how do i work out valency
Answers
Related Questions
Please help me on this question

Answers
1. What happens to the density of an object if the volume increases, but the mass stays the same?
Answers
Answer:
Its density becomes greater.
Explanation:
If the mass of the object stays the same but the volume of the object decreases then its density becomes greater. ... If the volume of the object stays the same but the mass of the object increases then its density becomes greater.
By definition of density, the density of an object if the volume increases, but the mass stays the same, will decrease.
You have to know that density is a quantity that allows us to measure the amount of mass in a certain volume of a substance.
In other words, the density of a material, be it liquid, chemical, or gaseous, is the ratio of its mass to its volume.
The expression for the calculation of density is the quotient between the mass of a body and the volume it occupies:
\(density=\frac{mass}{volume}\)
From this expression it can be deduced that density is inversely proportional to volume: the smaller the volume occupied by a given mass, the higher the density.
And the higher the volume occupied by a given mass, the smaller the density.
Finally, the density of an object if the volume increases, but the mass stays the same, will decrease.
Learn more about density:
brainly.com/question/952755?referrer=searchResults brainly.com/question/1462554?referrer=searchResultsA piece of copper with a volume of 100cm3 has a mass of 890g. Explain how you could use this information to find out how much mass would be in 13cm3 of copper.
Answers
The mass of the 13 cm³ of copper, given that 100 cm³ of it has a mass of 890 g is 115.7 g
How to determine mass of the copperWe'll begin by obtainig the density of the copper. This can be obtained as illustrated below:
Volume of copper = 100 cm³Mass of copper = 890 gDensity of copper =?Density = mass / volume
Density of copper = 890 / 100
Density of copper = 8.9 g/cm³
Finally, we shall determine the mass of the 13 cm³ of copper. This is obatined as follow:
Volume of copper = 13 cm³Density of copper = 8.9 g/cm³Mass of copper = ?Density = mass / volume
Cross multiply
Mass = Density × volume
Mass of copper = 8.9 × 13
Mass of copper = 115.7 g
Thus, the mass of the copper is 115.7 g
Learn more about density:
https://brainly.com/question/952755
#SPJ1
The mass of 13 cm³ of copper with a volume of 100 cm³ has a mass of 890 g is 115.7 grams.
What is mass?Mass is defined as a dimensionless number used to describe the mass of a particle or item.
We'll start by finding out how dense copper is.
Given, Volume = 100 cm³
Mass = 890 g
Density = Mass / Volume
= 890 g / 100 cm³
= 8.9 g/cm³
Now to determine mass is 13 cm³ of copper is
Density = Mass / Volume
So, Mass = Density x Volume
= 8.9 g/cm³ x 13 cm³
= 115.7 grams
Thus, the mass of 13 cm³ of copper with a volume of 100 cm³ has a mass of 890 g is 115.7 grams.
To learn more about mass, refer to the link below:
https://brainly.com/question/26789700
#SPJ1
Which best describes how decomposers recycle energy in an ecosystem?
A.
Decomposers break down energy sources within the ecosystem.
B.
Decomposers provide energy directly to producers in an ecosystem.
C.
Decomposers provide energy directly to consumers in an ecosystem.
D.
Decomposers break down decayed matter and return the energy to the ecosystem.
Answers
Answer:
The statement “Decomposers are those organisms that recycle matter in the ecosystem” describes decomposers.
Decomposers are organisms that break down dead or decaying organisms, and in doing so, they carry out the natural process of decomposition.
why neon is special from other chemical elements
Answers
Answer: it has full valence shells
Explanation: Hope this is it
Answer:
Neon, along with helium, argon, krypton and xenon, make up the group known as noble gases. These are the most stable and least reactive elements due to having full valence shells (the outer shell has the max number of electrons, two for helium, eight for the rest).
Explanation:
Hope it helps
PlS MARK BRAINLIEST <3
`
`
`
Tori
what is the mass of 7.525 x10^24 particles of sulfur difluoride SF2?
Answers
Answer:
Mass will be equal to 874.56 gram
Explanation:
Molar mass of sulfur = 32 u
Molar mass of Florine = 19 u
Therefore molar mass of \(SF_2\) \(=32+2\times 19=70u\)
So mass of \(6.023\times 10^{23}\) particle is 70 gram
Therefore mass of 1 particle
\(=\frac{70}{6.023\times 10^{23}}=11.62\times 10^{-23}g\)
Therefore mass of \(7.525\times 10^{24}\) particle is
\(7.525\times 10^{24}\times 11.62\times 10^{-23}=874.56gram\)
First, develop a procedure for determining the miscibilities of the six solutions. consider how you might sort the solutions into polar and non-polar groups.
