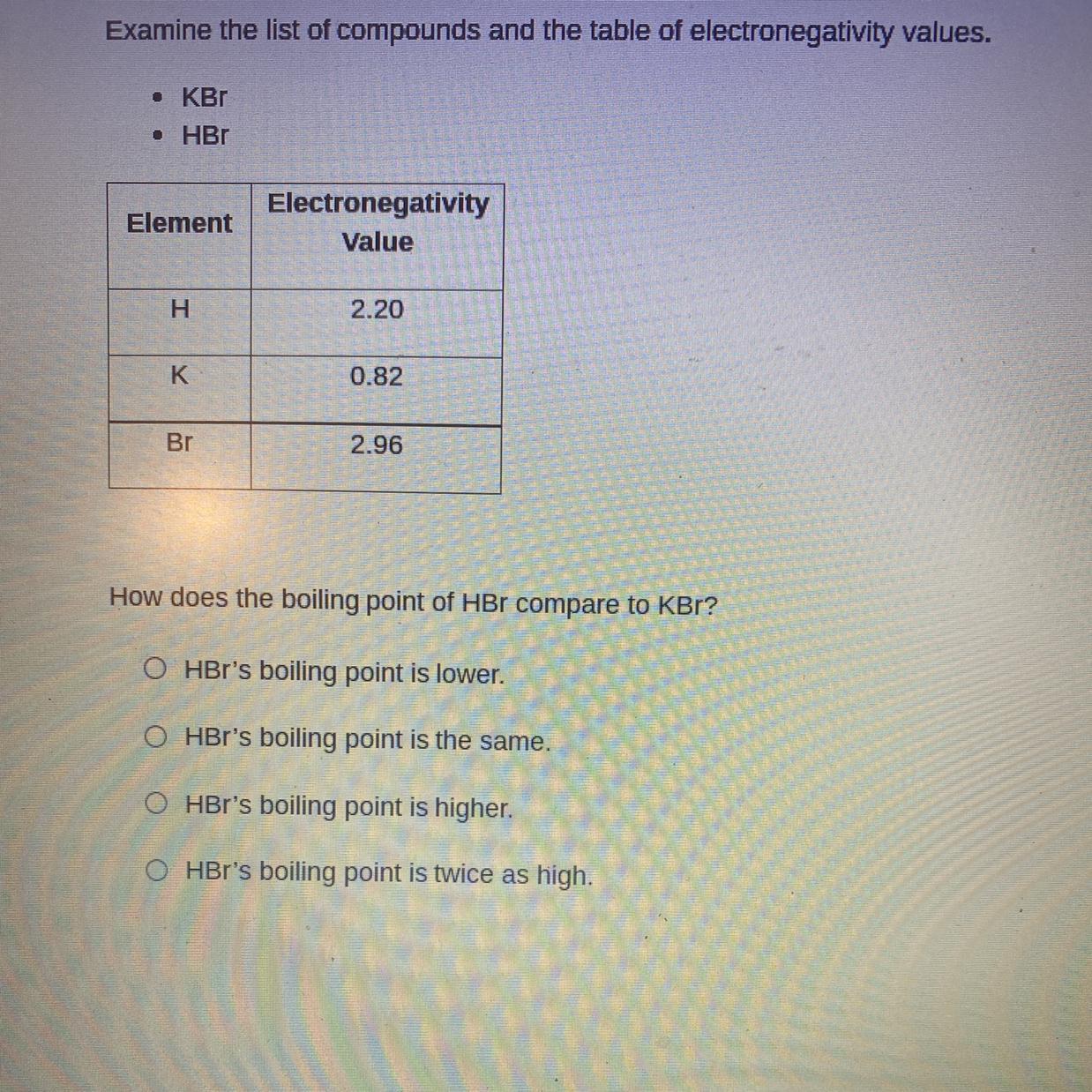
Answers
• Ionic bonds KBr are stronger than the in HBr hydrogen bonds. and we know tht ionic forces are stronger to covalent forces, which leads to higher boiling points .
So, the correct option would be A: Hbr boiling point is lower.
Related Questions
Describe the trend of the reactivity of the elements in group VII
Answers
The non-metal elements in Group 7 – known as the halogens – get less reactive as you go down the group
Answer & Explanation:
The reactivity of elements in Group VII, also known as Group 17, decreases with increasing atomic radius. This is because halogens have high electronegativities and a proclivity to gain electrons in noble gas configurations. Myths are traditional stories or beliefs that explain cultural or societal beliefs, customs, or natural phenomena. They can be passed down through generations and can be based on true or fictitious events. Mythology, on the other hand, is the collection of myths associated with a specific culture or religion. Mythology can be amplified through retelling, incorporation into religious practices; association with significant events or figures, and adaptation into other media forms such as literature, film, or art.
