How are Hfr strains of bacteria different from F+ strains? Select one: a. Cells of Hfr strains cannot initiate conjugation with F- cells. b. Cells of Hfr strains can initiate conjugation with F+ cells or other Hfr cells. c. Cells of Hfr strains are able to transfer chromosomal genes, whereas cells of F+ strains cannot. d. The F factor is integrated into the bacterial chromosome in all or most cells of an Hfr strain but in only a few cells in an F+ strain.
Answers
The F factor is integrated into the bacterial chromosome in all or most cells of an Hfr strain but in only a few cells in an F+ strain. Option d is correct.
Hfr strains of bacteria are different from F+ strains because the F factor is integrated into the bacterial chromosome in all or most cells of an Hfr strain but in only a few cells in an F+ strain. This means that Hfr strains are capable of transferring chromosomal genes during conjugation, while F+ strains are not.
During conjugation, the Hfr cell transfers a copy of its chromosomal DNA to the F- cell, but it is usually incomplete because the process is interrupted before the entire chromosome can be transferred. This results in a recombinant F- cell with new genes but still lacking the F factor. In contrast, an F+ cell can transfer only the F factor to an F- cell, not chromosomal genes. Thus, Hfr strains are an important tool for mapping bacterial chromosomes and studying gene transfer. Hence Option d is correct.
To learn more about conjugation, here
https://brainly.com/question/28175934
#SPJ4
Related Questions
ANSWER FAST! MARKING BRAINLIEST!
This system insulates and protects earth and its lifeforms by trapping thermal energy from the sun that bounces back from earths surface
A- Atmosphere
B- Hydrosphere
C- Geosphere
Answers
The answer is B hydro sphere
Geologists use radioactive dating to determine the absolute ages of rocks. *
True
False
Answers
Explanation:
define da term lsotopos
Answers
Answer:
atoms have same atomic number but different atomic masses is called isotopes
Explanation:
At the energy-generating station, the coal is burned. Burning
changes, the coal's chemical energy into sound energy.
true or
false
Answers
Answer:
The correct answer is - false.
Explanation:
At the energy-generating station, when the coal is burned, the chemical energy store in the coal converted into thermal energy and light or luminous energy.
The thermic energy generated used to heat and boil the water or liquid to generate the gas and generate the kinetic energy to move the fan that is desgined to generate the electrical energy.
Thus, the statment is false statement as it does not convert into sound energy.
predict what happens when lead is added to nitric acid
Answers
When Lead reacted with nitric acid, lead(II) nitrate, nitrogen oxide and water are produced.
What products are produced when lead reacts with nitric acid?
Lead(II) nitrate, nitrogen oxide and water are the products which are produced by reacting lead and nitric acid with each other. The nitrogen attach with the lead molecule as well as oxygen which produces Lead(II) nitrate and nitrogen oxide.
So we can conclude that when Lead reacted with nitric acid, lead(II) nitrate, nitrogen oxide and water are produced.
Learn more about lead here: https://brainly.com/question/26320301
The car has a rechargeable battery to drive it’s motor. The rechargeable battery provided a potential difference of 330 volts and can store up to 64 mega Jules it takes 8 hours for the battery to receive a full charge assume that the charging process is 100% efficient calculate the total charge the flows while the battery is being charged
Answers
The total charge that flows while the battery is being charged is approximately 193,939.39 Coulombs.
To calculate the total charge that flows while the battery is being charged, we can use the relationship between electrical energy, potential difference, and charge.
The electrical energy (E) stored in the battery is given as 64 mega Jules (64 MJ). The potential difference (V) provided by the battery is 330 volts. We know that the energy (E) is equal to the product of the potential difference (V) and the charge (Q):
E = V * Q
Since the charging process is 100% efficient, all the electrical energy supplied is stored in the battery. Therefore, we can rearrange the equation to solve for the charge (Q):
Q = E / V
Substituting the given values, we have:
Q = 64 MJ / 330 V
To perform the calculation, we need to convert mega Jules (MJ) to joules (J) since the SI unit of energy is joules. One mega Joule is equal to 1 million joules:
Q = (64 * 10^6 J) / 330 V
Calculating the division:
Q ≈ 193,939.39 Coulombs
Therefore, the total charge that flows while the battery is being charged is approximately 193,939.39 Coulombs.
This value represents the quantity of electric charge transferred during the charging process, and it indicates the amount of electricity that enters the battery.