Answers
To determine the miscibilities of the six solutions, gather information about the solutes and solvents to classify them as polar or non-polar. Perform a preliminary test by mixing small amounts of each solution with water to identify polar and non-polar groups based on the formation of homogeneous or heterogeneous mixtures. Confirm the classification by conducting further tests with appropriate solvents to observe the formation of homogeneous mixtures.
To determine the miscibilities of the six solutions and classify them into polar and non-polar groups, it is essential to analyze the chemical nature of the solutes and solvents involved. Polar solutes generally dissolve well in polar solvents, such as water, due to their ability to form hydrogen bonds and interact with the dipole moments of the solvent molecules. On the other hand, non-polar solutes tend to dissolve better in non-polar solvents, such as hexane or benzene, as they lack the necessary polarity for strong interactions with polar solvents.
To begin the procedure, gather information about the solutes and solvents used in each solution. Identify the functional groups or chemical structures present in the solutes to determine their polar or non-polar nature. For instance, compounds with hydroxyl (-OH) or amino (-NH2) groups are typically polar, while hydrocarbons or alkyl groups are non-polar.
Next, perform a preliminary miscibility test by mixing small amounts of each solution with water. Observe the formation of a homogeneous or heterogeneous mixture. Solutions that readily mix with water to form a uniform solution are likely polar in nature. Conversely, solutions that separate into distinct layers or show limited solubility in water are indicative of non-polar characteristics.
To confirm the miscibility classification, additional tests can be conducted using appropriate solvents. For polar solvents, such as ethanol or acetone, the polar solutions should mix well, while the non-polar solutions may show limited or no solubility. Conversely, non-polar solvents like hexane or toluene should readily dissolve non-polar solutions, while polar solutions may exhibit poor solubility.
By following this procedure, one can determine the miscibilities of the six solutions and categorize them into polar and non-polar groups based on their interactions with water and other suitable solvents.
Learn more about mass here:
brainly.com/question/11954533?
#SPJ11
using the enthalpy of reaction for two reactions with ozone, determine the enthalpy of reaction for the reaction of chlorine with ozone.
Answers
The enthalpy of reaction for the reaction of chlorine with ozone is +141.6 kJ/mol.
To determine the enthalpy of reaction for the reaction of chlorine with ozone, we can use the enthalpy of reaction for two other reactions involving ozone as a reactant or product.
The first reaction is the decomposition of ozone, which can be written as:
O3(g) -> 3O(g) ΔH1 = +142.7 kJ/mol
The enthalpy change, ΔH1, for this reaction is given as +142.7 kJ/mol, which represents the energy required to break down one mole of ozone into three moles of oxygen gas.
The second reaction is the reaction of chlorine gas with oxygen gas, which can be written as:
2Cl(g) + O2(g) -> 2ClO(g) ΔH2 = -143.8 kJ/mol
The enthalpy change, ΔH2, for this reaction is given as -143.8 kJ/mol, which represents the energy released when two moles of chlorine gas react with one mole of oxygen gas to form two moles of chlorine oxide gas.
To determine the enthalpy of reaction for the reaction of chlorine with ozone, we need to write the desired reaction as the sum of the two reactions above, with appropriate coefficients:
2O3(g) + 2Cl(g) -> 2ClO(g) + 3O2(g)
The enthalpy change for this reaction, ΔH3, can be calculated using Hess's law, which states that the overall enthalpy change for a reaction is the sum of the enthalpy changes for the individual steps involved. Thus, we can write:
ΔH3 = (2 × ΔH1) + ΔH2 = (2 × +142.7) + (-143.8) = +141.6 kJ/mol
Therefore, the enthalpy of reaction for the reaction of chlorine with ozone is +141.6 kJ/mol. This value represents the energy required to break down two moles of ozone and react two moles of chlorine gas with one mole of oxygen gas to form two moles of chlorine oxide gas and three moles of oxygen gas.
for more such question on enthalpy
https://brainly.com/question/16720480
#SPJ11
Which of these is not part of an atom?
A. proton
B. isotope
C.nucleus
D. electron
Answers
The isotope isn't a part of the atom
Answer:
B. Isotope, because an isotope is just two atoms that have the same atomic number
Help me please, I am confused

Answers
Answer:
.....
Explanation:....
There are ________ mol of carbon atoms in 4 mol of C4H8O2
Answers
There are 16 mol of carbon atoms in 4 mol of C4H8O2
The chemical formula C4H8O2 tells us that each molecule of this compound contains 4 carbon atoms, 8 hydrogen atoms, and 2 oxygen atoms. Therefore, to determine the number of carbon atoms in 4 mol of C4H8O2, we need to multiply the number of moles by the number of carbon atoms per molecule:
Number of carbon atoms = number of moles × number of carbon atoms per molecule
Number of carbon atoms = 4 mol × 4 carbon atoms per molecule
Number of carbon atoms = 16 mol
So there are 16 mol of carbon atoms in 4 mol of C4H8O2. It's important to note that the chemical formula of a compound gives us information about the ratio of atoms in the compound, which allows us to determine the number of atoms in a given amount of the compound.
