How many levels of biological classification are represented in the taxonomic biological name for an organism.
Answers
Answer:
There are two Two level represented in biological name....
Explanation:
There are two level of classification represented in biological name. Genus and specie.....
Related Questions
(b) The chemical equation for the reaction between lithium and oxygen is 4Li + 0,2L1,0 Write a chemical equation for the reaction between lithium and nitrogen
Answers
Answer:
Li3N
Explanation:
Li+N2=Li3N.........................
Answer:
4 Li(s)+O2(g) → 2 Li2O(s)
Explanation:
its right
(13)
QUESTION 5
5.1
Sulphur reacts with iron according to the equation:
Fe +S → FeS
The reaction requires large amount of heat
When 2,5g sulphur and 5 g iron were placed in a test tube and heated strongly, a
reaction took place to produce a greyish black solid. After the reaction was completed
the solid was found to be magnetic.
5.1.1 Why was this greyish black solid magnetic?
5.1.2 Calculate the mass of Fos produced
Answers
Answer:
The black solid is magnetic due to the special property of iron called ferromagnetism
6.9 g
Explanation:
The substance FeS is magnetic despite of the fact Fe^2+ is spin paired due to the special magnetic property of iron called ferromagnetism.
The equation of the reaction is;
Fe(s) + S(s) ----> FeS(s)
Number of moles of Fe = 5g/56g/mol = 0.089 moles
Since the reaction is 1:1, 0.089 moles of FeS is formed
For Sulphur;
Number of moles of sulphur = 2.5g/32 g/mol = 0.078 moles
Since the reaction is 1:1, 0.078 moles of FeS is formed
Hence sulphur is the limiting reactant.
So, mass of FeS produced = 0.078 × 87.91 g/mol = 6.9 g
Hydrogen gas and oxygen gas react to produce water. Both of these gases are present in
the air around us -so, how come water isn't being produced all around u?
Answers
Water isn't being produced all around us because the hydrogen and oxygen are present in stable form.
How come water isn't being produced all around us?Water molecule is produced when the unstable hydrogen reacts with unstable oxygen because they are very reactive. Due to their reactive nature, hydrogen and oxygen react with each other forming water molecule. So that's why we can say that water is not being produced around us due to stable form of hydrogen and oxygen.
So we can conclude that Water isn't being produced all around us because the hydrogen and oxygen are present in stable form.
Learn more about water here: https://brainly.com/question/1313076
#SPJ1
Please help,due in one hour I only need the number of atoms of each element

Answers
Answer:
Sodium sesquicarbonate
Sodium carbonate, Na2CO3, (also known as washing soda, soda ash and soda crystals) is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, water-soluble salts that yield moderately alkaline solutions in water. Is surrounded roughly tetrahedrally by four atoms, two sodiums and two oxygens. As commonly observed in hydrate structures, the water oxygen is surrounded in this way by two cations and two anions, and in this matter sodium sesquicarbonate obeys the usual rule.
Sodium (Na), chemical element of the alkali metal group (Group 1 [Ia]) of the periodic table. Sodium is a very soft silvery-white metal.
Sodium is a chemical element with the symbol Na (from Latin "natrium") and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable isotope is 23Na.
The common name for the chemical compound with the formula 3H2O is water. The H2O part is the formula for water. The 3 means that there are 3 molecules of water.
3H2O has 6 hydrogen atoms and 3 oxygen atoms.
Carbon monoxide (Co)
Carbon monoxide is a colorless, odorless gas. Prolonged exposure to carbon monoxide rich atmospheres may be fatal. It is easily ignited. It is just lighter than air and a flame can flash back to the source of leak very easily. Under prolonged exposure to fire or intense heat the containers may violently rupture and rocket.
Carbon monoxide is a colorless, odorless, tasteless gas. A molecule of carbon monoxide (CO) contains one carbon atom and one oxygen atom.
