Answers
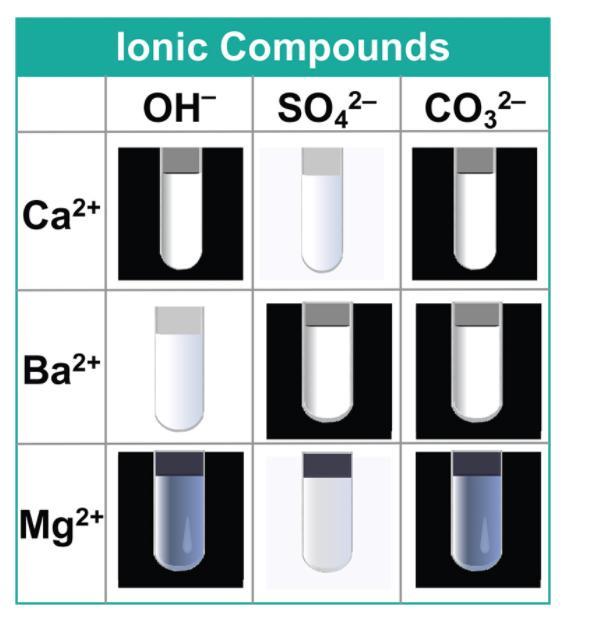
A confirmatory test of sodium hydroxide and potassium chromate can be used to distinguish between a solution of calcium ion and barium ion.
The test for cations can be used to distinguish calcium ion from barium ion.
Calcium ion (Ca²⁺) forms a white precipitate with a drop of sodium hydroxide which is insoluble in excess.Barium ion (Ba²⁺) forms a yellow precipitate with potassium chromate.Thus, a confirmatory test of sodium hydroxide and potassium chromate can be used to distinguish between a solution of calcium ion and barium ion.
Learn more about about confirmatory test of cations here: https://brainly.com/question/1969246
Related Questions
PLZ ANSWER QUICK PLZZZZZZZZZZZZZZZZZZZZZZZZZZZZZZZZZ

Answers
Answer:
D. Atom, Electron, and Proton
Explanation:
Because Atoms are the smallest and they make up everything.
because the Proton, stable subatomic particle that has a positive charge equal in magnitude to a unit of electron charge and a rest mass of 1.67262 × 10−27 kg, which is 1,836 times the mass of an electron.
Answer:
C
Explanation:
Electrons are the smallest part of matter. Protons are bigger than electrons. And the whole atom is bigger than everything inside it.
The boiling point at 1.00 atm of an aqueous solution of CaCl2 is 105.3 °C. What is the concentration of CaCl2
in the solution? Kb (H2O) = 0.512 °C/m. Assume an ideal van't Hoff factor for CaCl2.
Answers
d.2.93m.CaCl2 is present in the solution with a 2.93m concentration.
The boiling point of a solution is directly related to its concentration. The boiling point elevation of a solution, ΔTb, is equal to the product of the van't Hoff factor (i) and the molality of the solution (m).The quantity of moles of solute per kilogramme of solvent is known as molality.
Therefore, we can solve for the molality of the solution using the following equation:
ΔTb
\(= i *m\\105.3\°C= i * m\\\)
\(m =\frac{ 105.3 \°C }{i}\)
Assuming an ideal van't Hoff factor for CaCl2 (i = 2), the molality of the solution is:
\(m =\frac{ 105.3 \°C }{ 2}\\m = 52.65 m = 52.65 mol/kg\)
The concentration of CaCl2 in the solution is then:
\(C = m * Kb\\C = 52.65 mol/kg * 0.512 \°C/m\\C = 2.93 mol/kg\)
Therefore,The concentration of CaCl2 in the solution is 2.93m.
learn more about boiling point refer:brainly.com/question/24168079
#SPJ1
complete question:The boiling point at 1.00 atm of an aqueous solution of CaCl2 is 105.3 °C. What is the concentration of CaCl2 in the solution? Kb (H2O) = 0.512 °C/m. Assume an ideal van't Hoff factor for CaCl2.
a.3.45m
b.4.40m
c.8.79m
d.2.93m
Why do you think sodium bicarbonate is included to neutralize an acidic spill rather than sodium hydroxide?
