how can you tell how many valence electrons an element has
Answers
Answer:
the number of valence electrons is equal to the atom's main group number
Explanation:
Related Questions
the force of attraction between a divalent cation and an divalent anion is 1.92 x 10-8 n. if the ionic radius of the cation is 0.058 nm, what is the anion radius?
Answers
The anion radius is 0.072 nm. the cation has a charge of +2, the anion must have a charge of -2
The force of attraction between a divalent cation and an anion is proportional to the product of their charges and inversely proportional to the distance between them. The force can be expressed as F = k * q1 * q2 / r, where k is the Coulomb constant (8.99 x\(10^-9\) N \(m^2/C^2\)), q1 and q2 are the charges of the cation and anion, respectively, and r is the distance between the centers of the ions.
Since the cation has a charge of +2, the anion must have a charge of -2. Rearranging the equation and plugging in the known values, we can solve for the anion radius: r = sqrt(k * q1 * q2 / F) = sqrt(8.99 x \(10^9\) N \(m^2/C^2\) * 2 * 2 / (1.92 x \(10^-8\) N)) = 0.072 nm.
Learn more about anion radius here:
https://brainly.com/question/1835367
#SPJ4
On a cold winter morning when the temperature is -13 C, the air pressure in an automobile is 1.5 atm. Using Gay Lussac's Law, what is the pressure after the tire has warmed to 15 degrees C?
Answers
Answer:
The correct answer is 1.66 atm ≅ 1.7 atm
Explanation:
Given:
T₁ = -13°C + 273 = 260 K (initial temperature)
T₂ = 15°C + 273 = 288 K (final temperature)
P₁ = 1.5 atm
P₂= ?
We use the mathematical expression of Gay Lussac's Law to calculate P₂, as follows:
P₁/T₁ = P₂/T₂
P₂= P₁/T₁ x T₂ = (1.5 atm)/(260 K) x 288 K = 1.66 atm ≅ 1.7 atm
Since the law says that the temperature of a gas is directly proportional to the pressure, when the temperature is increased, the pressure increases.
i will give brainliest!! plz no links i wiill report
what experimentation,events and/or discoveries led to the development of Hydrogels?
Answers
during the process of roasting copper(i) sulfide, how many grams of sulfur dioxide form when 10.0 mol of copper(i) sulfide reacts?
Answers
To determine the number of grams of sulfur dioxide formed when 10.0 mol of copper(I) sulfide reacts during the process of roasting, we need to use the balanced chemical equation and the molar masses of the compounds involved.
The balanced chemical equation for the reaction of copper(I) sulfide (Cu2S) with oxygen (O2) to form copper(I) oxide (Cu2O) and sulfur dioxide (SO2) is:
2 Cu2S + 3 O2 → 2 Cu2O + 2 SO2
From the balanced equation, we can see that for every 2 moles of Cu2S, 2 moles of SO2 are formed.
Therefore, using the stoichiometry of the reaction:
10.0 mol Cu2S * (2 mol SO2 / 2 mol Cu2S) = 10.0 mol SO2
Next, we can calculate the mass of sulfur dioxide using the molar mass of SO2, which is approximately 64.06 g/mol:
Mass of SO2 = 10.0 mol SO2 * 64.06 g/mol
= 640.6 g
Therefore, when 10.0 mol of copper(I) sulfide reacts during roasting, approximately 640.6 grams of sulfur dioxide are formed.
To know more about stoichiometry, click here:
https://brainly.com/question/28780091
#SPJ11
a. 1.73 m =
cm
a. 7,651.27 m =
km
Answers
Answer:
A. 1.73 m = 172 cm
B. 7,651.27 m = 7.65127 Km
4. Antibiotics are effective on
____________________________________cells.
This means they would not work on which two
infections from question #1?
________________________________________
and ____________________________________.
(use the photo for question one)

Answers
Antibiotics are effective on bacterial cells. This means they would not work on which two infections from question 1? Common cold and influenza ("flu").