Iron oxide or ferric oxide is the inorganic compound with the formula Fe2O3. It is one of the three main oxides of iron, the other two being iron(II) oxide (FeO) the rarer form, and iron(II,III) oxide (Fe3O4) which naturally as magnetite. Since atoms are not divisible into halves, we should instead say that in the ferric molecule there are 3 oxygen atoms for every 2 iron atoms. Thus, (Fe is the symbol for an iron atom, and O is the symbol for an oxygen atom) the ferrous oxide molecule is FeO, and the ferric oxide molecule is Fe2O3.
Aluminium hydroxide, Al(OH)₃, is found in nature as the mineral gibbsite and its three much rarer polymorphs: bayerite, doyleite, and nordstrandite. Aluminium hydroxide is amphoteric in nature, i.e., it has both basic and acidic properties. 1 atom.
Hope this is what you are looking for!
Explanation:
Brainliest please?
En una botella hay 2x10 25 moléculas de vinagre puro ¿Cuántos mol y cuántos gramos de esta sustancia habrá en la botella?
Answers
Answer:
33.2 moles y 1994 gramos.
Explanation:
¡Hola!
En este caso, dado que es posible para nosotros relacionar moles con gramos por medio de la masa molar y moléculas con moles por medio del número de Avogadro, resulta factible para nosotros primero calcular las moles en las moléculas dadas y de esta manera luego calcular los gramos, teniendo en cuenta una masa molar de 60.05 g/mol ya que el vinagre se puede estudiar como ácido acético (CH3COOH):
\(2x10^{23}molec*\frac{1mol}{6.022x10^{23}molec}=33.2mol\)
Seguidamente, calculamos los gramos:
\(33.2mol*\frac{60.05g}{1mol}=1994g\)
¡Saludos!
help me please id appreciate it

Answers
Answer:
Thats the periodic table of elements
Explanation:
Give an example of gravitational potential
Answers
A raised weight.
Water that is behind a dam.
Have a nice day!
Answer:
When something has a high position, its gravitational potential energy is high. For example, a book on a high bookshelf has higher potential energy than a book on the bottom shelf because it has farther to fall. ... A book on a table before it falls. A child at the top of a slide.
Explanation:
U=m g h
sodium, and potassium react violently with water. the reaction becomes more explosive as you move from top to bottom down the group. what can you conclude about the rate laws for these reactions as you move down the group from lithium to potassium?
Answers
As you move down the group from lithium to potassium, the rate of the reaction between sodium and potassium with water increases. This suggests that the rate laws for these reactions change as you move down the group. Specifically, the rate of reaction is likely to be dependent on the concentration of the alkali metal and the concentration of water.
The more reactive metals such as sodium and potassium have a greater affinity for water, leading to a more explosive reaction. Therefore, the rate of reaction is likely to increase as you move down the group due to the increased reactivity of the metals. as you move down the group from lithium to potassium, the reaction with water becomes more explosive. This implies that the rate of reaction increases. The rate laws for these reactions can be concluded as follows:
1. The rate of reaction is directly proportional to the concentration of alkali metals (sodium and potassium in this case) and water.
2. As you move down the group from lithium to potassium, the reactivity of alkali metals increases. This is due to the increase in the size of the atom and the decrease in ionization energy, which makes it easier for the outermost electron to be lost.
3. Therefore, the rate constant (k) in the rate laws for these reactions increases as you move down the group.
In summary, the rate laws for the reactions of sodium and potassium with water indicate that the rate of reaction increases as you move down the group from lithium to potassium, due to an increase in reactivity resulting from atomic size and ionization energy factors.
Learn more about potassium here
https://brainly.com/question/13321031
#SPJ11
As you move down the group from lithium to potassium, the rate of reaction between sodium and potassium with water increases, resulting in a more explosive reaction.