Imagine a hypothetical situation in which 250 mL of diethyl ether (SDS) has spilled inside of a chemical fume hood onto a stir plate that is plugged in and stirring. Discuss the risks associated with this situation (location, size, compound spilled, and external hazards), and then explain how this spill should be managed.
Answers
Answer:
Acid spills should be neutralized with sodium bicarbonate and then cleaned up with a paper towel or sponge.
Explanation:
Cuánto es aproximadamente 29, 029 pies en millas
Answers
Seria 5.498 millas
Solo se debe dividir el valor de longitud entre 5280
Consider the reaction below: 4 NH3(g) + 5O2(g) 4 NO(g) + 6 H₂O(g) H°=-906 kJ How many moles of ammonia must react to produce 453 kJ? Show your work on a separate piece of paper or provide the answer in the space provided.
Answers
The given reaction releases 906 kJ of heat energy when 4 moles of ammonia react.
So, the amount of heat energy released when 1 mole of ammonia reacts is:
906 kJ ÷ 4 mol = 226.5 kJ/mol
How many moles of ammonia must react to produce 453 kJ?To produce 453 kJ of heat energy, we can use the following proportion:
226.5 kJ/mol = 453 kJ/x
where x is the number of moles of ammonia required.
Solving for x, we get:
x = (453 kJ × 4 mol) ÷ 906 kJ
x ≈ 2 mol
Therefore, 2 moles of ammonia must react to produce 453 kJ of heat energy.
Learn more about balanced equation from
https://brainly.com/question/28722049
#SPJ1
The given reaction releases 906 kJ of heat energy when 4 moles of ammonia react.
So, the amount of heat energy released when 1 mole of ammonia reacts is:
906 kJ ÷ 4 mol = 226.5 kJ/mol
How many moles of ammonia must react to produce 453 kJ?To produce 453 kJ of heat energy, we can use the following proportion:
226.5 kJ/mol = 453 kJ/x
where x is the number of moles of ammonia required.
Solving for x, we get:
x = (453 kJ × 4 mol) ÷ 906 kJ
x ≈ 2 mol
Therefore, 2 moles of ammonia must react to produce 453 kJ of heat energy.
Learn more about balanced equation from
brainly.com/question/28722049
#SPJ1
Write a balanced nuclear equation for the beta decay of 234/90Th

Answers
The balanced nuclear equation for the beta decay of 234/90Th can be represented as follows: ^234/90Th --> ^234/91Pa + e^0/-1β
In this equation, the nucleus of thorium-234 (234/90Th) undergoes beta decay. During beta decay, a neutron within the nucleus is converted into a proton, resulting in the emission of an electron (beta particle). As a result, the thorium-234 nucleus is transformed into protactinium-234 (234/91Pa) by gaining one proton.
The beta particle emitted during the decay process is represented as e^0/-1β, where the superscript 0 denotes that the electron has no charge (neutral), and the subscript -1 indicates that the electron carries a negative charge of -1.
It is important to note that in a nuclear equation, the total atomic mass and atomic number on both sides of the equation must be equal to maintain a balanced equation and conserve mass and charge.
For more such questions on balanced nuclear equation visit:
https://brainly.com/question/31505524
#SPJ8
Watch this video: G...
G Cotton gin - Wikipe... Copy of SOL 2 and..
2 pts
IN
Question 1
Determine Pxe in a container that has the following gases and respective pressures:
Gas
Pressure (kPa)
Xenon
?
Helium
77.3
Nitrogen dioxide
52.0
TOTAL
248.8

Answers
From the total pressure of the gaseous mixture, the partial pressure of xenon is 119.5 kPa
What is partial pressure of a gas?The partial pressure of a gas is the pressure that gas exerts when it is part of a mixture of gases which did not chemically react together.
The sum of the partial pressure of each gas in the mixture gives the total pressure.
In the gaseous mixtures above:
Total pressure = 248.8 kPa
Partial pressure of xenon = Total pressure - (Sum of partial pressure of the other gases)
Partial pressure of xenon = 248.8 - (77.3 + 52.0)
Partial pressure of xenon = 119.5
Therefore, the partial pressure of xenon is 119.5 kPa.
Learn more about partial pressure at: https://brainly.com/question/14119417
Sodium azide (NaN3) is a substance that can be used to inflate airbags. An electrical impulse causes the sodium azide to decompose, producing elemental sodium and nitrogen gas. Write the balanced chemical equation for this reaction.