Why won't it be effective on those infections?Antibiotics are a type of medication that will fight bacterial infections by killing the bacteria and preventing their growth. It will then affect living microorganisms.
It will then not be effective in viral infections since viruses are not living beings, which will not do anything to them and what will generate is that in the individual who is taking the antibiotics, the bacteria generate antibiotic resistance making it more difficult to eradicate them when it is generated by a bacterial infection.
Therefore, we can confirm that in a common cold and influenza the use of antibiotics is not going to be effective because they are caused by viral agents that will not be eradicated.
To learn more about antibiotics visit: https://brainly.com/question/10868637
#SPJ1
chemicals x and y (both gases) react to form the gas xy, but it takes a bit of time for the reaction to occur. both x and y are placed in a container with a piston (free to move), and you note the volume. as the reaction occurs, what happens to the volume of the container? explain.
Answers
As the reaction between the two gases proceeds, the volume of the container will decrease due to the formation of a new gas, XY, which takes up less space than the two gases combined.
In a closed system, the decrease in volume is accompanied by an increase in pressure, as the molecules of XY have less space to move around in than the molecules of X and Y.
This is because the increased pressure will cause the molecules to move faster, thereby increasing the kinetic energy of the system. This increase in temperature will cause the reaction to speed up, leading to more molecules of XY being formed. As the reaction continues, the pressure, temperature, and volume will continue to change in a dynamic equilibrium until all the reactants are used up.
Learn more about gases:
https://brainly.com/question/27660102
#SPJ4
what is a limitation of using a chemical formula, such as C6H12O6, to represent a compound?
A.The chemical formula does not show the types of elements that make up the compound.
B.The chemical formula does not show how the atoms are connected to one another.
C.The chemical formula does not show the number of atoms of each element in a molecule.
D.The chemical formula does not show the chemical symbols of the elements in the compound.
Answers
Answer:
B
The chemical formula does not show how the atoms are connected to one another
Answer:
b
Explanation:
edge 2020
Look at the electrophoresis sample below. Which DNA samples are the
most similar (A-F)? What could these similarities mean?
If Band 8 in Sample F represents the mutated gene that causes baldness,
which other DNA samples may also have the baldness gene?
PLEASE HELP WILL GIVE BRAINLIEST!!
Answers
Based on the electrophoresis sample, it appears that DNA samples B and E are the most similar. Electrophoresis separates DNA fragments based on size and charge.
These similarities could mean that the DNA in these samples may have similar sequences or may come from related individuals or species. Regarding the presence of the baldness gene, it is not possible to determine from the electrophoresis which other DNA samples may also have the baldness gene. This is because the electrophoresis technique only separates DNA fragments based on size, and it cannot determine the sequence or identity of the DNA fragments.
Therefore, to determine which other DNA samples may have the baldness gene, additional genetic testing would be required, such as PCR or DNA sequencing, to detect the presence of the specific mutation associated with baldness. Overall, electrophoresis is a powerful technique used in molecular biology to separate and analyze DNA fragments. While the technique has its limitations, such as the inability to determine the identity of the separated fragments, it is still widely used to study DNA and other biomolecules.
Learn more about DNA here:
https://brainly.com/question/30993611
#SPJ11
The half-life of the carbon 14 isotope is 5730 years. If there were 4 billion atoms of C-14 in a particular organism at the time it died, how many atoms of C-14 would there be in the remains of that organism 11,460 years after it died?
Answers
Answer:
1 billion
Explanation:
The computation of the number of atoms for C-14 is shown below:
Because it is mentioned
Half the isotope for carbon 14 is 5,730 years
Currently if there had been 4 billion it would equate to 2 billion
Now that the 2 billion would've been 1 billion in the next 5,730 years
And it'll become 3 half-life
Therefore the number of C-14 atoms is 1 billion
if 75.0 grams of carbonic acid are sealed in a 2.00 l soda bottle at room temperature (298.15 k) and decompose completely via the equation below, what would be the final pressure of carbon dioxide (in atm) assuming it had the full 2.00 l in which to expand?