For more such questions on charge visit:
https://brainly.com/question/18102056
#SPJ8
Which pair of elements are nonmetals and gases at room temperature and normal atmospheric pressure?.
Answers
The pair of elements which are non-metals and at the same time; gases at room temperature and normal atmospheric pressure are as follows:
N2, O2, He, Ar
What is an element?An element is a substance which cannot be split into simpler units by any ordinary process.
Some elements are metalsSome are non-metalsSome are gasesSome few examples of elements include:
Hydrogen
Helium
Lithium
Beryllium
Boron
Carbon
Nitrogen
Oxygen
Fluorine
Learn more about elements:
https://brainly.com/question/14514242
Which of the following is considered the first receiver for the nicotine in a smoking person?
1.left atrium
2.right atrium
3.left ventricle
4.right ventricle
Answers
Answer:
i think right artrium but not full aure
Which of the following is an example of
positive feedback?
A Regulation of blood glucose levels
B Regulation of body temperature
C Contractions in childbirth
Answers
Answer:
Regulation of body temperature
Explanation:
The following galvanic cell has a potential of 1.214 V at 25∘C:
Hg(l)|Hg2Br2(s)|Br−(0.10M)||MnO4−(0.10M),Mn2+(0.10M),H+(0.10M)|Pt(s)
Calculate the value of Ksp for Hg2Br2 at 25∘C.
Express your answer using one significant figure.
Answers
The value of Ksp for Hg2Br2 at 25∘C is 1.0 × 10^-12.
The given galvanic cell involves the reaction between Hg(l), Hg2Br2(s), Br−(0.10M), MnO4−(0.10M), Mn2+(0.10M), H+(0.10M), and Pt(s). The potential of the cell is given as 1.214 V at 25∘C.
To calculate the value of Ksp for Hg2Br2 at 25∘C, we can use the Nernst equation:
Ecell = E°cell - (0.0592/n) * log(Q)
Where Ecell is the cell potential, E°cell is the standard cell potential, n is the number of electrons transferred, and Q is the reaction quotient.
Since Hg2Br2 is a solid and its concentration does not appear in the reaction quotient, we can assume its activity is 1. Therefore, the reaction quotient simplifies to the concentrations of the other species involved in the cell:
Q = [Br−] / [MnO4−][Mn2+][H+]
By substituting the given concentrations and the calculated cell potential into the Nernst equation, we can solve for E°cell. Then, using the Nernst equation at equilibrium (Q = Ksp), we can solve for Ksp. In this case, the value of Ksp for Hg2Br2 at 25∘C is found to be 1.0 × 10^-12, rounded to one significant figure.
Learn more about galvanic cell here: brainly.com/question/29784751
#SPJ11
Which statement best compares potential and kinetic energy?
Objects always have more potential energy than kinetic energy.
Kinetic energy increases and potential energy decreases when the velocity of an object increases.
Only potential energy decreases when an object’s height increases.
Objects always have more kinetic energy than potential energy.
Answers
Answer:
Its B) Kinetic energy increases and potential energy decreases when the velocity of an object increases.
Explanation:
because i took the test on edge 2020
Taking into account the definition of kinetic, potencial and mechanical energy, the tatement that best compares potential and kinetic energy is: Kinetic energy increases and potential energy decreases when the velocity of an object increases.
Kinetic energyKinetic energy is a form of energy. It is defined as the energy associated with bodies that are in motion.
Kinetic energy is defined as the amount of work necessary to accelerate a body of a given mass and at rest, until it reaches a given speed. Once this point is reached, the amount of accumulated kinetic energy will remain the same unless there is a change in speed or the body returns to its state of rest by applying a force.
The kinetic energy is represented by the following expression:
Ec= ½ mv²
Where:
Ec is the kinetic energy, which is measured in Joules (J).m is the mass measured in kilograms (kg). v is the speed measured in meters over seconds (m/s).Potential energyPotential energy is the energy that measures the ability of a system to perform work based on its position.
Gravitational potential energy is the energy associated with the gravitational force. This will depend on the relative height of an object to some reference point, the mass, and the force of gravity as follow:
Ep= m×g×h
Where:
Ep is the potential energy in joules (J).m is the mass in kilograms (kg).h is the height in meters (m).g is the acceleration of fall in m/s² (approximately 9.81 m/s²).Mechanical energyFinally, mechanical energy is that which a body or a system obtains as a result of the speed of its movement or its specific position, and which is capable of producing mechanical work. Then:
Potential energy + kinetic energy = total mechanical energy
Principle of conservation of mechanical energyThe principle of conservation of mechanical energy indicates that the mechanical energy of a body remains constant when all the forces acting on it are conservative (a force is conservative when the work it does on a body depends only on the initial and final points and not the path taken to get from one to the other.)