Answers
Answer:
2NaN₃ → 2Na + 3N₂
Explanation:
First we use the information given by the problem to write an unbalanced equation:
NaN₃ → Na + N₂There are 3 N atoms on the left side and only 2 on the right, to remedy that we put a 2 coefficient on NaN₃ and a 3 coefficient on N₂:
2NaN₃ → Na + 3N₂Now there are 6 N atoms on both sides of the equation. What's left is to balance Na atoms:
2NaN₃ → 2Na + 3N₂The equation is now balanced.
The properly balanced chemical equation for this chemical reaction is given by \(2NaN_3 \rightarrow 2Na + 3N_2\)
What is a balanced chemical equation?
A balanced chemical equation can be defined as a chemical equation wherein the number of atoms on the reactant side (left) is equal to the number of atoms on the product side (right).
In this scenario, Sodium azide (\(NaN_3\)) undergoes a decomposition reaction due to an electrical impulse, which causes it to produce elemental sodium and nitrogen gas.
The properly balanced chemical equation for this chemical reaction is given by:
\(2NaN_3 \rightarrow 2Na + 3N_2\)
Read more on chemical equation here: brainly.com/question/13750908
4 Some elements have symbols that do not appear to match their names. For
example, the symbol for sodium is Na. Why is this?
Answers
Answer:
It is because some elements have the symbol of their Latin names for example - Latin name of sodium is Natrium and potassium is Kalium .Thatswhy their symbol differs from their modern name .
Without doing any calculations, arrange the elements in CF2Cl2 in order of decreasing mass percent composition. Rank from highest percent to lowest.
a. C > F > Cl
b. F < Cl > C
c. Cl > C > F
d. Cl > F > C
Answers
Answer:
a. C > F > Cl
Explanation:
We know that atomic mass of Chlorine is greater than of Florine than that of carbon. Moreover, in CF2Cl2, therefore, there are two atoms of Cl, F and one atom of C. Therefore, in CF2Cl2 in order of decreasing mass percent composition C > F > Cl. Therefore, the correct option is a.
atoms and ions are held together by..
A.) nuclear bonds
B.) Stick bonds
C.) physical bonds
D.) Chemical bonds
Answers
Answer:
chemical bonds
Explanation:
The atoms in chemical compounds are held together by attractive electrostatic interactions known as chemical bonds. Ionic compounds contain positively and negatively charged ions in a ratio that results in an overall charge of zero. The ions are held together in a regular spatial arrangement by electrostatic forces.
1. A sample of commercial concentrated hydrochloric acid is 11.8 M HCl and has a density of 1.190 g/mL. Calculate (a). the mass % of HCI (b). the molality of HCI (c). the mole fraction of HCI
Answers
(a) The mass percent of HCl in the solution is approximately 36.1%.
(b) The molality of HCl in the solution is approximately 15.5 mol/kg.
(c) The mole fraction of HCl in the solution is approximately 0.218.
(a) To calculate the mass percent of HCl, we need to determine the mass of HCl in a given volume of the solution.
Given: Concentration of HCl = 11.8 M
Density of the solution = 1.190 g/mL
First, we need to calculate the mass of the solution. Since density is mass per unit volume, the mass of 1 mL of the solution is 1.190 g.
Next, we need to calculate the mass of HCl in 1 mL of the solution. Since the concentration is given in moles per liter (M), and the molar mass of HCl is 36.46 g/mol, we can calculate the mass of HCl in 1 mL as follows:
Mass of HCl = concentration × volume × molar mass
= 11.8 mol/L × 0.001 L × 36.46 g/mol
= 0.430 g
Now, we can calculate the mass percent of HCl using the following formula:
Mass percent = (mass of solute ÷ mass of solution) × 100
= (0.430 g ÷ 1.190 g) × 100
≈ 36.1%
(b) The molality of HCl is calculated by dividing the moles of solute (HCl) by the mass of the solvent (water) in kilograms.
Since the density of the solution is given as 1.190 g/mL, the mass of 1 mL of the solution is 1.190 g. However, we need to consider the density of the solvent (water) to calculate the mass of water in the solution.
Assuming the density of water is 1 g/mL, the mass of water in 1 mL of the solution is (1.190 g - 0.430 g) = 0.760 g.