To know more about the Carbon, here
https://brainly.com/question/30282405
#SPJ4
A solution of NaCl was prepared in the following manner: 0.0842 g of NaCl is massed out on an analytical balance. The solid is transferred to a 25.00 mL volumetric flask. Deionized water is added to the flask such that the bottom of the meniscus is at the line. A 1.00 mL aliquot of the stock solution is transferred to a 50.00 mL volumetric flask using a volumetric pipet and diluted to volume. 6. Calculate the concentration of NaCl in the resulting solution in mg/L NaCl. (answer = 67.4 mg/L) 7. Calculate the concentration of NaCl in the resulting solution using propagation of error through the calculation. Use the manufacturer's tolerance values as the absolute error. The tolerances can be found in Chapter 2 of the Harris text. Assume a Class 1 balance and Class A glassware. Treat the tolerances as random error. (answer = 67.4+0.4 mg/L) 8. Identify 2 possible sources of random (indeterminate) error. Identify 2 possible sourses of systematic (determinate) error.
Answers
Two possible sources of systematic (determinate) error in the experiment are; Incorrect calibration of volumetric glasswareIncorrect mass of NaCl
To calculate the concentration of NaCl in the resulting solution in mg/L NaCl, we can use the formula; Concentration (mg/L) = (Mass of solute ÷ Volume of solution in L) × 1000 g / 1 mg NaCl is present in the stock solution of 25 mL. So, the mass of NaCl in the solution would be;0.0842 g ÷ 25 mL = 0.00337 g/mL. Now, in the resulting solution, a 1.00 mL aliquot of the stock solution is transferred to a 50.00 mL volumetric flask and diluted to volume. Therefore, the volume of the resulting solution is 50.00 mL. We will substitute these values in the formula, Concentration (mg/L) = (0.00337 g/mL ÷ 50 mL) × 1000 g / 1 mg concentration (mg/L) = 67.4 mg/L. Therefore, the concentration of NaCl in the resulting solution in mg/L NaCl is 67.4 mg/L.7. Concentration = 67.4 mg/LTolerance = 4.28 mg/LTotal concentration = 67.4 + 4.28 mg/L = 71.68 mg/LWe round off this value to one decimal place; Total concentration = 71.7 mg/LTherefore, the concentration of NaCl in the resulting solution using propagation of error through the calculation is 67.4+0.4 mg/L.8. Two possible sources of random (indeterminate) error in the experiment are; Errors in temperature measurement. Errors in measurement of water volume. Two possible sources of systematic (determinate) error in the experiment are; Incorrect calibration of volumetric glasswareIncorrect mass of NaCl.
Learn more about NaCl
https://brainly.com/question/32275922?
#SPJ11
At a certain temperature, SO,(9) and (o) react to produce SO, (s) according to the chemical equation shown above. An evacuated rigid vessel is originally filled with SO, () and O.), each with a partial pressure of 1 atm. Which of the following is closest to the partial pressure of 0,9) after the system has reached equilibrium, and why? A) O atm because K, is very large, nearly all the SO, () and 0,6) are consumed before the system reaches equilibrium B) 0.5 atm, because K is very large, nearly all the SO, () is consumed before the system reaches equilibrium, but an excess amount of O,() remains at equilibrium C) 1 atm, because K, is very large, the system is already near equilibrium, and there will be very little change to the partial pressure of O,G). increases the amount of O,Co) tequilibrium D) 1.5 atm because K, is very lwg, the decomposition of any so, (o) that for
Answers
The closest to the partial pressure of O₂ after the system has reached equilibrium is 0.5 atm, because K is very large, nearly all the SO₂ is consumed before the system reaches equilibrium, but an excess amount of O₂ remains at equilibrium. the correct answer is B.
Chemical equilibrium is the condition in which the concentrations of the reactants and products are equal and have no further tendency to fluctuate over time, with no discernible change in the features of the system.
The equilibrium moves to the side of the reaction where there are less moles of gas as the pressure increases. When the pressure decreases, the equilibrium moves to favor the reaction that produces more gas molecules.
Partial pressure is inversely proportional to volume, according to the ideal gas law. Additionally, it correlates with both the temperature and the amount of moles.
To learn more about equilibrium refer to :
brainly.com/question/14001401
#SPJ4
If you make up a solution of 150ml of 0.1m tris-hydrochloride, what will be the ph?
Answers
The pH of a solution of 150 mL of 0.1 M Tris-Hydrochloride is 1.