Answers
The final pressure of carbon dioxide in the soda bottle, assuming it had the full 2.00 L in which to expand, is 1.20 atm.
The equation for the decomposition of carbonic acid is: H2CO3 → H2O + CO2.
When 75.0 g of carbonic acid is sealed in a 2.00 L soda bottle at room temperature (298.15 K), the decomposition reaction will occur and the carbon dioxide (CO2) will expand to fill the available space in the bottle.
The final pressure of carbon dioxide (in atm), the ideal gas law equation:
PV = nRT, where P is the pressure, V is the volume, n is the number of moles, R is the universal gas constant, and T is the temperature.
Since we know the initial amount of carbonic acid (75.0 g), the number of moles present: n = (75.0 g H2CO3) / (84.01 g/mol), giving us a value of 0.894 moles.
The volume of the bottle (2.00 L) and the temperature (298.15 K). Thus, we can plug these values into the ideal gas law equation to calculate the final pressure of carbon dioxide:
P = (0.894 mol CO2) (0.08206 L*atm/K*mol) (298.15 K) / (2.00 L), which gives us a pressure of 1.20 atm.
Therefore, the final pressure of carbon dioxide in the soda bottle, assuming it had the full 2.00 L in which to expand, is 1.20 atm.
to know more about carbon dioxide refer here:
https://brainly.com/question/13528931#
#SPJ11
_____of gas molecules with an object is the cause of
pressure by a gas.
Answers
Answer:
Gas Pressure
Explanation:
Gas pressure is caused by the force exerted by gas molecules colliding with the surfaces of objects (Figure 5.2. 1). Although the force of each collision is very small, any surface of appreciable area experiences a large number of collisions in a short time, which can result in a high pressure.
Answer:
Collisions
Explanation:
It says that it was the answer.
Which of the following descriptions and/or equations best represent the enthalpy change of a system? There may be more than one correct answer.Choose one or more:A. The sum of the internal energy and the pressure-volume product of a system B. The heat absorbed or released during a phase change or chemical reaction at constant volume C. The heat absorbed or released during a phase change or chemical reaction at constant pressure D. The amount of energy required to raise the temperature of 1 mole of a substance by 1°C at constant pressure E. ΔH = ΔE + PΔVF. H = E + PVG. ΔE = q + wH. The work done on or by a phase change or chemical reaction at constant pressure F. H = E + PVG. ΔE = q + wH. The work done on or by a phase change or chemical reaction at constant pressure
Answers
The following descriptions and/or equations best represent the enthalpy change of a system: C. The heat absorbed or released during a phase change or chemical reaction at constant pressure; E. ΔH = ΔE + PΔV; F. H = E + PV.
Enthalpy (H) is a thermodynamic property that represents the total heat content of a system. It takes into account both the internal energy (E) of the system and the work done by or on the system in the form of pressure-volume work (PV).
Option C states that enthalpy change occurs during a phase change or chemical reaction at constant pressure. This is because at constant pressure, the heat absorbed or released by the system is equal to the change in enthalpy.
Option E, ΔH = ΔE + PΔV, represents the equation for calculating the change in enthalpy, where ΔE is the change in internal energy and PΔV is the pressure-volume work done.
Option F, H = E + PV, directly defines enthalpy (H) as the sum of internal energy (E) and pressure-volume work (PV).
These options highlight the relationship between enthalpy, internal energy, and work in the context of phase changes, chemical reactions, and constant pressure conditions.
To learn more about enthalpy change of a system, here
https://brainly.com/question/31635338
#SPJ4
Suppose you've been asked to design a study about this town. You want to know how their efforts have affected climate
change. What is one question you can ask that can be solved by scientific investigation? What types of data would you
need to answer your question?
Answers
One question that can be solved by scientific investigation is:
To what extent have the town's efforts impacted CO₂ levels and the balance of oxygen to CO₂ ?How can we answer this question with scientific investigation?To answer this question, you would need data on CO₂ levels from different areas around the town including measurements taken over a period of time. It will be important to consider the intake of the surrounding plantations or green spaces to assess the overall carbon balance.