To calculate the molality of HCl, we need to convert the mass of water to kilograms:
Mass of water (kg) = 0.760 g ÷ 1000 = 0.000760 kg
The molality (m) is calculated using the formula:
Molality = (moles of solute ÷ mass of solvent in kg)
= (11.8 mol/L × 0.001 L) ÷ 0.000760 kg
≈ 15.5 mol/kg
(c) The mole fraction (X) of HCl is calculated by dividing the moles of HCl by the total moles of all components in the solution.
To calculate the mole fraction, we need to consider the volume of the solution and convert it to liters.
Given: Concentration of HCl = 11.8 M
Volume of the solution = 1 mL
Volume of the solution (L) = 1 mL ÷ 1000 = 0.001 L
To calculate the mole fraction of HCl, we need to calculate the moles of HCl and the moles of water (solvent) in the solution.
Moles of HCl = concentration × volume
= 11.8 mol/L × 0.001 L
= 0.0118 mol
Moles of water = mass of water ÷ molar mass of water
= 0.760 g ÷ 18.015 g/mol (molar mass of water)
= 0.0422 mol
Total moles in the solution = moles of HCl + moles of water
= 0.0118 mol + 0.0422 mol
= 0.054 mol
Mole fraction of HCl = moles of HCl ÷ total moles
= 0.0118 mol ÷ 0.054 mol
≈ 0.218
For such more questions on molality
https://brainly.com/question/14366957
#SPJ8
If salt water has a density of 1.2 g/mL, which object listed below would SINK? *
Object 1 with a density of 1.14 g/cm3
Object 3 with a density of 1.62 g/cm3
Object 4 with a density of 0.8 g/cm3
Object 2 with a density of 0.92 g/cm3
Answers
which of the following statements regarding membranes is true? which of the following statements regarding membranes is true? both faces of membranes tend to have similar compositions. transverse diffusion occurs rapidly. bilayer formation is largely driven by the hydrophobic effect. lateral diffusion is largely dependent on an enzyme-mediated process.
Answers
It is accurate what is said about membranes below: an enzyme-mediated mechanism is mostly responsible for lateral diffusion.
What is the difference between hydrophilic and hydrophobic substances?A substance can be either hydrophobic or hydrophilic. Given that the word "hydr" is derived from the Greek word "hydor," which means "water," hydrophobic materials are "water-fearing" and do not blend with water, whereas hydrodynamic materials are "water-loving" and have a propensity to become wetted by water.
What does hydrophobic substance mean?Non-polar substances with a low affinity for water are referred to as hydrophobic substances and are water-repellent. As opposed to a hydrophobic interaction, which is indicated by a contact angle larger than 90°, a hydrophilic interaction is indicated by a contact angle less than 90°.
To know more about Hydrophobic visit:
https://brainly.com/question/16003692
#SPJ1
PLEASE HELP ME!! sue tomorrow:(

Answers
Answer:
If you are looking for the answer, for the question.
Earthquakes trigger tsunamis when the seismic activity causes the land along fault lines to move up or down
How many grams of carbon are in 6.32 moles?
Answers
Answer:
17.18 moles of NaCl are in 2,719 mL of a 6.32
Explanation:
What needs to be done to fight climate change?
Answers
1. Eliminate Food Waste
Food waste in the US occurs mostly in stores and at home—either because it spoils on the store shelf or before we can eat it. According to an NRDC study, Americans throw away up to 40 percent of the food they buy. We can combat food waste by shopping for what you need, eating leftovers, composting scraps, and donating excess to food banks. You can find a local food bank at FeedingAmerica.org. Project Drawdown estimates that curbing food waste could avoid a whopping 70.5 gigatons of CO2—that’s a bigger impact than restoring 435 million acres of tropical forest.
2. Eat Plant-Based
Transitioning to a vegetarian diet can cut your carbon footprint in half, and going vegan, even lower. Even shifting from high to low meat consumption can shrink your footprint by a third, according to a University of Oxford study. If half of the world’s population reduced meat consumption and avoided the associated deforestation caused by agriculture, we could reduce carbon emissions by 66 gigatons.