To determine the pH of a solution of 150 mL of 0.1 M Tris-Hydrochloride, you need to use the following equation:
pH = -log[H+]
Where [H+] is the concentration of hydrogen ions in the solution. In this case, the concentration of hydrogen ions is 0.1 M.
So, to calculate the pH of the solution, you simply plug the concentration of hydrogen ions into the equation:
pH = -log[0.1]
pH = -(-1)
pH = 1
Therefore, the pH of a solution of 150 mL of 0.1 M Tris-Hydrochloride is 1.
It's important to note that Tris-Hydrochloride is a buffer, meaning that it resists changes in pH. This means that the pH of the solution will remain relatively constant, even if small amounts of acid or base are added. This is useful in many biological and chemical applications, as it allows for more consistent and reliable results.
Learn more about solution: https://brainly.com/question/172153
#SPJ11
2 h2(g) o2(g) 2 h2o(g) if 8.08 g of h2(g) and an excess of o2(g) react to form 54.06 g of h2o(g), what is the percent yield for this reaction? enter your answer as a percent, not a decimal.
Answers
The percentage yield of the reaction is 37.16%.
The balanced chemical equation is,
2H₂ + O₂ → 2H₂O
It is quite clear from the reaction,
2 moles of H₂ gives us 2 moles of H₂O
Hence, it can be written,
Moles of H₂ = Moles of H₂O
we know,
Moles = (Formed/reacted mass)/Molar mass.
Molar mass of water is 18 g/mol
Molar mass of hydrogen is 1g/mol
Reacted mass of Hydrogen is 8.08grams.
Hence the formed mass of water should be.
8.08/1 = Formed mass of water/18
Formed mass of water = 14.44 grams.
The percentage yield is given by,
Percentage yield = actual mass/theoretical mass x 100
Putting the values,
Percentage yield = 54.06/145.44 x 100
Percentage yield = 37.16%.
The percentage yield of the reaction is 37.16%.
To know more about percentage yield, visit,
https://brainly.com/question/25996347
#SPJ4
Question 1 of 10
Match the term in Column 1 to the corresponding statement in Column 2.
BRUH CAN SOMEONE HELP!?!?!?
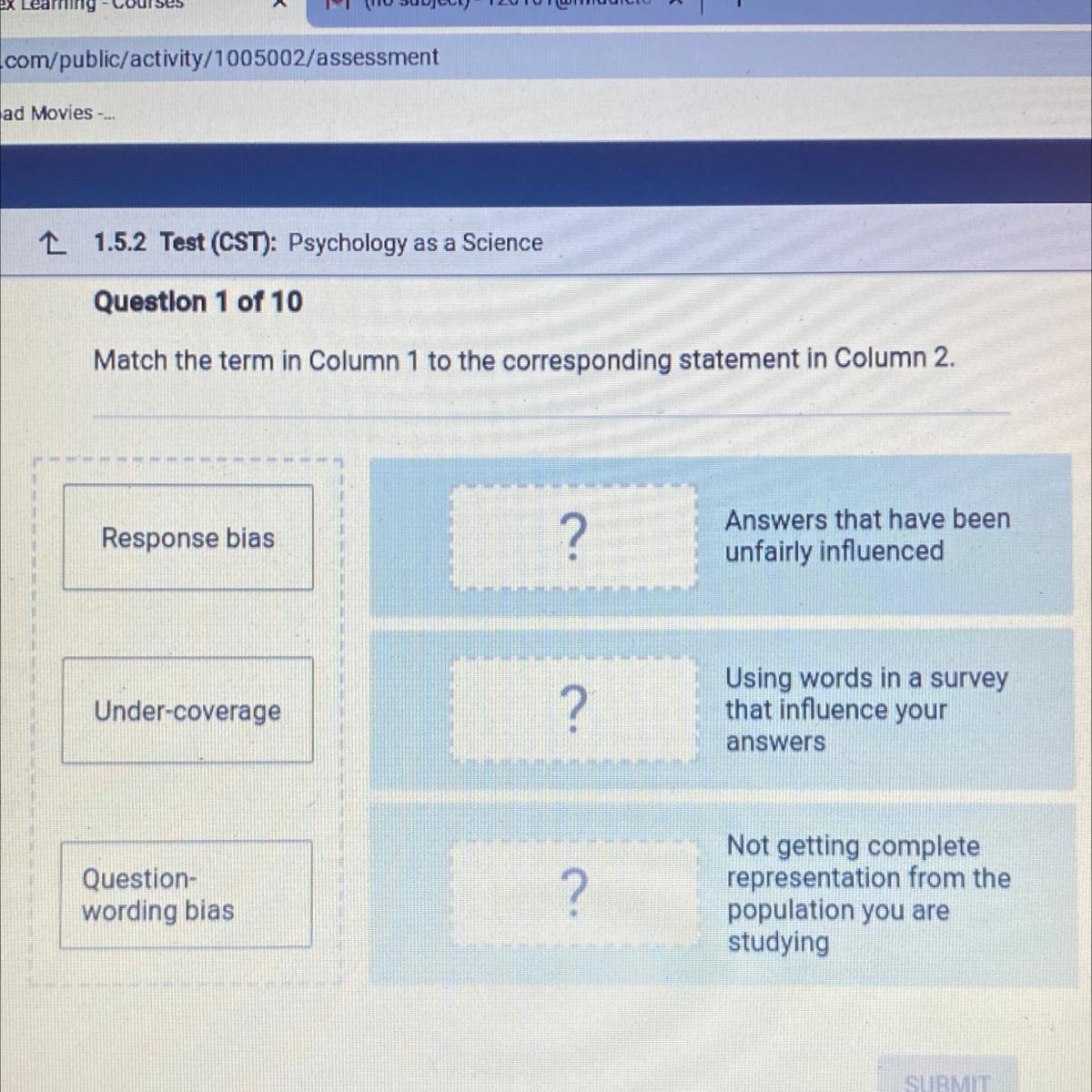
Answers
What is the solubility of silver iodide in grams per milliliter at a temperature at which the Kₛₚ of Agl is 1.47 x 10 ⁻¹⁶?