Which sentence most accurately describes electrically charged objects?
A. They are attracted to one other without coming into contact.
B. They are negatively charged objects that are attracted to each other.
C. They attract or repel other charged objects without touching them.
D. They attract other objects after they have been in contact with them.
Answers
Answer:
A.
Explanation:
In conclusion, an electrically neutral object is an object that has a balance of protons and electrons. In contrast, a charged object has an imbalance of protons and electrons. ... The type of charge(positive or negative) is determined by whether the protons or the electrons are in excess
Dear God, the person reading this is kind and I’m proud of them . Please help them live life to the fullest and bless her in their chosen field. Now, you’re on the clock.❤❤❤ In 9 minutes something will make you happy. Please share this with 15 people you love. Remember, . If I don’t get this back I’m obviously not a close friend. Now, I have a game for you, it’s been played since 1977. Once you read this, you have to send it to 15 people. Your next 5 days will be like this: Day 1 - you will wake up to the biggest shock of your life. Day 2 - you will cross paths with an old friend you have missed. Day 3 - you will find yourself with a lot of money. Day 4 - your day will be perfect. Day 5 - the person u like the most in your life will spend lots of time with you. If you don’t forward this, your next 5 days will be the exact
What is the mass of 6.02 x 1024 molecules of the compound HCl?
Answers
Answer:
First, we need to determine the molar mass of HCl.
The molar mass of HCl = the mass of hydrogen (1.008 g/mol) + the mass of chlorine (35.45 g/mol) = 36.45 g/mol.
Next, we can use Avogadro's number (6.02 x 10^23 molecules/mol) to convert the number of molecules to moles:
6.02 x 10^24 molecules / 6.02 x 10^23 molecules/mol = 10 moles
Finally, we can use the molar mass to convert moles to grams:
10 moles x 36.45 g/mol = 364.5 grams
Therefore, the mass of 6.02 x 10^24 molecules of HCl is 364.5 grams.
:. It means 1 mole of Hcl
:. To find the mass of HcL
no of moles = mass/ molar mass
To get the molar mass of HCL {H=1 CL=35.5}
:. H+CL = 1+ 35.5 =36.5
So we have our molar mass and number of moles now
Then we input it in the eqn
1=x/36.5
X= 36.5g of HCl
Which metal can be extracted from bauxite ore?
Answers
Bauxite ore is the world's primary source of aluminum. The ore must first be chemically processed to produce alumina (aluminum oxide). Alumina is then smelted using an electrolysis process to produce pure aluminum metal. Bauxite is typically found in topsoil located in various tropical and subtropical regions.
Answer:
Aluminum can be extracted from bauxite ore.
In Bohr's model, what does the period and the groups represent?
Answers
Explanation:
The atomic number of each element is written above the symbol. A period is a horizontal row of the periodic table. ... A group is a vertical column of the periodic table, based on the organization of the outer shell electrons. There are a total of 18 groups.
Question 4 (1 point)
If the decomposition of (NH4)2(CO3) is a first-order process with a rate constant of
0.196 s-1, how much ammonium carbonate would remain after 39.0 s, starting from
a concentration of 0.957 M?
Your Answer in units:
Answers
The final concentration of the reactant of a first order reaction can be determined from the rate constant equation. The concentration of ammonium carbonate after 39 s will be 0.003 M.
What is rate constant?Rate constant of a reaction is the rate of reaction when one molar concentration of the reactant is involved in the reaction. The expression for the rate constant k for first order reaction is :
k = 1/t ln (C0/Ct)
Where C0 be the initial concentration and Ct be the concentration after t seconds.
Given that C0 of ammonium nitrate = 0.957 M
rate constant = 0.196 /s
t = 39 s.
The concentration after 39 seconds is calculated as follows:
0.196 /s = 1/39s ln (0.957 M / Ct)
Ct = 0.957 / (ln⁻¹ (0.196 × 39))
= 0.003 M.
Therefore, the concentration of ammonium carbonate after 39 seconds will be 0.003 M.
To find more on rate constant, refer here:
https://brainly.com/question/20305871
#SPJ1
Why do you think it is necessary to break up the fat into tiny droplets?
How could you explain why soap is able to clean the oil and dirt off our bodies?