3. Use Clean Energy
Renewable energy is fundamental to powering the world as we move away from fossil fuels. Modeled after World War II “war bonds,” Clean Energy Victory Bonds—a bill introduced to Congress by Sen. Udall (D-NM), Reps. Lofgren (D-CA), and Reps. Matsui (D-CA)—would offer Treasury bonds as low as $25 to finance the government’s clean energy programs. Ask your representatives to support this bill to make Clean Energy Victory Bonds a reality. Additionally, you can purchase renewable energy from installers such as Blue Pacific Solar and RGS Energy, as well as plug into renewable utilities with Clean Choice Energy and Arcadia Power, which don’t require you to install any new hardware in your home to get sun- and wind-power.
4. Participate in the Democratic Process
Climate change has implications on local, national, and global levels. While the average person isn’t responsible for governing a nation, we are responsible for deciding who does. Vote for a climate activist, support comprehensive climate policies, and use your citizen voice to contact legislators when you disagree. The results of upcoming elections will determine how Americans and their elected leaders grapple with catastrophic climate change.
5. Divest
The largest source of greenhouse gas emissions come from fossil fuels. Divesting means taking your money out of institutions that fund fossil fuel expansion, which could eventually dry up funding to those projects. So far, the fossil fuel divestment movement has removed $9.94 trillion dollars from fossil fuel companies because of institutional divestments and $5.2 billion thanks to 58,000 individual divestments. You can build a fossil-free portfolio with our nationwide network of socially-responsible investing financial advisors which you can find on GreenPages.org and by encouraging your faith organization or alma mater to divest.
6. Improve Insulation
One of the most cost-effective and accessible tactics to combating the climate crisis is better insulation. Older homes can lose up to 35 percent of heat through their walls. Modern insulation reduces the energy needed to heat a home, therefore reducing emissions and saving you money. If even half of existing buildings installed thicker insulation, 8.3 gigatons of emissions could be avoided—that’s more than overhauling efficiency for the entire international shipping industry.
8. Rethink Transportation
Overhauling the world’s transportation systems, both commercial and personal, would save as much CO2 as one billion acres of regenerative agriculture. Commercial trucks alone account for six percent of the world’s emissions—more than the collective emissions of airplanes around the globe. While individuals can’t revolutionize the shipping, flight, and automobile industries overnight, we can demand they change by voting with our dollars for public transit, using electric or hybrid vehicles, and reducing our total trips taken.
9. Recycle
Acquiring virgin resources—from logging trees to mining minerals—exploits more resources than recycling existing materials. For example, recycled aluminum products use 95 percent less energy than creating new ones. About 50 percent of recycled materials come from households; if that number were to increase to 65 percent, at-home recycling could prevent 2.8 gigatons of carbon emissions. However, recycling wrong can slow the system and create more waste, so be sure to rinse out your recyclables and stay up to date on local regulations to make sure what you recycle isn’t causing contamination.
Connor turns on a hair dryer to style his hair in the morning. Which choice identifies all of the energy transformations in the system?
electrical -- heat sound, and motion
electrical -- sound and motion
heat-electrical and sound
heat and sound -motion
Answers
Answer:
It is electrical → heat, sound, and motion
because a hair dryer makes heat to dry your hair and sound is the blowing and motion is how it goes
Explanation:
Answer:
A
Explanation:
electrical -- heat sound, and motion
A certain liquid has a normal boiling point of and a boiling point elevation constant . A solution is prepared by dissolving some glycine () in of . This solution boils at . Calculate the mass of that was dissolved. Round your answer to significant digit.
Answers
The question is incomplete, the complete question is:
A certain substance X has a normal freezing point of \(-6.4^oC\) and a molal freezing point depression constant \(K_f=3.96^oC.kg/mol\). A solution is prepared by dissolving some glycine in 950. g of X. This solution freezes at \(-13.6^oC\) . Calculate the mass of urea that was dissolved. Round your answer to 2 significant digits.
Answer: The mass of glycine that can be dissolved is \(1.3\times 10^2g\)
Explanation:
Depression in the freezing point is defined as the difference between the freezing point of the pure solvent and the freezing point of the solution.
The expression for the calculation of depression in freezing point is:
\(\text{Freezing point of pure solvent}-\text{freezing point of solution}=i\times K_f\times m\)
OR
\(\text{Freezing point of pure solvent}=\text{Freezing point of solution}=i\times K_f\times \frac{m_{solute}\times 1000}{M_{solute}\times w_{solvent}\text{(in g)}}\) ......(1)
where,
Freezing point of pure solvent = \(-6.4^oC\)
Freezing point of solution = \(-13.6^oC\)
i = Vant Hoff factor = 1 (for non-electrolytes)
\(K_f\) = freezing point depression constant = \(3.96^oC/m\)
\(m_{solute}\) = Given mass of solute (glycine) = ?