Answers
The solubility product constant expression for AgI is:
AgI(s) ⇌ Ag⁺(aq) + I⁻(aq)
The Ksp expression for AgI is given as 1.47 x 10⁻¹⁶.
Since AgI dissociates into 1 Ag⁺ ion and 1 I⁻ ion, the molar solubility (s) of AgI is equal to the concentration of Ag⁺ and I⁻ ions in the solution.
Let's assume the molar solubility of AgI is s M.
Since the molar solubility (s) of AgI is equal to the concentration of Ag⁺ and I⁻ ions, we have:
[Ag⁺] = s M
[I⁻] = s M
Using the stoichiometry of the balanced equation, the expression for the solubility product constant is:
Ksp = [Ag⁺][I⁻] = s^2
Substituting the given Ksp value, we have:
1.47 x 10⁻¹⁶ = (s)^2
Taking the square root of both sides, we get:
s = √(1.47 x 10⁻¹⁶)
Calculating the square root, we find:
s ≈ 3.83 x 10⁻⁹ M
Since the solubility is given in grams per milliliter (g/mL), we need to convert the molar solubility to grams per milliliter using the molar mass of AgI.
The molar mass of AgI is:
Ag: 107.87 g/mol
I: 126.90 g/mol
AgI: 107.87 g/mol + 126.90 g/mol = 234.77 g/mol
To convert the molar solubility (s) to grams per milliliter (g/mL):
s (g/mL) = (molar solubility (M) * molar mass of AgI (g/mol)) / 1000
Substituting the values, we have:
s (g/mL) = (3.83 x 10⁻⁹ M * 234.77 g/mol) / 1000
Calculating the value, we find:
s (g/mL) ≈ 9.0 x 10⁻¹² g/mL
Therefore, the solubility of silver iodide (AgI) in grams per milliliter (g/mL) at the given temperature is approximately 9.0 x 10⁻¹² g/mL.
The solubility of silver iodide (AgI) in grams per milliliter can be calculated using the concept of solubility product constant (Kₛₚ). Given that the Kₛₚ of AgI is 1.47 x 10⁻¹⁶.
The solubility product constant (Kₛₚ) is a measure of the equilibrium between a solid and its dissolved ions in a saturated solution. For silver iodide (AgI), the equilibrium equation can be expressed as:
AgI(s) ⇌ Ag⁺(aq) + I⁻(aq)
The Kₛₚ expression for this equilibrium is:
Kₛₚ = [Ag⁺][I⁻]
Given the Kₛₚ value of AgI as 1.47 x 10⁻¹⁶, it indicates that the product of the concentrations of Ag⁺ and I⁻ ions in the saturated solution is equal to 1.47 x 10⁻¹⁶.
To determine the solubility of AgI in grams per milliliter, we need to know the molar mass of AgI and the volume of the saturated solution. The molar mass of AgI is 234.77 g/mol, which is the sum of the atomic masses of silver (Ag) and iodine (I).
To convert the concentration of Ag⁺ or I⁻ ions to grams per milliliter, we need to divide the concentration (in moles per liter) by the molar mass (in grams per mole) and multiply by the solution volume (in milliliters).
However, without the given volume of the saturated solution, it is not possible to calculate the solubility in grams per milliliter directly using the Kₛₚ value. The solubility information typically depends on both temperature and the presence of other ions or substances in the solution. Therefore, additional data or an experimental approach would be needed to determine the solubility of AgI in grams per milliliter at the given temperature.