Answers
Answer:
for the first question, the context is necessary in order to answer...
Fat is immiscible (does not mix) with water because fat is nonpolar and water is polar (remember like dissolves like: polar mixes with polar, nonpolar with nonpolar).
soap bubbles by having many soap molecules surround fat molecules with their nonpolar end, and direct their polar ends outwards.
For your second question:
Dirt and oil are nonpolar. When we wash our bodies with water (which is polar) it does not do a good job in removing these nonpolar molecules. Soap, on the other hand, has a long nonpolar end and a small polar end. The nonpolar end of the soap molecule attaches to the dirt and oil on your skin and when the water from the shower head hits the soap, it pulls the dirt, oil, and soap off of you by attaching itself to the polar end of the soap molecule.
Explanation:
Does anyone know Chemistry
Answers
Answer:
so so
Explanation:
this your question?? <_>
How many moles of Ne contain 61,000,000 atoms?
Answers
Answer:
1.01×10⁻¹⁶ moles (to 3 significant figures)
Explanation:
Using avocado's constant, we can use it to find the number of moles (x) for 61,000,000 atoms of Ne:
1 mole = 6.02×10²³ atoms
x = 61,000,000 atoms
We can solve this by cross-multiplying:
6.02×10²³ × x = 1 × 61,000,000
6.02×10²³x = 61,000,000
To solve for x, divide both sides by 6.02×10²³x:
\( \frac{6.02×10²³x}{6.02×10²³} = \frac{61,000,000}{6.02×10²³ } \)
This cancels out both 6.02×10²³ on the left
\(x = \frac{61,000,000}{6.02×10²³ } \)
\(x = 1.01 \times 10⁻¹⁶\)
A 100. g strip of alloy containing iron and copper in equal amounts is placed in a burner that supplies 4.0 kJ of heat. If the initial temperature of the alloy is 20.0 oC, what is the final temperature of the alloy? (cp for iron is 0.460 J/g oC. and cp for copper is 0.385 J/g oC).
Answers
The final temperature of a 100g strip of alloy made up of iron and copper is 67.34°C.
How to calculate temperature?Calorimetry is the science of measuring the heat absorbed or evolved during the course of a chemical reaction or change of state.
The temperature of a substance using calorimetry procedure can be calculated as follows:
Q = mc∆T
Where;
Q = quantity of heat absorbed or releasedm = mass of substance∆T = change in temperaturec = specific heat capacityAccording to this question, a 100g strip of alloy containing iron and copper in equal amounts is placed in a burner that supplies 4.0kJ of heat.
4000 = 100 × (0.46 + 0.385) × {T - 20°C}
4000 = 84.5 {T - 20°C}
4000 = 84.5T - 1690
84.5T = 5690
T = 67.34°C
Therefore, 67.34°C is the final temperature of the alloy.
Learn more about calorimetry at: https://brainly.com/question/1407669
#SPJ1
1) Aquatic algae remove huge amounts of carbon dioxide from the atmosphere. These algae live in thesurface regions of the world's oceans. If 1 acre of algae can remove 2.7 tons of carbon dioxide in a singleday, how many moles of carbon dioxide are removed in a year?
Answers
They give us the weight of carbon dioxide that removes 1 acre of algae. To determine moles we are going to convert the weight from tons to grams and then divide it by the molar mass of carbon dioxide.
We take in count following:
1ton=1x10^6g
1molCO2=44.01g
1year=365days
So, the moles that 1 acre of algae removes in a year will be:
\(molCO_2=2.7\frac{tonCO_2}{day}\times\frac{10^6g}{1ton}\times\frac{1molCO_2}{44.01gCO_2}\times\frac{365day}{1year}\)\(molCO_2=2.2\times10^7molCO_2\)Answer: In a year, are removed 2.2 x10^7 moles of carbon dioxide
Calculate the mass of HCl that is contained in 54.0 mL of an aqueous HCl solution that has a density of 1.19 g cm^–3 and contains 37.21% HCl by mass. Use the correct number of significant figures in your answer.
Answers
Answer:
Explanation:
Since density = mass/volume
Mass of solution = density x volume = (1.19)(54.0) = 64.26 g
Mass of HCI = (64.26)(0.3721) = 23.9 g
_______ occurs when a body’s molecular wavelength sends vibrations to another body, resulting in the production of another sound wave
Answers
Answer:
Natural frequency
Explanation:
Answer:
Resonance
Explanation:
PLS HELP
Which of the following atoms is smallest? Vanadium, Chromium or Tungsten
Which has the highest ionization energy?