Therefore, if the potential energy decreases, the kinetic energy will increase. In the same way, if the kinetics decreases, the potential energy will increase.
Statement best compares potential and kinetic energyTaking into account the definition of kinetic energy, when the velocity of an object increases the kinetic energy increases.
Therefore, considering the principle of conservation of mechanical energy, the potential energy decreases.
So, the correct answer is the second option: Kinetic energy increases and potential energy decreases when the velocity of an object increases.
Learn more about mechanical energy:
brainly.com/question/17809741
brainly.com/question/14567080
brainly.com/question/12784057
brainly.com/question/10188030
brainly.com/question/11962904
Use to answer Question 1-2. The potential energy diagram for a chemical reaction
is shown below. Each interval on the axis labeled "Potential energy (kJ) represents
40kJ.
Potential Energy (k
Reaction Coordinate
Answers
The activation energy of the reaction is 40 Kj/mol
What is the activation energy?Activation energy is the amount of energy required for a chemical reaction to occur. In other words, it's the minimum energy that molecules must possess in order to react with one another. The activation energy can be thought of as the height of a hill that the reactants must climb in order to reach the other side.
The activation energy is a key factor in determining the rate of a chemical reaction. Generally, reactions with higher activation energies occur more slowly than those with lower activation energies.
Learn more about activation energy:https://brainly.com/question/28384644
#SPJ1

How many molecules are in 0.87 mole of carbon dioxide?
Answers
Answer:
1 moles Carbon Dioxide = 44.0095 gram using the molecular weight calculator and the molar mass of CO2
Explanation:
The molecules in 0.87 mole of carbon dioxide are 5.2 x 10²³.
What are moles?The mole is a SI unit of measurement that is used to calculate the quantity of any substance.
If there are 6.023 × 10²³ molecules present in one mole of carbon dioxide then for 0.87 moles
\(6.023 \times 10^2^3 \times 0.87 = 5.2 \times 10^2^3\)
Thus, the molecules in 0.87 mole of carbon dioxide are 5.2 x 10²³
Learn more about moles
https://brainly.com/question/26416088
#SPJ2
CO₂
How to find the chemical or iupac name
Answers
Answer: Carbon oxide
Explanation:
H2CO: → H2O + CO2
Answer the following question about the equation above.
1. How many atoms of Hydrogen are in the reactant?
2. How many atoms of Carbon are in the reactant?
3. How many atoms of Oxygen are in the reactant?
4. How many atoms of Hydrogen are in the product?
5. How many atoms of Oxygen are in the product?
6. How many atoms of Carbon are in the product?
7. Does the number of atoms of each element on the reactant side match that of the product side?

Answers
Answer:
1) 2
2) 1
3)1
4) 2
5)3
6)1
7)yes except Oxygen
who will go out with me
Answers
Answer:
i want to see a pic but if you got nice toes all give you my number
Explanation:
Answer:
nah Im good
Explanation:
single forever
A particular reaction has a positive ΔS∘ and a negative ΔH∘. Under what temperature conditions the reaction occurs spontaneously? At no temperature range Above a temperature of ΔSoΔH∘ Above a temperature of ΔSoΔH∘ All temperatures
Answers
Under all temperatures the reaction occurs spontaneously. Option D is correct.
A reaction occurs spontaneously when the Gibbs free energy change of the system is negative, which implies a decrease in the system's free energy.
The following equation expresses the relationship between Gibbs free energy, enthalpy, and entropy:
ΔG∘=ΔH∘−TΔS∘
where ΔG∘ is the change in Gibbs free energy,
ΔH∘ is the change in enthalpy,
T is the temperature in kelvin,
and ΔS∘ is the change in entropy.
The Gibbs free energy change, enthalpy change, and entropy change all have a positive value for a spontaneous reaction.ΔS∘ is positive and ΔH∘ is negative in this specific reaction. This means that the temperature at which the reaction will occur spontaneously is all temperatures.
Therefore, we can say that this reaction is spontaneous at all temperatures.
To determine whether a reaction is spontaneous or nonspontaneous, the Gibbs free energy change (ΔG) must be calculated, and the sign of ΔG indicates the spontaneity. If ΔG is negative, the reaction is spontaneous; if ΔG is positive, the reaction is nonspontaneous, and if ΔG is zero, the reaction is at equilibrium.