\(M_{solute}\) = Molar mass of solute (glycine) = 75.07 g/mol
\(w_{solvent}\) = Mass of solvent = 950. g
Putting values in equation 1, we get:
\(-6.4-(-13.6)=1\times 3.96\times \frac{m_{solute}\times 1000}{75.07\times 950}\\\\m_{solute}=\frac{7.2\times 75.07\times 950}{1\times 3.96\times 1000}\\\\m_{solute}=129.66g=1.3\times 10^2g\)
Hence, the mass of glycine that can be dissolved is \(1.3\times 10^2g\)
The kinetic energy of a 23.2-g object moving at a speed of 98.7 m/s is ________ J. The kinetic energy of a 23.2-g object moving at a speed of 98.7 m/s is ________ J. 0.950 145 113 1450 113000
Answers
The kinetic energy of a 23.2-g object moving at a speed of 98.7 m/s is 113.30 J. Option D
The kinetic energy of an object can be calculated using the formula: KE = (1/2)mv^2, where KE is the kinetic energy, m is the mass of the object, and v is the velocity or speed of the object.
Given:
Mass (m) = 23.2 g = 0.0232 kg
Speed (v) = 98.7 m/s
Substituting these values into the formula, we can calculate the kinetic energy:
KE = (1/2)(0.0232 kg)(98.7 m/s)^2
KE = (1/2)(0.0232 kg)(9756.09 m^2/s^2)
KE ≈ 113.30 J
Therefore, the kinetic energy of a 23.2-g object moving at a speed of 98.7 m/s is approximately 113.30 J.
It's worth noting that the question is repeated twice, but the answer remains the same. The kinetic energy of the object is determined by its mass and speed, and both calculations yield the same result. Option D
For more sucu questions on kinetic energy visit:
https://brainly.com/question/25959744
#SPJ8
9. When 1.10 g of magnesium reacted with unlimited amount of HCl, the products were
hydrogen gas and magnesium chloride. What volume of hydrogen gas would be collected
if the reaction had been run at STP?
Answers
As per the balanced equation of the reaction one mole of Mg give s one mole of H2. 1.10 g of magnesium is 0.045 moles. Thus it gives 0.045 moles of H2 which is 1.02 litres.
What is magnesium chloride?Magnesium chloride is an ionic compound formed by the reaction of magnesium metal with two moles of hydrochloric acid. The reaction also produce one mole hydrogen gas.
The atomic mass of magnesium is 24 g/mol. Thus number of moles in 1.10 g is 1.10/24 = 0.045 moles.
As per the reaction equation one mole of magnesium gives one mole of hydrogen gas. Thus 0.045 moles of magnesium gives 0.045 moles of hydrogen gas.
At STP, the volume of one mole of any gas will be equal to 22.4 L. Thus volume of 0.045 moles of hydrogen at STP is:
volume = 0.045 × 22.4
=1.02 L.
Hence, the volume of hydrogen produce here is 1.02 L.
To find more on magnesium, refer here:
https://brainly.com/question/1533548
#SPJ1
lphins... Acid. (b) Chlorine reacts with red hot iron powder to give Iron(III) Chloride but not Iron (II) Chloride. Explain. (1Mark)
Answers
(a) Because acid is caustic, dolphins can perish from exposure to it. Acids are compounds that give other things protons (H+). Acid can react with the proteins and lipids in dolphins' skin when they come into touch with it, leading to chemical burns and damage to the underlying tissue. Systemic consequences from this include death.
(b) Because chlorine is a potent oxidizer, it interacts with red-hot iron powder to produce Iron(III) chloride (FeCl3) rather than Iron(II) chloride (FeCl2). FeCl3 is created when chlorine at high temperatures rapidly accepts electrons from iron atoms. Contrarily, iron interacts with HCl, a less potent oxidizer than chlorine, to produce FeCl2.