This can be concluded from the fact that the rate laws for these reactions become more favorable as you move down the group. The increased reactivity can be attributed to the lower ionization energies and larger atomic radii of the alkali metals, making it easier for them to lose electrons and react with water.This suggests that the rate laws for these reactions change as you move down the group, with the rate increasing significantly. Additionally, it is important to note that the increase in rate is likely due to an increase in the reactivity of these alkali metals with water, as well as an increase in the size and mass of the atoms themselves.
Learn more about reactivity here: https://brainly.com/question/30843855
#SPJ11
the ph of a 0.124m solution of weak base is 10.89 at 25C. calculate the ph of a 0.040 M solution of base at 25
Answers
he pH of a 0.040 M solution of base at 25°C is:pH = 14 - pOH, pH = 14 - 1.15, pH = 12.85
The pH of a 0.124M solution of a weak base is 10.89 at 25C. The task is to calculate the pH of a 0.040M solution of the base at 25°C.
Let us assume that the weak base is represented by B and its dissociation in water is given by:
B + H2O ↔ BH+ + OH-
For any weak base B, the base dissociation constant Kb is given by:
Kb = [BH+] [OH-] / [B]
Where, [B] represents the initial concentration of the weak base and [BH+] and [OH-] represent the concentrations of the conjugate acid and hydroxide ion, respectively, at equilibrium.
The relationship between the pOH and OH- concentrations can be represented by:
pOH = -log [OH-]
Similarly, the relationship between pH and H+ concentration is:
pH = -log [H+]
However, for any aqueous solution:pH + pOH = 14
Therefore, pH = 14 - pOH
Hence, in the given problem, we can use the following formula: pH = 14 - pOH
Kb = [BH+] [OH-] / [B]
The pOH of the given 0.124M solution of weak base is:
pOH = 10.89
pH = 14 - 10.89
pH = 3.11
Let x be the concentration of OH- ions in 0.040M solution of the base, then:
[OH-] = x Kb = [BH+] [OH-] / [B]
0.124 = x^2 / 0.040
x^2 = 0.124 * 0.040
x^2 = 0.00496
x = √0.00496
x = 0.0702
Therefore, the concentration of OH- ions in 0.040M solution of the base is 0.0702 M
The pOH of the solution would be:
pOH = -log [OH-]
pOH = -log (0.0702)
pOH = 1.15
Therefore, the pH of a 0.040 M solution of base at 25°C is:
pH = 14 - pOH
pH = 14 - 1.15
pH = 12.85
To learn more about Concentration visit;
brainly.com/question/3045247
#SPJ11
2.) the lym.an series is brighter than the balmer series because this series of transitions ends up in the most common state for hydrogen, the ground state. why then was the balmer series discovered first?
Answers
The balmer series for hydrogen was discovered first because it lies in the visible domain of the spectrum, although the lym.an series is brighter because it exhibits a series of transitions in its more common state.
What is the balmer series?It is the set of lines derived from the emission of the hydrogen atom when an electron transits from a level n ≥ 3 to n = 2. n represents the principal quantum number referring to the energy level of the electron.
What is the lyman or lym series?It is the set of lines produced by the emission of the hydrogen atom when an electron transits from n ≥ 2 to n = 1.
There is another series called Paschen series, which corresponds to a level less than or equal to 3
Learn more about the balmer series at https://brainly.com/question/14915323
#SPJ4

how are solar eruptions influenced by the sunspot cycle
Answers
changes in the Sun's magnetism produce a greater number of sunspots, more energy and cause solar eruptions of particles
Green plants use light from the Sun to drive photosynthesis. Photosynthesis is a chemical reaction in which water and carbon dioxide chemically react to form the simple sugar glucose and oxygen gas . What mass of carbon dioxide is consumed by the reaction of of water? Round your answer to significant digits.
Answers
3.0g mass of carbon dioxide is consumed by the reaction of water.