To learn more about solubility - brainly.com/question/32565143
#spj11
A pharmacist works with a 1. 75 M solution of sodium bromide (NaBr) and water. The volume of the solution is 84. 0 milliliters. If the pharmacist dilutes the solution to 1. 00 M, what is the volume of the new solution? Express your answer to three significant figures. The volume of the new solution is milliliters.
Answers
Dilution is the method of decreasing the solute concentration in the solution by adding solvent. The new volume after dilution is 147 mL.
What is the relation between molarity and dilution?Molarity and dilution are inversely proportional as the increase in the dilution of the solution the molarity of the solution will decrease as the number of moles of the solute will decrease.
The volume of the diluted solution can be calculated as:
\(\rm M_{1}V_{1} = M_{2}V_{2}\)
Where,
Initial molarity = 1.75 M
Initial volume = 84 mL
Final molarity = 1.0 M
Final volume = ?
Substituting values in the equation:
\(\begin{aligned}\rm V_{2} &= \dfrac{\rm M_{1}V_{1} }{\rm M_{2}}\\\\&= \dfrac{1.75 \times 84.0}{1}\\\\&= 147\;\rm mL\end{aligned}\)
Therefore, 147 mL is the new volume after dilution.
Learn more about molarity and dilution here:
https://brainly.com/question/4123280
Which of the waves below have more energy? How do you know?

Answers
Wave B has higher energy since it has a greater magnitude of disturbance.
How do you know which wave has more energy from the sketch of a wave motion?The energy of a wave is directly proportional to its amplitude (height), frequency (number of cycles per second), and wavelength (distance between two successive crests or troughs of the wave). Generally, waves with higher amplitudes, frequencies, and shorter wavelengths carry more energy than those with lower values.
In summary, to determine which wave has more energy from a sketch of a wave motion, you should consider the amplitude, frequency, and wavelength of the wave, as well as the medium through which it is traveling.
Learn more about waves:https://brainly.com/question/25954805
#SPJ1
PLEASE HELP ASAP!! {40 POINTS}
Classify EACH possible hypothesis about a medical aloe Vera plant as falsifiable or non-falsifiable
A) Aloe Vera gel is the best natural skin moisturizer.
B) Aloe Vera gel can heal wounds by boosting cell renewal.
C) Aloe Vera juice tastes better than carrot juice.
D) Drinking aloe juice can reduce the risk of lung cancer.

Answers
Answer:
The correct answer is -
FALSIFIABLE:
Aloe vera gel can heal wounds by boosting cell renewal.
Drinking aloe juice can reduce the risk of lung cancer.
NON-FALSIFIABLE:
Aloe vera gel is the best natural skin moisturizer.
Aloe vera juice tastes better than carrot juice.
Explanation:
Falsifiable is the ability or chances of any hypothesis, claim or statement to be proved wrong. In such a hypothesis, it is possible to carry an experimental observation that disproves the idea in claim or question.
In the given examples
Aloe vera gel can heal wounds by boosting cell renewal.
Drinking aloe juice can reduce the risk of lung cancer.
There is no observation in the favor of the claim so there more likely to be falsifiable whereas, Aloe vera gel is the best natural skin moisturizer.
Aloe vera juice tastes better than carrot juice are more non-falsifiable as these are based on personal choice or experimental observation.
Answer:
FALSIFIABLE IS *DOWNARROW
Aloe vera gel can heal wounds by boosting cell renewal.
Drinking aloe juice can reduce the risk of lung cancer.
NON-FALSIFIABLE IS *DOWNARROW*
Aloe vera gel is the best natural skin moisturizer.
Aloe vera juice tastes better than carrot juice.
Explanation:
yes
which ph value is consistent with the indicator results
Answers
The color of the indicator will correspond to the pH values of either acidic or basic substances.
What are pH values and Indications?pH values are values which are obtained from taking the negative logarithm to base ten of the hydrogen ions concentration of a substance.
pH values of acidic substances are less than 7 while the pH of basic or alkaline substances are greater than 7.
An indicator is an organic dye which changes colour according to the pH of a substance.
Examples of indicators are methyl orange and phenolphthalein.
Therefore, the color of the indicator will correspond to pglh values of either acidic or basic substances.
Learn more about indicators at: https://brainly.com/question/1918667
#SPJ1
What happens to atomic radius on going from left to right in a period in a periodic table?
A. Remains constant
B. Decreases first and then remains constant
C. Decreases
D. Increases
Answers
Please give brainliest I need two more!!!!!!!!!!!!!!!