Answers
I need help on this Chem problem

Answers
Four Hydrogen atoms are present in the molecule (Furan) shown below.
Furan is a heterocyclic organic compound with a five-membered ring containing four carbon atoms and one oxygen atom. It has the chemical formula C4H4O. Furan is a colorless, volatile liquid with a distinctive aromatic odor. Coal tar and organic material burning make it.
Furan is utilised in resins, polymers, and solvents. Furan is poisonous and carcinogenic, hence its use and exposure must be carefully controlled.
Learn more about Furan, here:
https://brainly.com/question/29753641
#SPJ1
Please help me please I beg someone please help me !!!

Answers
Answer:
Object 1 = Moving Towards Earth
Object 2 = Moving Away From Earth
Object 3 = Moving Towards Earth
Explanation:
When you look at emission spectras, look at where the observed lines are in comparison to the element (stationary) lines. Ones that have observed lines closer to the left are moving towards you and ones with observed lines closer to the right are moving away from you.
Lab 7 questions for disturbing equilibrium
1. What is the color of bromosphenol blue in an acid? In a base?
2. How did you get the solution to change from blue to yellow?
3. Why did the solution change from blue to yellow (what chemical reaction happened?)
4. How did you get the solution to change from yellow to blue?
5. Why did the solution change from yellow to blue (what chemical reaction happened?)
6. After procedure step 3 the solution in well A1 was in equilibrium. what happened to the solution in well A1 during procedure step 5? did the equilibrium shift?
Answers
Based on the nature of the indicator Bromophenol blue,
it is yellow in an acid and blue in a base.Adding an acid will change the blue colour to yellow.On addition of an acid, hydrogen ion concentration increases, equilibrium shifts towards removal of excess hydrogen ions and the solution will turn yellow.The solution can be made to change from yellow to blue by addition of base.On addition of a base, hydroxide ion concentration increases, equilibrium shifts towards removal of excess hydroxide ions and the solution will turn blue.equilibrium shifts towards removal of excess hydroxide ions.What is an indicator?An indicator is a organic dye which changes colour according to the pH of the medium it is present in.
Examples of indicators include:
Methyl OrangeMethyl redBromophenol blueBromophenol blue is an acid–base indicator whose range of colour change lies between pH 3.0 and 4.6.
It changes from yellow at pH 3.0 to blue at pH 4.6.
Bromophenol is yellow in an acid and blue in a base.Adding an acid will change a blue colour of bromophenol in a basic solution to yellow.On addition of an acid, hydrogen ion concentration increases, equilibrium shifts towards removal of excess hydrogen ions and the solution will turn yellow.The solution can be made to change from yellow to blue by addition of base.On addition of a base, hydroxide ion concentration increases, equilibrium shifts towards removal of excess hydroxide ions and the solution will turn blue.When hydroxide ion is added to the solution in step 3, equilibrium shifts towards removal of excess hydroxide ions.Therefore, Bromophenol blue is a useful indicator between pH range 3.5 ⇌ 4.6.
Learn more about indicators at: https://brainly.com/question/1820795
Answer:
1. It is blue in a base and yellow in an acid
2. Additional acid will change the blue color into a yellow color.
3. The acid included has hydrogen ions that provide concentration that increases and makes the equilibrium shift towards it which removes the excess hydrogen ions and causes the solution to become yellow.
4. The addition of the base causes the yellow to go to a blue color.
5. Hydroxide ion concentration means the solution will become blue as well.
6. The equilibrium will become blue since it has th reoccurring change at the end of the lab.
Explanation:
Hope this helps, have a great summer!
Which sample represents a solution?

Answers
Tyndall effect is the scattering of light by colloidal particles. Colloids are mixtures in which one substance is dispersed evenly throughout another substance.
Solutions are a type of mixture that appear homogeneous and do not exhibit the Tyndall effect. On the other hand, suspensions and colloids do not appear homogeneous and do exhibit the Tyndall effect.
Based on the information given, Sample 1 shows yes for both filtering and settling, which means it is a heterogeneous mixture, and therefore not a solution.
Sample 2 does not show the Tyndall effect and does not settle, which means it is a homogeneous mixture, and therefore a solution. Sample 3 shows the Tyndall effect but does not settle, which means it is a heterogeneous mixture, and therefore not a solution.
Therefore, the sample that represents a solution and does not exhibit the Tyndall effect is Sample 2, and the answer is A. Sample 2.