What are moles?A mole is defined as 6.02214076 × \(10^{23}\) of some chemical unit, be it atoms, molecules, ions, or others. The mole is a convenient unit to use because of the great number of atoms, molecules, or others in any substance.
The reaction equation is given as:
6CO₂ + 6H₂O → C₆H₁₂O₆ + 6O₂
Parameters that are known:
Mass of CO₂ used = 7.3g
Unknown: mass of water consumed =?
Solution:
To solve this kind of problem, we simply apply some mole concept relationships.
First, we work from the known to the unknown. From the problem, we have 7.3g of CO₂ that was used. We can find the number of moles from this value using the expression below:
Number of moles of CO₂ = \(\frac{mass}{molar \;mass}\)
From this number of moles of CO₂, we can use the balanced equation to relate the number of moles of CO₂ to that of H₂O:
6 moles of CO₂ reacted with 6 moles of H₂O(1:1)
We can then use the mole relationship with mass to find the unknown.
Workings
A number of moles of CO₂ =?
The molar mass of CO₂ :
The atomic mass of C = 12g
The atomic mass of O = 16g
Molar mass of CO₂ = 12 + (2 x16) = 44gmol⁻¹
Number of moles of CO₂ = = 0.166moles
If 6 moles of CO₂ reacted with 6 moles of H₂O, then 0.166moles of CO₂ would produce 0.166moles of H₂O
Mass of water consumed = number of mole of H₂O x molar mass
Mass of H₂0 = 0.166 x ?
The molar mass of H₂O:
The atomic mass of H = 1g
The atomic mass of O = 16
Molar mass of H₂O = (2x1) + 16 = 18gmol⁻¹
Mass of H₂O = 0.166 x 18 = 3.0g
Hence, 3.0g mass of carbon dioxide is consumed by the reaction of water.
Learn more about moles here:
https://brainly.com/question/8455949
#SPJ1
what is the role of the secondary coolant in a nuclear power plant?
Answers
The secondary coolant is converted to steam, which runs the steam turbine to generate electricity.
Secondary coolant:
creates steam to turn a turbine and generate electricityfluid is cooled by a condenser and recycleddoes not contact the reactorThe reactor coolant flows from the reactor to the steam generator. Inside of the steam generator, the hot reactor coolant flows inside of the many tubes. The secondary coolant, or feedwater, flows around the outside of the tubes, where it picks up heat from the primary coolant.
The secondary system is designed to transport heat from primary system to the atmosphere via an evaporative cooling tower. The typical system is designed to furnish 12.6 m3/min of water to the plate type heat exchanger at an inlet temperature of about 33 °C and an outlet temperature of about 42 °C.
Learn more about secondary coolant here:
https://brainly.com/question/29472057
#SPJ4
What is the answer for 250 K = ? °C
Answers
Answer:
This is your answer ☺️☺️☺️

Answer:
-23.15
Explanation:
Which sample is a pure substance? zinc oxide sugar dissolved in water pond water soil
Answers
Answer:
Its (A) zinc oxide
Explanation:
just took the quiz
Among the given option, Zinc Oxide is a Pure substance.
What is Pure Substance ?A substance that has a fixed chemical composition throughout is called a pure substance such as water, air, and nitrogen.
A pure substance does not have to be of a single element or compound.
Hence, Among the given option, Zinc Oxide is a Pure substance.
Learn more about pure substance here ;
https://brainly.com/question/24462192
#SPJ2
the chemical equation for reduction of phosphorite Ca3(PO4)2
Answers
Answer:
The chemical equation is;
Ca3(PO4)2 + 8C ———> Ca3P2 + 8CO
Explanation:
Here, we are interested in writing a chemical equation that is useful in the reduction of phosphorite.
We should understand that to be able to reduce phosphorite, there is need for a reducing agent.
Solid carbon can be used here.
The products formed are shown in the equation as follows;
Ca3(PO4)2 + 8C ———> Ca3P2 + 8CO
Distinguish between pure and applied science.