How many grams of Cu(OH)2 will precipitate when excess KOH solution is added to 65.0 mL of 0.728 M CuSO4 solution
Answers
4.60 grams of Cu(OH)2 will precipitate when excess KOH solution is added to 65.0 mL of 0.728 M CuSO4 solution.
The balanced equation for the reaction between CuSO4 and KOH is:
CuSO4 + 2KOH → Cu(OH)2 + K2SO4
From the balanced equation, we can see that 1 mole of CuSO4 reacts with 2 moles of KOH to form 1 mole of Cu(OH)2.
Therefore, we need to determine how many moles of CuSO4 are present in 65.0 mL of 0.728 M CuSO4 solution:
n = C x V = (0.728 mol/L) x 0.0650 L = 0.0473 mol
This is the number of moles of CuSO4 that will react with the KOH to form Cu(OH)2.
Since there is excess KOH, all of the CuSO4 will react, so the number of moles of Cu(OH)2 formed will be equal to the number of moles of CuSO4:
moles of Cu(OH)2 = 0.0473 mol
To convert moles to grams, we need to use the molar mass of Cu(OH)2:
molar mass of Cu(OH)2 = 97.56 g/mol
mass of Cu(OH)2 = moles of Cu(OH)2 x molar mass of Cu(OH)2
= 0.0473 mol x 97.56 g/mol
= 4.60 g
to know more about precipitate reaction refer here:
https://brainly.com/question/29762381#
#SPJ11
Calculate the number of moles of liquid water that were present if 50 000. J
of heat energy were released as the temperature is dropped from 20.00 °C
to 1.00 °C. (the molar heat capacity of liquid water is 75.3 J/K mol)
Answers
According to contemporary thermodynamics, heat is the system's overall internal energy. Two properties were defined in order to measure the heat energy related to matter and its relationship to temperature.
The amount of heat needed to raise a substance's temperature by one degree is known as its heat capacity. The entire internal energy of a system is measured in terms of heat energy. Both the system's overall kinetic energy and the molecules' potential energy are included in this.
The heat required is:
q = mc (T₂ - T₁)
50000 = m × 75.3 (1.00 - 20.00)
m = 50000 / 75.3 (1.00 - 20.00)
m = -34.9 = 34.9
Molar mass of water = 18 g/mol
n = 34.9 / 18 = 1.93 mol
To know more about heat capacity, visit;
https://brainly.com/question/16738485
#SPJ1
briefly tell what is meant by the drift velocity and mobility of a free electron.
Answers
Drift velocity is the average velocity attained by an electron under the influence of an electric field. Mobility refers to the ease of flow of an electron in a given direction.
Drift velocity is the average velocity attained by an electron under the influence of an electric field. It is the net velocity of the free electron in the direction of the electric field. It is directly proportional to the electric field and inversely proportional to the mass of the electron and time taken between collisions. It is a vector quantity.
Mobility refers to the ease of flow of an electron in a given direction. It is the ratio of drift velocity to the electric field intensity. It is a scalar quantity. It is affected by the number of free electrons, the magnitude of the electric field, the mass of the electrons and the time taken between collisions. Mobility is an important parameter in designing electronic devices and in understanding the behavior of semiconductors.
Learn more about Drift velocity here:
https://brainly.com/question/28498217
#SPJ11
Theme parks can snap a crystal clear picture of you on a roller coaster at 70 mph, but bank cameras can't get a clear shot of a robber standing still.
bruh.
Answers
Answer:
I know right
Explanation:
Hiiiiiiiiiiiiiiiiiii
Which molecule has stronger intermolecular forces acetone or vegetable oil? and Why?
Answers
Vegetable oil has a higher intermolecular force than acetone. This is due to the difference in the types of molecules present in each substance.
Acetone consists of molecules with only a single carbon-oxygen bond, while vegetable oil consists of molecules with multiple carbon-carbon and carbon-hydrogen bonds. The multiple bonds in vegetable oil create stronger intermolecular forces due to the increased number of electron-pair bonds between each molecule.
This is because more electrons are shared between molecules, creating a stronger attraction. The result is a greater intermolecular force, which is why vegetable oil has stronger intermolecular forces than acetone.
know more about intermolecular force here
https://brainly.com/question/31797315#
#SPJ11
correct question is :
what molecule has stronger intermolecular forces acetone or vegetable oil? and Why?
Phases of Matter—Comic Strip Template
Instructions: Create a comic strip detailing the adventure of your character as the character is exposed to thermal energy, causing it to undergo phase changes from a solid, to a liquid, to a gas. Place drawings inside the boxes and written content on the lines below each box.