So, by analyzing these data, we can evaluate the impact of the town's efforts on climate change most specifically in terms of CO₂ levels and the oxygen to CO₂ ratio.
Read more about Climate change
brainly.com/question/1789619
#SPJ4
Calculate the pH and the equilibrium concentration of S²- in a 6.89x10-2 M hydrosulfuric acid solution, H₂S (aq). For H₂S, Ka1 = 1.0x10-7 and Ka_2 = 1.0×10-1⁹ pH = [S²] = M
Answers
Therefore, the pH and the equilibrium concentration of S²⁻ in a 6.89x10⁻² M hydrosulfuric acid solution are pH = 7.78 and [S²⁻] = 2.31x10⁻¹¹ M.
Hydrosulfuric acid (H₂S) is a weak acid that dissociates in water to produce hydrogen ions (H⁺) and bisulfide ions (HS⁻). H₂S(aq) + H₂O(l) ⇌ H₃O⁺(aq) + HS⁻(aq)
The bisulfide ions (HS⁻) in turn reacts with water to produce hydronium ions (H₃O⁺) and sulfide ions (S²⁻).
HS⁻(aq) + H₂O(l) ⇌ H₃O⁺(aq) + S²⁻(aq) Ka1
= 1.0x10⁻⁷,
Ka2 = 1.0x10⁻¹⁹
To calculate the pH and the equilibrium concentration of S²⁻ in a 6.89x10⁻² M H₂S(aq) solution, we must first determine if H₂S(aq) is a strong or weak acid.
It has Ka1 = 1.0x10⁻⁷, which is a very small value; thus, we can conclude that H₂S(aq) is a weak acid.
To calculate the equilibrium concentration of S²⁻ in a 6.89x10⁻² M H₂S(aq) solution, we need to use the Ka2 value (Ka2 = 1.0x10⁻¹⁹) and a chemical equilibrium table.
[H₂S] = 6.89x10⁻² M[H₃O⁺] [HS⁻] [S²⁻]
Initial 0 0 0Change -x +x +x
Equilibrium (6.89x10⁻² - x) x xKa2 = [H₃O⁺][S²⁻]/[HS⁻]1.0x10⁻¹⁹
= x² / (6.89x10⁻² - x)
Simplifying: 1.0x10⁻¹⁹ = x² / (6.89x10⁻²)
Thus: x = √[(1.0x10⁻¹⁹)(6.89x10⁻²)]
x = 2.31x10⁻¹¹ M
Thus, [S²⁻] = 2.31x10⁻¹¹ M
To calculate the pH of the solution, we can use the Ka1 value and the following chemical equilibrium table.
[H₂S] = 6.89x10⁻² M[H₃O⁺] [HS⁻] [S²⁻]
Initial 0 0 0
Change -x +x +x
Equilibrium (6.89x10⁻² - x) x x
Ka1 = [H₃O⁺][HS⁻]/[H₂S]1.0x10⁻⁷
= x(6.89x10⁻² - x) / (6.89x10⁻²)
Simplifying: 1.0x10⁻⁷ = x(6.89x10⁻² - x) / (6.89x10⁻²)
Thus: x = 1.66x10⁻⁸ M[H₃O⁺]
= 1.66x10⁻⁸ M
Then, pH = -log[H₃O⁺]
= -log(1.66x10⁻⁸)
= 7.78 (rounded to two decimal places)
To know more about concentration visit:
https://brainly.com/question/30862855
#SPJ11
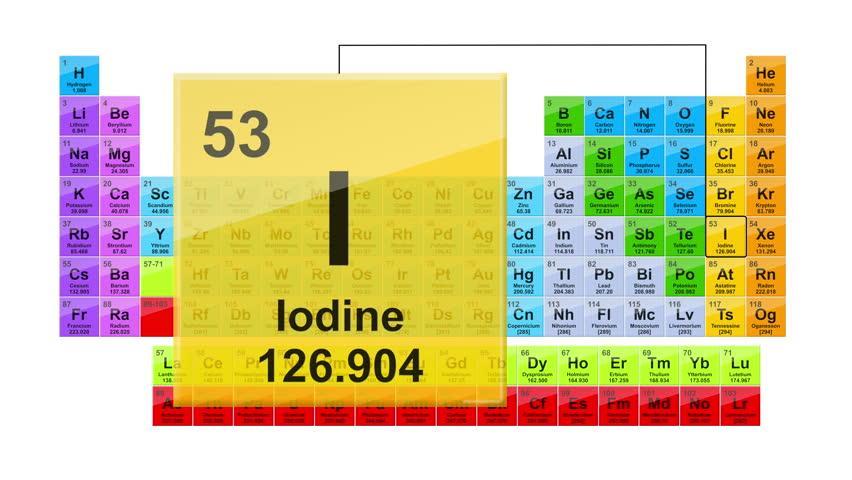
Which element is in Group 17 and has more than 50 protons but less than 75 protons?