Answers
Answer:
pure science is the study of nature and its environment while applied science is the acquisition of scientific knowledge from pure science to solve practical problems
Porrrrrrffffffaaaaaavvvvvvooooorrrrrrr

Answers
Answer:
2.2)solido
2.3)Sólido cristalino
2.4)Sólido amorfo
How many atoms are there in 3.3 moles of strontium?
Answers
Explanation:
Multiply the moles Fe by 6.022×1023 atoms/mol
Using examples, explain which electrochemistry technology you think is the most cost efficient.
Answers
Among various electrochemistry technologies, lithium-ion batteries are considered the most cost-efficient due to their widespread use, decreasing prices, and high energy density.
Lithium-ion batteries have emerged as the dominant technology for energy storage in portable electronics, electric vehicles, and renewable energy systems. They offer a combination of high energy density, long cycle life, and relatively low self-discharge rates compared to other electrochemical technologies. These factors make them highly cost-efficient in a variety of applications.
One example of the cost efficiency of lithium-ion batteries can be seen in the electric vehicle (EV) market. Over the years, advancements in lithium-ion battery technology and increased production scale have led to significant cost reductions. This has resulted in a decline in the prices of EVs, making them more accessible to consumers. The cost efficiency of lithium-ion batteries has also been demonstrated in the renewable energy sector. Energy storage systems based on lithium-ion batteries allow for efficient integration of intermittent renewable energy sources, such as solar and wind power, into the grid. This helps stabilize the grid and reduce reliance on fossil fuels.
Furthermore, the high energy density of lithium-ion batteries enables compact and lightweight designs, making them suitable for portable electronics like smartphones and laptops. This not only enhances user convenience but also contributes to cost efficiency by reducing material and transportation costs. Additionally, the long cycle life of lithium-ion batteries ensures durability and longevity, further enhancing their cost efficiency as they require fewer replacements over their lifespan.
Learn more about lithium-ion batteries here:
https://brainly.com/question/13651147
#SPJ11
10.0 g of acetic acid (ch3cooh) is dissolved in a 500.0 ml solution. what is the molarity?
Answers
The molarity of the acetic acid solution is 0.332 M.
To calculate the molarity of the acetic acid (CH3COOH) solution, follow these steps:
1. Determine the molecular weight of acetic acid. The molecular weights of C, H, and O are 12.01 g/mol, 1.01 g/mol, and 16.00 g/mol, respectively. For CH3COOH, the molecular weight is (2 × 12.01) + (4 × 1.01) + (2 × 16.00) = 24.02 + 4.04 + 32.00 = 60.06 g/mol.
2. Convert the mass of acetic acid to moles. Divide the given mass (10.0 g) by the molecular weight (60.06 g/mol):
10.0 g / 60.06 g/mol = 0.166 moles of acetic acid.
3. Convert the volume of the solution to liters. The given volume is 500.0 mL, which is equal to 0.500 L (since 1 L = 1000 mL).
4. Calculate the molarity. Divide the moles of acetic acid by the volume of the solution in liters:
0.166 moles / 0.500 L = 0.332 M (moles per liter).
learn more about molarity Refer: https://brainly.com/question/8732513
#SPJ11
Study the image.
Which phrase describes this plate boundary?
does not occur in oceans
may form rift valleys
is a type of convergent boundary
is a region where earthquakes occur
Answers
The phrase that describes the plate boundary shown is D. is a region where earthquakes occur .
What is the plate boundary shown ?A transform boundary is the name given to this tectonic plate boundary. Two or more plates slip past one another in such a border. This shift is typically accompanied by some highly active earthquakes.
At a transform plate border, the grinding motion between the plates causes shallow earthquakes, significant lateral rock displacement, and a wide zone of crustal deformation. Essentially While no new crust is produced, subducted, or formed, and no volcanoes are formed, the fault produces earthquakes.