To know more about Tyndall effect, visit :
https://brainly.com/question/23487849
#SPJ1
someone pls help T-T
100 points

Answers
Layer 1 because it is closest to the surface
Define exothermic and endothermic. What are the mathematical signs of the internal energy and enthalpy when a process is exothermic?
Answers
Exothermic refers to chemical interactions that aerobic respiration. Combustion reactions release higher energy. Endothermic refers to atoms and molecules that either use or absorb reactive power.
What is an exothermic explanation?A chemical process known as an endothermic releases energy as heat or light. It is an endothermic reaction's opposite. Chemical equation expressed as reactants + products + energy. An reaction mechanism is one in which electricity is given off as light or warmth.
Exothermic example: What is it?A response is deemed to be exothermic if it produces heat while also undergoing a net decrease in basic enthalpy change. Samples include those type of combustion, iron rust, including water froze. Exothermic processes are those that discharge heat and energy into the surroundings.
To know more about exothermic visit:
https://brainly.com/question/13243759
#SPJ1
A gas has a volume of 50.0 mL at a temperature of 10.0 K and a pressure of 760. kPa. What will be the new volume when the temperature is changed to 20.0 K and the pressure is changed to 380. kPa?
Answers
To solve this problem using the gas laws, we need to use the Ideal Gas Law. This law states that the product of the pressure and the volume of a gas is proportional to the absolute temperature.
The equation of the Ideal Gas Law is the following:
\(\boxed{\large\displaystyle\text{$\begin{gathered}\sf \bf{\dfrac{P_1V_1}{T_1}=\frac{P_2V_2}{T_2} } \end{gathered}$} }\)
Where:
P₁ = initial pressure = 760 kPaV₁ = initial volume = 50.0 mL = 0.050 LT₁ = initial temperature = 10.0 KP₂ = Final pressure = 380 kPaT₂ = final temperature = 20.0 KV₂ = Final volume = ?We clear for V₂:
\(\boxed{\large\displaystyle\text{$\begin{gathered}\sf \bf{V_2=\frac{P_1V_1T_2}{P_2T_1 } } \end{gathered}$} }\)
Where:
P₁ = initial pressure V₁ = initial volumeT₁ = initial temperatureP₂ = Final pressureT₂ = final temperatureV₂ = Final volumeSubstituting the known values:
\(\boxed{\large\displaystyle\text{$\begin{gathered}\sf \bf{V_2=\frac{760\not{kPa}\times0.050 \ L\times20.0\not{k} }{ 380\not{kPa}\times10.0\not{k} } } \end{gathered}$} }\)
\(\boxed{\large\displaystyle\text{$\begin{gathered}\sf \bf{V_2=\frac{760 \ L}{3800 } } \end{gathered}$} }\)
\(\boxed{\boxed{\large\displaystyle\text{$\begin{gathered}\sf \bf{V_2\approx0.2 \ Liters} \end{gathered}$} }}\)
When the temperature changes to 20.0 K and the pressure changes to 380 kPa, the new volume will be approximately 0.2 L (200.0 mL).what two-step process separated the cans into aluminum, steel, and tin?
Answers
Answer:
Magnetivity and melting point.
Explanation:
Aluminum, steel and tin cans can be separated by two step process of magnetisation and melting point, because the three cans have different magnetic properties.
Steel attract to magnet easily because of it's has magnetic properties and these separate steel from aluminum.
Neither steel and aluminum melted at 300°C but Tin melt at that temperature.
What the volume of 59.2 grams of number 94. The density of plutonium is 19.8 g/cm^ 3
A) 377 cm^3
B) 0.0537 cm^3
C) 2.99 cm^3
D) 3.33 cm^3
Answers
Answer:
C) 2.99 cm^3
Explanation:
ρ=m/V
V=m/ρ=59.2/19.8=2.99 cm^3
hellooooooooooooooooooooo
Answers
Answer:
hiiiiiiiiiiiiiiiiiiiiiiiii THANK YOU SO MUCH FOR THE POINT
WIL GIVE AS MANY POINTS POSSIBLE!!!!!! A. A U.S. state needs to increase its available electricity. Almost half of the state
is covered in desert, and there are strong winds most of the year. There are no
large or fast-moving rivers, and very few crops are grown.
i. Name two renewable energy sources, and state and explain whether the
state should use them based on the description above. (6 points)
1
ii. If the state wants to minimize environmental damage, what energy sources
should it consider using? Explain your position. (2 points)
Answers
it takes specific amount of energy to remove exactly one electron from an atom.