Answers
Answer:
Iodine
Explanation:
Iodine is in group 17 on the periodic table (first picture attached). The second picture shows you in the top left-hand corner of Iodine's little square, there is the number 53. This is the atomic number, it is also the number of protons and electrons in an element.
Hope this helped :)

Sometimes a new product is test-marketed. A limited amount of the product is released to a select group of users. Then the group is observed carefully to see how the product performs. Which step might occur in the design process after a product is test-marketed and before large numbers are produced for sale?
Answers
Answer:
Modification Step
Explanation:
The entire reason why test-marketing exists is to make sure that the product will be well received by the public. Once the test marketing concludes, the data from the test is analyzed. Then the company decides whether or not they will make changes on how the product looks, feels, performs, or even rebranding the product, all to make sure that the final consumer will like the product enough to want to purchase and continuously use the product. Which will in term increase profits for the company.
If a solution of 0.1 M HCI and 0.1M 2-Bromopentane are mixed, the following reaction has a rate of 25 mM/s. What would be the new rate if the concentration of HCl and 2-Bromopentane increased by 75%. Round to the nearest whole number. HC and 2sroapenta mM/s
Answers
The new rate of the modified reaction will be 44mmmM/s which can be calculated by the rate of disappearance.
For this response, the rate of disappearance was calculated. The concentration will drop as it is consumed. After 54 minutes, the concentration dropped to 1.58 molar.
What is the reaction's speed?
Units are a way to express the reaction rate. HCl is characterized by a change in polarity of the compound per unit of time or per change in time. We have 1.85 Molar moving to 1.58 Molar in 54 minutes. Ten times as much negative three molar HCl would be produced each minute. People should subtract the initial polarity from the end one to do it correctly.
Because of the drug's concentration, we experience a negative rate of reaction. The fact that the rate is negative indicates that we are consuming.
To know more about reaction rate, please refer:
https://brainly.com/question/12904152
#SPJ4
In a separate location, take notes from the sources you’ve identified. The notes will provide details for your paper. While taking notes, you may want to use these reading strategies. Use these sources if you find them helpful: Using your notes, compare the features of analog and digital signals in the table provided. Include one to three points for each feature and type of signal.