Find out more on plate boundaries at https://brainly.com/question/22005072
#SPJ1
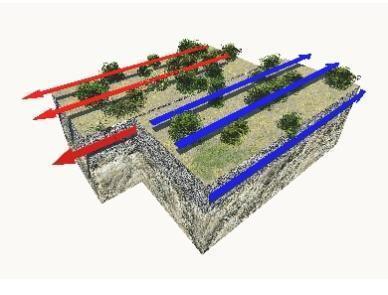
Plz help!
What is 1 item that is an element?
Give an example of how the 1 item is an element
Answers
Answer:
its a
Explanation:ok
The mass ratio of hydrogen to oxygen in water (formula H20) is 0.126 g hydrogen for every 1 g of oxygen. In another compound of hydrogen and oxygen called hydrogen peroxide, the mass ratio of hydrogen to oxygen is 0.063 g hydrogen for every 1 g of oxygen. What is a possible formula of hydrogen peroxide?
Answers
Answer: H2O2
Explanation:
1,3-Butadiene is a conjugated diene with the chemical formula C4H6true or false
Answers
It is true that 1,3-butadiene has the chemical formula C4H6 and is a conjugated diene. A popular monomer in the creation of synthetic rubber, polymers, and resins, it is a colourless, flammable gas.
A conjugated diene with the chemical formula C4H6 is 1,3-butadiene. A popular monomer in the creation of synthetic rubber, polymers, and resins, it is a colourless, flammable gas. Because of the butadiene's conjugated double bond system, pi-electrons are delocalized over the whole molecule, giving it special characteristics including high reactivity, a low boiling point, and a propensity for addition reactions. Butadiene is a crucial industrial chemical that is created by the catalytic dehydrogenation of butene or butane. It finds usage in a variety of petrochemical, polymer, and automotive applications.
learn more about chemical formula here:
https://brainly.com/question/29031056
#SPJ4
What volume of carbon dioxide gas is produced at STP when 125 mL of a 0.10 M nitric acid solution reacts with excess calcium carbonate
Answers
The volume of carbon dioxide gas produced at STP when 125 mL of a 0.10 M nitric acid solution reacts with excess calcium carbonate is 140 mL.
The balanced chemical equation for the reaction between nitric acid and calcium carbonate is:
2HNO3(aq) + CaCO3(s) → Ca(NO3)2(aq) + CO2(g) + H2O(l)
From the equation, we can see that 2 moles of nitric acid react with 1 mole of calcium carbonate to produce 1 mole of carbon dioxide gas.
To find the number of moles of nitric acid in 125 mL of a 0.10 M solution, we can use the formula:
moles = concentration x volume (in liters)
Converting the volume of the solution to liters:
125 mL = 0.125 L
Substituting the values into the formula:
moles of nitric acid = 0.10 M x 0.125 L = 0.0125 moles
Since 2 moles of nitric acid produce 1 mole of carbon dioxide gas, we can calculate the moles of carbon dioxide produced as:
moles of CO2 = 0.0125 moles of HNO3 ÷ 2 = 0.00625 moles
At STP (Standard Temperature and Pressure), 1 mole of any gas occupies 22.4 L. Therefore, the volume of carbon dioxide gas produced at STP can be calculated as:
volume of CO2 = moles of CO2 x 22.4 L/mol = 0.00625 mol x 22.4 L/mol = 0.14 L or 140 mL (rounded to 2 significant figures)
For more such questions on carbon dioxide
https://brainly.com/question/25385913
#SPJ11
If 21.2260 mL of 906.6430 mM H2SO4 reacts completely with FeCl3, how many grams of Fe2(SO4)3 are formed
Answers
Approximately 7.598 grams of Fe2(SO4)3 are formed when 21.2260 mL of 906.6430 mM H2SO4 reacts completely with FeCl3.