Therefore, Option D is correct.
Learn more about reaction -
brainly.com/question/25769000
#SPJ11
average atomic mass of zync
Answers
Answer:
The average atomic mass of zinc is 65.38 u.
Explanation:
Hope this helps.
Give brainliest please!
can someone do this for me i wasent there when we learned it
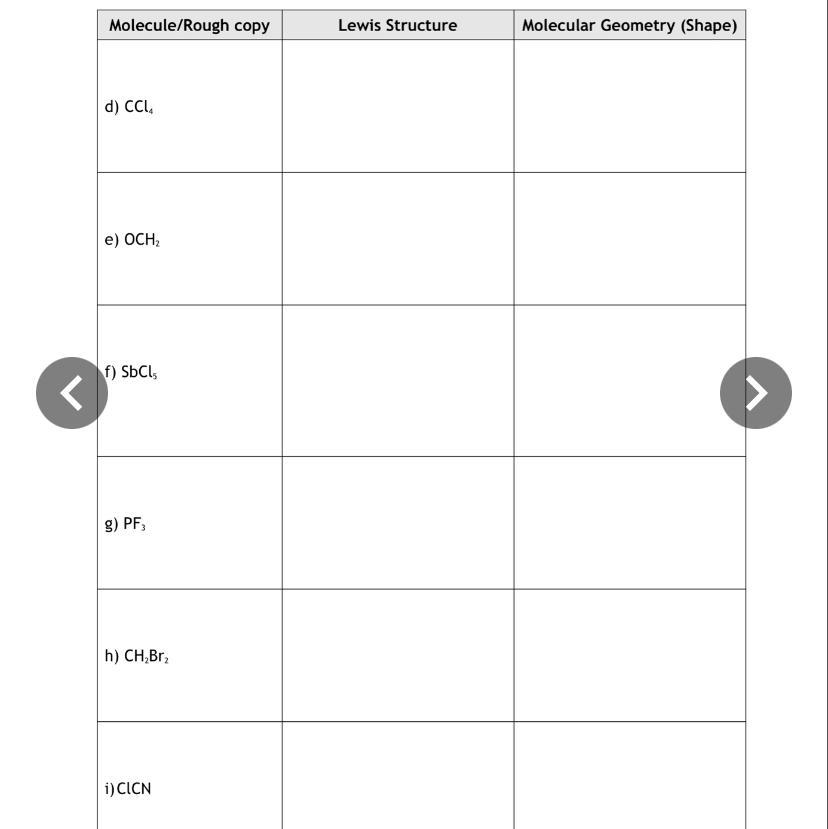
Answers

Consider a test for a rare disease present at 2% of the population. The test is 95% accurate (positive) in people who have the disease. A false positive result appears in a healthy person with 6% chance. (a) What is the probability that someone who has a positive test actually has the disease? (b) In your answer, you will observe that the probability of actually having the disease for those who tested positive is very low despite that the test quite accurately detect the disease (95\%). Explain the reason behind this low probability.
Answers
The probability that someone who has a positive test actually has the disease is approximately 24%.
The low probability that someone who tests positive actually has the disease despite the test's accuracy can be attributed to two factors: the rarity of the disease and the rate of false positives.
Firstly, let's consider the rarity of the disease, which is present in only 2% of the population. This means that even if the test is highly accurate, a positive result is more likely to be a false positive due to the larger proportion of healthy individuals in the population.
Secondly, we need to take into account the rate of false positives, which occurs in 6% of healthy individuals. When we combine this with the small percentage of the population actually having the disease, the number of false positives can outnumber true positives.
To understand this better, let's consider a hypothetical group of 1000 individuals. Out of these, only 20 (2%) would have the disease, while the remaining 980 (98%) would be healthy. The test's accuracy suggests that 19 out of the 20 individuals with the disease would test positive, and approximately 59 (6% of 980) healthy individuals would also test positive falsely.
Therefore, out of the total 78 positive test results (19 true positives + 59 false positives), only 19 (approximately 24%) would actually indicate the presence of the disease. This demonstrates why the probability of having the disease, given a positive test result, is relatively low.
Learn more about disease:
brainly.com/question/943439
#SPJ11
Fill in the blank.