Answers
Answer:
Here is every answer for analog and digital (in order)
Analog:
Signal Shape: Smooth and continuousNumerical Values for Signal Measurements: Analog signals represent one continuous variable as the result of another continuous time-based variable. They are capable of outputting continuous information with a theoretically infinite number of possible values.Amount of Data that can be transmitted: analog circuits can conduct only fairly low-speed data communications. The maximum data rate over an analog facility is 33.6K bps when there are analog loops at either end. With 56K bps modems, only one end of the loop can be analog. The other end of the connection has to be digital. Energy requirements: light, sound, temperature, position, and pressurePrivacy and security (ability of the signal to be encoded in a secret code): not encryptedClarity of Signal: noise affects clarity and quality, noise is amplified, amplified noise causes more random information in the signal, signal bandwidth is lowDigital (in order):
Signal shape: stepping, square, and discreteNumerical Values for Signal Measurements: digital communication methods transmit a complete measured value, in other words both a numeric value and a unit of measurement Amount of data that can be transmitted: First of all, it is theoretically possible to transmit digital signals directly. Unfortunately when we use capacitors and inductors (energy storage devices) to match the impedance from the transmitter to the air (low impedance transistor say 5 ohms to 388 ohms (air)) these components introduce a bandwidth limiting match. Since all impulse functions have infinite bandwidth (transmitting a 1 and a zero) the bandwidth of the transceiver must be multi octave in order to have any reasonable efficiency. Systems engineers simplify the problem by introducing direct sequence modulation where a carrier is modulated 0/180 degrees dependent on the data rate.Energy requirements: voltage, accoustic pressure, and magnetization of a magnetic storage mediaPrivacy and security: encryptedClarity of signal: noise is lower in amplitude, electronics can ignore the noise, quality of signal is maintained, signal is highExplanation:
I did mine in bullet points, hopefully this helped!
Analog:
Signal Shape: Smooth and continuous
Numerical Values for Signal Estimations: Analog signals speak to one ceaseless variable as the result of another ceaseless time-based variable.
Amount of Information that can be transmitted: analog circuits can conduct as it were reasonably low-speed information communications.
Energy necessities: light, sound, temperature, position, and pressure Privacy and security : not encrypted
Clarity of Signal: clamor influences clarity and quality, commotion is amplified,
Digital:
Signal shape: stepping, square, and discrete
Numerical Values for Signal Measurements: digital communication methods transmit a complete measured value, in other words both a numeric value and a unit of measurement
Amount of data that can be transmitted: First of all, it is theoretically possible to transmit digital signals directly.
Energy requirements: voltage, accoustic pressure.
Privacy and security: encrypted
Clarity of signal: noise is lower in amplitude, electronics can ignore the noise, quality of signal is maintained, signal is high
Analog and digital is :Analog signal could be a continuous signal which speaks to physical estimations.
Digital signals are discrete time signals created by computerized balance.
Example :Human voice in discuss, analog electronic gadgets. Computers, CDs, DVDs, and other advanced electronic gadgets.
Suppose that you experimentally determine the molar extinction coefficient (ε) of a complex ion sample to be 8171 L/mol-cm at 510 nm. The literature value for the ε of the complex ion sample is reported as 8400 L/mol-cm. What is the percent purity of your experimental sample
Answers
The percent purity of your experimental sample is 97.3 %
The percentage purity % = impure value/pure value × 100 %.
Actual value of molar extinction coefficient = impure value = 8171 L/mol-cm, pure value = literature value = 8400 L/mol-cm.
Substituting the values of the variables into the equation, we have
% purity = 8171 L/mol-cm/8400 L/mol-cm × 100 %
% purity = 0.9727 × 100 %
% purity = 97.27 %
% purity ≅ 97.3 %
The percent purity of your experimental sample is 97.3 %
Learn more about percentage purity here:
https://brainly.com/question/17836636
TRUE OR FALSE
Measurement of the position and velocity of a particle cannot be measured simultaneously with the same precision and accuracy.
Answers
Answer:
True.
Explanation:
To describe the problem of trying to locate a subatomic particle that behaves like a wave, Werner Heisenberg formulated what is now known as the Heisenberg uncertainty principle: it is impossible to know simultaneously both the momentum p (defined as mass times velocity) and the position of a particle with certainty.
Making measurement of the momentum of a particle more precise means that the position must become correspondingly less precise. Similarly, if the position of the particle is known more precisely, its momentum measurement must become less precise.
Which material has selective permeability a sponge or a shovel?.