What is electron?
Electron is a stable sub atomic particle with a charge of negative electricity found in all atoms and acting as the primary Carrier of electricity in solid.
1-This is shown by the equation where the orbit of the electron around a proton is equal to the quantum. The quantities energy level, times a wavelength.
2-So this is this equation and then abruptly hypothesis links quantities. Energy states with a standing wave function so as that the energy of the election on increases its orbit must also increase.
3- And as considering the wavelength, we're assuming station is constant. Two pies are the only constants, and they aren't changing. It does.
4-As the energy or quantifies energy level of an electron increases its radius around the nucleus must increase.
5- Also, if its energy level decreases, its radius must also decrease. So this hypothesis ties together quantities, energy levels and the relationship and its relationship with the size of the circumference around it. A nucleus which is also the electron.
To know more about electron click-
https://brainly.com/question/25674345
#SPJ1
How many moles of O2 did you produce the 8.31 moles of H2O
Answers
the equation is not present.
How many atoms are in 12 g of Carbon-12 (12C)?
Answers
There are approximately 6.022 × 10^23 atoms in 12 grams of Carbon-12 (12C).
The number of atoms in a given amount of a substance can be calculated using Avogadro's number, which represents the number of atoms or molecules in one mole of a substance. Avogadro's number is approximately 6.022 × 10^23.
Carbon-12 is a specific isotope of carbon, with an atomic mass of 12 atomic mass units (amu). One mole of Carbon-12 has a mass of 12 grams. Since one mole of any substance contains Avogadro's number of particles, in the case of Carbon-12, it contains 6.022 × 10^23 atoms.
Therefore, if we have 12 grams of Carbon-12, which is equal to one mole, we can conclude that there are approximately 6.022 × 10^23 atoms in this amount of Carbon-12.
In summary, 12 grams of Carbon-12 contains approximately 6.022 × 10^23 atoms. Avogadro's number allows us to relate the mass of a substance to the number of atoms or molecules it contains, providing a fundamental concept in chemistry and enabling us to quantify and understand the microscopic world of atoms and molecules.
for such more questions on atoms
https://brainly.com/question/6258301
#SPJ8
What mass of sodium hydroxide, NaOH, would be required to produce 16 g of the antacid milk of magnesia [magnesium hydroxide, Mg(OH)2] by the following reaction? MgCl2(aq) + 2NaOH(aq) ⟶ Mg(OH)2(s) + 2NaCl(aq)
Answers
It would take 22g of sodium hydroxide (NaOH) to make 16g of the antacid milk of magnesia (magnesium hydroxide).
Simply put, what is stoichiometry?In the field of chemistry known as stoichiometry, desired quantitative data is ascertained by using relationships between the reactants and/or products of a chemical reaction. Stoichiometry literally translates as the measure of elements because the Greek words stoikhein and metron both mean element and measure, respectively.
What is the stoichiometric law?In a chemical reaction, the total mass of reactant and product are equal, according to the statement, and neither is generated nor destroyed. This is the stoichiometric law, and also the law of conservation of mass.
\(16 \mathrm{~g} \text { of } \mathrm{Mg}(\mathrm{OH})_2 \times \frac{1 \mathrm{molMg}(\mathrm{OH})_2}{58.3 g \mathrm{gg}(\mathrm{OH})_2} \times \frac{2 \mathrm{~mol} \mathrm{NaOH}}{1 \mathrm{molMg}(\mathrm{OH})_2} \times \frac{40 \mathrm{gNaOH}}{\mathrm{molNaOH}}=22 \mathrm{~g}\)
To learn more about Stoichiometry visit:
brainly.com/question/29775083
#SPJ1
Why are many chemical names so complex?
Answers
Answer:
cause they like it that way
Explanation:
btw cant tell if this is legit or not
how many moles of NaOH in 125.0 mL of 0.190M NaOH?
Answers
Answer:
0.0238 moles NaOH
Explanation:
To find the amount of moles, you need to
(1) convert the volume from mL to L (1,000 mL = 1 L)
(2) calculate the amount of moles (using the molarity equation)
It is important to arrange the conversions in a way that allows for the cancellation of units. The final answer should have 3 sig figs.
125.0 mL NaOH 1 L
--------------------------- x --------------------- = 0.1250 L NaOH
1,000 mL
Molarity = moles / volume (L) <----- Molarity equation
0.190 M = moles / 0.1250 L <----- Insert values
0.0238 = moles <----- Multiply both sides by 0.1250