7.95x10^7 = [?]x 10^6
=
Х
Answers
Explanation:
7.95x10^7 = [?]x 10^6
[?] = 79.5
hope this helps you.
what is milliliter the same as?
Answers
Answer:
1 cubic centimeter
Explanation:
Answer:
milliliter is the same as cm^3
Explanation:
how many atoms are in each of the following
Answers
Answer:
can we get more of the problem?
What type of energy is caused by positive and negative charges in matter
Answers
Answer:
Static electricity is the result of an imbalance between negative and positive charges in an object. These charges can build up on the surface of an object until they find a way to be released or discharged.
Explanation:
Please help (: List and describe the locations and conditions in the Four zones of a lake
Answers
Explanation:
littoral horizantal raw curated
What mass of NaCl formed
when 0.25 g Na react
completely with 0.39 g Cl2?
A. 0.59 g NaCl
B. 0.64 g NaCl
C. -0.14 g NaCl
D. 0.14 g NaCl
Answers
Answer:
The answer is B
Explanation:
0.25 + 0.39= 0.64
Some substances like chalk, duster, paper, wood, air are not found in the periodic table. why?
Answers
Answer:
They are not pure elements, instead they are compounds, hence why they are not found on the periodic table. (Periodic table only consists of pure elements)
Calculate the amount of HEAT needed to increase the temperature of 300g of water (specific heat 4.18J/g°C) from 27°C to 50°C.
Answers
The amount of heat is 288 kJ needed to increase the temperature of 300g of water (specific heat 4.18J/g°C) from 27°C to 50°C
Heat is the transfer of kinetic energy from one medium to the object from another medium
Here given data is
Temperature = 27°C to 50°C
Specific heat = 4.18J/g°C
Mass = 300g
WE have to calculate the amount of heat =?
The formula is
the amount of heat
Q = mCΔT
Q = 300g × 4.18J/g°C × (27°C - 50°C)
Q = 300g × 4.18J/g°C × 23
Q = 288 kJ
Know more about amount of heat
https://brainly.com/question/3155189
#SPJ1
When a food source is missing an essential amino acid it is called a _____________amino acid. The essential amino acid _____________is not common in foods.
Answers
When a food source is missing an essential amino acid, it is called a limiting amino acid. The essential amino acid lysine is not common in foods. In simpler words, an amino acid is essential if it cannot be produced by the human body and must be obtained through the diet.
Foods with complete proteins are those that contain all the essential amino acids that the body requires.When a food source is lacking in an essential amino acid, it limits the body's ability to synthesize new proteins. This essential amino acid is referred to as the limiting amino acid. The limiting amino acid can vary depending on the source of the protein that is being consumed.
There are nine essential amino acids: histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine. These are amino acids that the human body cannot synthesize, and so they must be obtained through the diet.Lysine is not as common in foods as other essential amino acids. It is found in protein-rich foods such as beans, peas, lentils, and other legumes, as well as in some seeds and grains. Meat and dairy products are also excellent sources of lysine, but a vegetarian or vegan diet may require lysine supplementation.
To learn more about lysine, visit:
https://brainly.com/question/32240062
#SPJ11
What is the product of the synthesis reaction between calcium and iodine?
Answers
Answer:
The product of the synthesis reaction is CaI.
Explanation:
In chemistry, chemical equations are the representation of chemical reactions between elements, compounds, substances, or molecules.
They are written using element symbols (and sometimes including their current state of matter).
Therefore, if we wanted to show the reaction of sodium and chlorine to make sodium chloride, or table salt, we would illustrate it as such:
\(\bullet \ \text{Na + Cl} \rightarrow \text{NaCl}\)
A synthesis reaction in chemistry is the combination of two or more elements, compounds, molecules, or substances to make one compound, molecule, or substance.
The parent equation for a synthesis reaction is:
\(\bullet \ \text{A + B}\rightarrow \text{AB}\)
Therefore, we need to find out what the element symbols are for both calcium and iodine.
If we reference a periodic table, we will see that calcium is atomic number 20 and has the symbol Ca.
Additionally, when we locate iodine, we will see that it is atomic number 53 and has the atomic symbol I.
Now, we just need to create the reaction equation.
\(\text{Ca + I}\rightarrow \text{?}\)
We know that we are dealing with a synthesis reaction, or a combination, so the elements will combine to make one substance.
With this information, we can make an equation:
\(\text{Ca + I} \rightarrow \text{CaI}\)
Therefore, the product of the synthesis reaction is CaI.
Note: Please note that "I" is an uppercase i.