Answers
a sponge because it lets in some of the dirt and mess but it doesn't let in all of it as in a shovel doesn't really take in anything
A sponge has selective permeability, while a shovel does not. Selective permeability refers to the ability of a material or membrane to allow certain substances to pass through while restricting the passage of others.
In the case of a sponge, it has a porous structure with small openings that can absorb and hold liquids, while allowing water to pass through. This selective permeability enables a sponge to soak up and retain liquids while filtering out larger particles.
A shovel is typically made of solid materials like metal or plastic, which do not possess selective permeability. A shovel's main purpose is to scoop and transport materials such as soil, sand, or snow, but it does not have the ability to selectively allow substances to pass through its structure.
To learn more about the permeability, follow the link:
https://brainly.com/question/32006333
#SPJ6
If 70.9 g of chlorine reacts with sufficient cesium to produce cesium chloride, what is the theoretical yield?
Answers
336.69 g ≈337 g
337 g is the theoretical yield.
2 Cs + Cl2 ---> 2 CsCl
Two moles gives two moles
Convert moles into mass:
molar mass of cs = 132.91 g/mol
molar mass of cl = 35.453 g/mol
molar mass of 2CsCl
= 2 * 168.36
= 336.72
Now,
2 Cs + Cl2 ---> 2 CsCl
cl2 =70.906 2 * 168.36
70.906 336.72
If 70.906g of chlorine produces 336.72 g of 2CsCl,
70.9 g of chlorine produces how much 2CsCl?
On applying Unity formula70.906 g of Cl ≅ 336.72 g of 2CsCl
70.9 g of Cl ≅ X of 2CsCl
∴X = 70.9 × 336.72 / 70.906
X = 336.69 g
The theoretical yield of 2CsCl = 336.69 g
To learn more about theoretical yield visit:
https://brainly.com/question/14966377
#SPJ4
Ca + Cl - > 2CaCl3
the two is a subscript, coefficient, reactant, or superscript?
Answers
100 PTS IM DESPERATE NEED BY TOMMOROW


Answers
Answer:
rest will do later mom is calling me plz understand promise will do it
Explanation:
Elements and compounds are similar in that they are both made of atoms and in some cases molecules.Methods of Breaking Down CompoundsThe only way to break down a compound is through a chemical change. Sometimes, energy is needed for a chemical change to happen. Two ways to add energy to break down a compound are to apply heat and to apply an electric current.Answer:
lol
Explanation:
its 5 points
5.(08.02 MC)
A 0.0400 M NaCl solution was formed when 32.0 grams of NaCl was dissolved in enough water. What was the total volume of the solution formed? (5 points)
O 7.20 liters
9.80 liters
13.7 liters
18.1 liters
Answers
Answer:
13.7 liters
Explanation:
I took the test and got it right
The total volume of the NaCl solution whose molarity is 0.04 M is 13.7 liters.
What is molarity?Molarity of any solution is define as the number of moles of solute present in per liter of the solution and it will be represented as:
M = n/V
Mass from moles will be calculated as:
n = W/M, where
W = given mass of NaCl = 32g
M = molar mass of NaCl = 58.44g/mol
mole = 32g / 58.44g/mol = 0.547 mol
Molarity of solution = 0.04 M
On putting values on the above equation, we get volume as:
V = 0.547 mol / 0.04mol/L
V = 13.67 L = 13.7L
Hence required value of volume is 13.7 L.
To know more about molarity, visit the below link:
https://brainly.com/question/489225
Kardi, age 65, and Kanye, age 62, are married with a 23-year-old daughter who lives in their home. They provide over half of their daughter's support, and their daughter earned $4,600 this year from a part-time job. Their daughter is not a full-time student. The daughter can/cannot be claimed as a dependent because:a.She can be claimed because she is a qualifying child.b.She cannot be claimed because she fails the gross income test.c.She can be claimed because she lives in their household for 12 months.d.She can be claimed because she is a qualifying relative.