Your presentation must include the following:
• title and introduction of your character, including what substance it is made of
• source of thermal energy your character encountered (conduction, convection, and/or radiation)
• detailed description and/or diagram of the particle transformation from solid to liquid phase
• detailed description and/or diagram of the particle transformation from liquid to gas phase
Title of your comic strip: James thermal energy Adventure__________________________
________________________
________________________
________________________
________________________
________________________
________________________
________________________
________________________
________________________
________________________
________________________
________________________
________________________
________________________
________________________
________________________
________________________
________________________
Answers
Answer:
This will be a story. (comic can also be done with this. In fact it is better)
The title is the journey of the ice cube. (change it if you want)
Main character - ice cube
There was an ice cube made by the water from the freezer in an ice shop.
The ice cube, with a bunch of other ice cubes, were taken to be packed and sold to a restaurant. The ice cube was excited to where he was going while the other ice cubes are just meh. Now that the ice cubes arrived, a restaurant worker stores the ice cubes in a cooler and later used to put ice in a lemonade. While the lemonade in a glass cup was carried outside the porch of the restaurant by the waiter, the ice cube was fascinated by the environment of the restaurant. The waiter finally serves the thirsty customer and the customer drank almost half of the lemonade but the ice cube did not get in the man's mouth. Instead the other ice cubes went. The ice cube feared the person. Shortly after checking his phone, the customer was about to reach on to the glass until the hands accidentally pushes the glass over and falls. The ice cube and the remaining ice cubes spread away with the wasted lemonade liquid. No one bothered cleaning up the ice cubes, only the glass shards because the floor is a smooth concrete. Outside was really hot from the sun anyways so it would melt the ice cube and sooner evaporate. As it was in liquid form, the "ice cube" was feeling relaxed because it was just chilling on the floor. And when it evaporates, the ice cubes becomes more fascinated since it goes up to the sky while having a sky-view from above.
use the titration curve for the weak acid to calculate the ph of a 0.150 m solution of that weak acid.
Answers
To use the titration curve for a weak acid to calculate the pH of a 0.150 M solution of that acid, you would need to know the pKa value of the acid and the volume of the titrant added during the titration.
To calculate the pH of a 0.150 M solution of a weak acid using the titration curve, follow these steps:
1. Identify the weak acid and its corresponding Ka value (acid dissociation constant). The titration curve should provide this information or you can find it in a reference table.
2. Write the chemical equation for the dissociation of the weak acid (HA) in water:
HA + H₂O ⇌ H₃O⁺ + A⁻
3. Set up an equilibrium table (ICE table) to represent the initial concentrations, the change in concentrations, and the equilibrium concentrations of the species involved:
[HA] [H₃O⁺] [A⁻]
I: 0.150 0 0
C: -x x x
E: 0.150-x x x
4. Write the expression for the Ka using the equilibrium concentrations:
Ka = ([H₃O⁺][A⁻])/([HA])
5. Substitute the expressions from the equilibrium table into the Ka expression:
Ka = (x^2)/ (0.150-x)
6. Solve for x, which represents the [H₃O⁺] concentration at equilibrium. Since the weak acid is only slightly dissociated, you can assume that x is much smaller than 0.150, and the equation simplifies to:
Ka = (x^2)/0.150
7. Calculate the pH of the solution using the equilibrium [H₃O⁺] concentration:
pH = -log₁₀([H₃O⁺])
Following these steps will help you calculate the pH of a 0.150 M solution of a weak acid using the titration curve.
Learn more about the titration curve at https://brainly.com/question/31308997
#SPJ11
a car is traveling 35 miles/hr for 17 miles. How much time has the car been on the road?
a
595 hours
b
2.1 hours
c
1.5 hours
d
0.49 hours
Answers
Answer:
The car has been on the road for 0.49 hours
Explanation:
A car is traveling 35 miles/hr for 17 miles.
Speed of a car = 35 miles/hr
Distance travelled = 17 miles
The relationship between speed, distance, and time is given by
Distance = Speed × Time
Put Speed = 35 miles/hr, Distance = 17 miles
\(17=35\) × Time
Time = \(\frac{17}{35}=0.49\) hours
So, the car has been on the road for 0.49 hours
The time car has been on the road has been 0.49 hours. Thus, option D is correct.
Speed can be defined as the distance traveled by the object in unit time. The speed can be expressed as:
\(\rm Speed\;=\;\dfrac{Distance}{Time}\)
The car has been given to be traveling at:
Speed = 35 miles/hr
Distance traveled = 17 miles.
The time taken by car to travel 17 miles can be calculated by substituting the values as:
\(\rm 35\;miles/hr\;=\;\dfrac{17\;miles}{Time}\\\\\\\\Time\;=\;\dfrac{17\;miles}{35\;miles/hr}\)
Time = 0.49 hr.
The time taken by car to travel 17 miles has been 0.49 hours. Thus, the correct option has been D.
For more information about speed, refer to the link:
https://brainly.com/question/7359669