To determine the number of grams of Fe2(SO4)3 formed, we need to consider the stoichiometry of the reaction between H2SO4 and FeCl3. The balanced equation for the reaction is as follows:
H2SO4 + 2 FeCl3 → Fe2(SO4)3 + 2 HCl
From the equation, we can see that 1 mole of H2SO4 reacts with 2 moles of FeCl3 to produce 1 mole of Fe2(SO4)3.
First, let's calculate the number of moles of H2SO4. We can use the given concentration (906.6430 mM) and the volume (21.2260 mL) to find the moles of H2SO4:
Moles of H2SO4 = Concentration * Volume
Moles of H2SO4 = 906.6430 mM * 21.2260 mL * (1 L / 1000 mL) * (1 mol / 1 L)
Moles of H2SO4 ≈ 0.019 mol
Since the reaction is stoichiometric, the moles of Fe2(SO4)3 formed will be equal to the moles of H2SO4 reacted. Therefore, approximately 0.019 moles of Fe2(SO4)3 are formed.
To calculate the mass of Fe2(SO4)3, we can use its molar mass. Fe2(SO4)3 has a molar mass of 399.88 g/mol.
Mass of Fe2(SO4)3 = Moles of Fe2(SO4)3 * Molar mass of Fe2(SO4)3
Mass of Fe2(SO4)3 ≈ 0.019 mol * 399.88 g/mol
Mass of Fe2(SO4)3 ≈ 7.598 g
Therefore, approximately 7.598 grams of Fe2(SO4)3 are formed when 21.2260 mL of 906.6430 mM H2SO4 reacts completely with FeCl3.
To know more about stoichiometry, visit;
brainly.com/question/28780091
#SPJ11
Which of the following is an example of a combustion reaction?
A) Mixing of an acid and base
B) Photosynthesis in plants
C) Lighting of a Matchstick
D) Reacting sodium and chlorine
Answers
Answer:
C) Lighting a matchstick
Explanation:
Mixing an acid and base will cause a neutralization reaction, a type of double displacement.
Photosynthesis is just a series of chemical reactions, but don't deal with the use of O2, but rather it's creation.
Reacting sodium and chlorine would make NaCl in a synthesis reaction.
Answer:
C) Lighting a matchstick
Explanation:
What are 2 mixtures
Air
Orange juice
Silver
Sodium chloride
Water
Answers
Orange juice and Sodium chloride
What are the mole fraction and the mass percent of a solution made by dissolving 0.30 g of KI in 0.400 L of water (d = 1.00 g/mL)?
Answers
The mole fraction of solute exists at \($1.83 * 10^{-3}.\)
The mass percent of a solution exists at 1.66%.
How to find the mole fraction of solute and the mass percent of a solution?Mass of solute: 6.77 g of KI
Molar mass of KI = 166g/mol
Moles of solute = 6.77 g / 166 g/mol = 0.0408 moles
Density = Mass / Volume
Unit conversions:
1L = 1000mL
1kg = 1000g
Moles of solvent = 400 / 18 g/ mol = 22.2 moles
Mole fraction of solute = Moles of solute / Total moles
Total moles = Moles of solute + Moles of solvent
Total moles = 0.0408 moles + 22.2 moles
Total moles = 22.2408 moles
a) Mole fraction of solute = 0.0408 mol / 22.2408 moles
Mole fraction of solute \($$= 1.83 * 10^{-3}\)
b) Now, calculate the mass percent of a solution.
Mass of solvent + mass of solute = Mass of solution
400 g + 6.77 g = 406.77g
In 406.77 g of solution, we have 6.77 g of solute
Mass percentage \($= \frac{100*6.77}{406.77}\) = 1.66%
Therefore, the mole fraction of solute exists at \($1.83 * 10^{-3}.\)
The mass percent of a solution exists at 1.66%.
To learn more information about the "Number of moles" refer to:
brainly.com/question/5493941
#SPJ4