Answers
The daughter cannot be claimed because she fails the gross income test
In the United States of America, an individual can claim a relative of theirs as dependent. This helps reduce the tax imposed on them by the IRS. However, one of the requirements for claiming a relative as a dependent is that: their gross income must not be more than $4,300 in 2020 or 2021. This is called the gross income test and the limit changes every year. In the case of this family, their daughter's gross income this year is $4,600. This value means that the daughter has failed the gross income test and hence, cannot be claimed as a dependent.Learn more: https://brainly.com/question/15515745?referrer=searchResults
Question 19 of 25
What is specific heat capacity?
A. The energy required to completely melt 1 g of a substance
B. The energy needed to change the temperature of a substance
C. The energy absorbed or given off in a chemical reaction
D. The energy stored within the chemical bonds of a substance
Answers
Answer:
The answer is D
Explanation:
Dont think you need one
Answer:
The correct option is B The energy needed to change the temperature of a substance.
Explanation:
Specific heat capacity is the amount of energy required to raise the temperature of one unit mass of a substance by one degree Celsius or Kelvin. This means that it is the energy needed to change the temperature of a substance, which is option B.
Some key points about specific heat capacity include:
- It is a property of a substance and can vary depending on the material.
- It is typically measured in units of J/(g·°C) or J/(kg·K).
- The specific heat capacity of water is relatively high, meaning that it requires a lot of energy to heat up or cool down compared to other substances.
- Specific heat capacity is often used in calculations involving thermal energy transfer, such as calculating the amount of heat needed to heat up a substance.
Examples of how specific heat capacity is used include calculating the energy needed to heat up a pot of water on the stove or determining the amount of heat released by a reaction based on the specific heat capacity of the products and reactants.
To learn more about specific heat capacity
https://brainly.com/question/29766819?referrer=searchResults
https://brainly.com/question/1747943?referrer=searchResults
What is the nature of new elements added to the periodic table in the last hundred years.
Answers
Hope this helps! :)
the reaction of 1.40 g of carbon monoxide with excess water vapor to produce carbon dioxide and hydrogen gases in a bomb calorimeter causes the temperature of the calorimeter assembly to rise from to . the calorimeter assembly is known to have a total heat capacity (calorimeter constant) of . (a) write a balanced equation for the reaction. (b) calculate the internal energy change, , for the reaction of .
Answers
(a) The balanced chemical equation for the reaction can be given as:
CO (g) + H2O (g) → CO2 (g) + H2 (g)
(b) The internal energy change (∆U) for the given reaction of 1.40 g of carbon monoxide with excess water vapor to produce carbon dioxide and hydrogen gases in a bomb calorimeter is 0 J.
How to determine the balanced equation(a) The balanced equation for the given reaction of 1.40 g of carbon monoxide with excess water vapor to produce carbon dioxide and hydrogen gases in a bomb calorimeter is given below.
Carbon monoxide (CO) reacts with water (H2O) to produce carbon dioxide (CO2) and hydrogen (H2) gas.
CO (g) + H2O (g) → CO2 (g) + H2 (g)
(b) To calculate the internal energy change (∆U) for the given reaction of 1.40 g of carbon monoxide with excess water vapor to produce carbon dioxide and hydrogen gases in a bomb calorimeter, we can use the following equation:
∆U = -q
where q is the heat energy absorbed by the calorimeter.
The heat absorbed by the calorimeter (q) can be calculated using the following formula:
q = C∆T
where C is the total heat capacity (calorimeter constant) of the calorimeter assembly and ∆T is the change in temperature observed in the calorimeter assembly during the reaction.
The value of C is given as 150 J/°C. The initial temperature of the calorimeter assembly is not provided. So we assume the initial temperature to be same as the final temperature in the calorimeter assembly (30.2°C).
Hence, ∆T = 30.2 - 30.2 = 0°C.
The heat absorbed by the calorimeter (q) is thus given as:
q = C∆T= 150 J/°C × 0°C= 0 J
Substituting the value of q in the equation for ∆U, we get:
∆U = -q= -0 J= 0 J
Learn more about chemical reaction at
https://brainly.com/question/11231920
#SPJ11