm enough for Emma to swim in? Show all work to support your answer.
Answers
Answer:
the water is not warm enough for her
Explanation:
Given that
Her preferred temperature = 82°F
Thermometer in water reads = 24°C
Convert the preferred temperature to °C
°C=5/9 (82-32)
°C = 27.8 °C
Hence the water is not warm enough for her
The water is not warm enough for her to swim in.
She loves to swim in water at temperature = 82°F.Thermometer in water reads = 24°C.We have to convert Emma's preferred temperature to °C
°C= 5/9 x (F - 32)
°C=5/9 (82-32)
°C = 27.8
Therefore the temperature is 27.8°C
This means that the temperature of the water is not warm enough for her
to swim in.
Read more about Temperature here https://brainly.com/question/1852859
what is the number of moles in 2.43 g Mg
Answers
Mass of Mg - 2.43g
Mr of Mg - 24.3
Moles= 2.43/24.3
=0.1mol
pls help!! I need the answer to this ASAP

Answers
Answer:
I think the element is carbon and the atomic mass is 12.011
(7th grade science question)
Describe how you can feel all 3 forms of heat transfer while boiling ramen on the stove
Answers
Answer:
Thermal is the heat coming through the pot . Radiation is the waves of heat coming off of the pot. Convection is the water that is being heated.
Explanation:
Answer:
Radiation: heat of the steam
Conduction: pot on your hand
Convection: ????
Explanation:
helo
Boron
B
10.811
Number of neutrons
Answers
Answer:
6
Explanation:
To determine the number of neutrons we round 10.8 to 11 and subtract the atomic number (5) and get 6; therefore, boron has 6 neutrons.
A construction company clears away 75 percent of a forest area for urban development. What will most likely happen to the carrying capacity and the populations of organisms in the remaining forest area?
Answers
Answer:
B. Their populations will decrease, and the carrying capacity will increase.
Explanation:
What is the most common element all stars are made of?
Answers
Answer:
The most common elements, like carbon and nitrogen, are created in the cores of most stars, fused from lighter elements like hydrogen and helium. The heaviest elements, like iron, however, are only formed in the massive stars which end their lives in supernova explosions.
Answer:
You might not be surprised to know that stars are made of the same stuff as the rest of the Universe: 73% hydrogen, 25% helium, and the last 2% is all the other elements. So your answer is hydrogen and helium.
Explanation:
Mica has two metal blocks made from the same substance. She heats both blocks on the same stove for the same amount of time. She then measures the temperature. Metal Block A has a higher temperature than Metal Block B.
Answers
This situation is possible if Metal Block A has a lower specific heat capacity than Metal Block B. Specific heat capacity is the amount of heat energy required to raise the temperature of a substance by one degree Celsius per unit of mass.
What is specific heat?Specific heat is the amount of heat energy required to raise the temperature of one unit of mass of a substance by one degree Celsius or Kelvin. It is a characteristic property of a substance and is expressed in units of J/(g·°C) or J/(g·K). Substances with a higher specific heat require more energy to raise their temperature compared to substances with a lower specific heat.
Here,
If a substance has a lower specific heat capacity, it means that it requires less heat energy to raise its temperature. So, if both blocks are made from the same substance but have different specific heat capacities, heating them for the same amount of time on the same stove would result in Metal Block A having a higher temperature than Metal Block B.
To know more about specific heat,
https://brainly.com/question/11297584
#SPJ1
what characteristic of an element differs between isotopes?
Answers
to what direction does the rope move?
Answers
Answer:
This question appears incomplete
Explanation:
However, the direction in which the tension acting on the rope is directed is the direction the rope will move. Hence, when a rope is attached to an object, the rope will only "realistically" move in the opposite direction because the rope cannot push an object but can only pull an object, hence can only move in the direction away from the object.
NOTE: Tension can be defined as the pulling force of an object through the use of a string, chain or anything with a potential to stretch fully.
what is a colloid in chemistry
Answers
Answer:
Colloid, any substance consisting of particles substantially larger than atoms or ordinary molecules but too small to be visible to the unaided eye; more broadly, any substance, including thin films and fibres, having at least one dimension in this general size range, which encompasses about 10−7 to 10−3 cm.

Colloid has particles bigger than that of solution and larger than that of suspension. It shows tyndall effect unlike solution and does not settle down unlike suspension. Its particles are between 1 to 1000 manometer.
How does living six months with 24 hour's of sunlight or darkness affect the human body
Answers
Answer:
you could become ill and die from certain chronic illnesses which would be caused by the lack of sun
if carbon dioxide is broken down. what element will it give?
Answers
Which one of the following statements is not correct about MSDS?
Select one:
a.
It does not contain information on physical and chemical properties of the material, potential hazards of the material and how to work safely with these materials.
b.
It provides single reference for all information about hazardous chemicals.
c.
Material Safety Data sheet forms the important elements of effective Chemical Hazards Communication System.
d.
It is a document prepared by the manufacturers/suppliers of the chemical
Answers
Option B. It provides a single reference for all information about hazardous chemicals.
MSDS does not provide a single reference for all information about hazardous chemicals, but it does provide information on physical and chemical properties, potential hazards, and safe work practices.
Understanding the Role of MSDS in Chemical Hazard CommunicationThe Material Safety Data Sheet (MSDS) is a document prepared by the manufacturer or supplier of a hazardous chemical which provides information about the physical and chemical properties of the hazardous chemical, potential hazards associated with it, and how to work safely with it. The MSDS is an important element of an effective chemical hazard communication system as it provides information on how to identify and protect against potential health hazards, proper storage and handling procedures, and proper disposal methods.
Learn more about MSDS: https://brainly.com/question/18638385
#SPJ4
Which of the following would
MOST LIKELY be a habitat for
moss?
A a forest
B. a desert
C. a tundra
D. a grassland
Answers
Answer: A forest
Explanation:
Moss are more likely to be found in moist shady locations. They are best known for those species that carpet woodland and forest floors.
The temperature of a 0.65L sample of carbon dioxide gas is 580K. If the pressure remains constant, what is the new volume of the gas if the temperature increases to 1300K?