Learn more about chlorine at :
https://brainly.com/question/31560014
#SPJ1
A stock solution of HNO3 is prepared and found to contain 14.9 M of HNO3. If 25.0 mL of the stock solution is diluted to a final volume of 0.500 L, what is the concentration of the diluted solution
Answers
Answer:
\(0.745~M\)
Explanation:
In this case, we have a dilution problem. So, we have to use the dilution equation:
\(C_1*V_1=C_2*V_2\)
Now, we have to identify the variables:
\(C_1~=~14.9~M\)
\(V_1~=~25~mL\)
\(C_2~=~?\)
\(V_2~=~0.5~L\)
Now, we have different units for the volume, so we have to do the conversion:
\(0.5~L\frac{1000~mL}{1~L}=~500~mL\)
Now we can plug the values into the equation:
\(C_2=\frac{14.9~M*25~mL}{500~mL}=0.745~M\)
I hope it helps!
c) Discuss precision and Accuracy as they relate to types of errors.
what is the answer
Answers
Precision relates to the consistency and reproducibility of measurements, while accuracy reflects how close measurements are to the true value.
Precision and accuracy are two important concepts in the context of errors in measurements. While they both pertain to the quality of data, they refer to different aspects.
Precision refers to the degree of consistency or reproducibility in a series of measurements. It reflects the scatter or spread of data points around the average value. If the measurements have low scatter and are tightly clustered, they are considered precise. On the other hand, if the measurements have a high scatter and are widely dispersed, they are considered imprecise.
Accuracy, on the other hand, refers to the closeness of measurements to the true or target value. It represents how well the measured values align with the actual value. Accuracy is achieved when measurements have a small systematic or constant error, which is the difference between the average measured value and the true value.
Errors in measurements can be classified into two types: random errors and systematic errors.
Random errors are associated with the inherent limitations of measurement instruments or fluctuations in the measurement process. They lead to imprecise data and affect the precision of measurements. Random errors can be reduced by repeating measurements and calculating the average to minimize the effect of individual errors.
Systematic errors, on the other hand, are caused by consistent biases or inaccuracies in the measurement process. They affect the accuracy of measurements and lead to a deviation from the true value. Systematic errors can arise from factors such as instrumental calibration issues, environmental conditions, or experimental techniques. These errors need to be identified and minimized to improve the accuracy of measurements.
In summary, precision refers to the degree of consistency or reproducibility of measurements, while accuracy refers to the closeness of measurements to the true value. Random errors affect precision, while systematic errors affect accuracy. To ensure high-quality measurements, both precision and accuracy need to be considered and appropriate techniques should be employed to minimize errors.
Know more about Precision here:
https://brainly.com/question/30461151
#SPJ8
17. Which of the following
hydrocarbon undergo addition
reaction:
С3Н6
С2Н6
ОООО
С3Н8
CH4
Answers
Answer:
С3Н6.
Explanation:
Hello!
In this case, since addition reactions imply that a radical or some radicals are added to the parent chain, we notice that only unsaturated hydrocarbons are able to undergo addition whereas saturated ones undergo substitution reactions as they already have all the carbon bonds bonded to leaving groups.
In such a way, we can rule out C2H6, C3H8 and CH4 as they are all alkanes; therefore, only С3Н6 is able to undergo an addition reaction due to the C=C which is able to lose one of those bonds and allow an incoming radical to get included into the parent chain.
Best regards!
lonic bonding can only occur between which two types of elements
a
Metal and metal
b
non metal and non metal
c
metal and non metal
d
none of the above
Answers
Answer:
C.
Explanation:
An ionic bond is a type of chemical bond formed through an electrostatic attraction between two oppositely charged ions. Ionic bonds are formed between a cation, which is usually a metal, and an anion, which is usually a nonmetal. A covalent bond involves a pair of electrons being shared between atoms.
HELP PLEASE
Why doesn't the Earth's shadow fall on the Moon during the Full Moon phase?
The Moon's orbit isn't flat, it's tilted
The Earth is much larger than the Moon
The Moon is much smaller than Earth.
The Moon's orbit is flat and isn't tilted
Answers
The plane of the Moon's orbit around the Earth is tilted by 5° with respect to the plane of the Earth's orbit around the Sun, the ecliptic. This tilt prevents an eclipse from occurring at every new and full moon. In a lunar eclipse, the observer watches the Earth's shadow fall on the Moon.
Answer:
Because there is no light in space so there is no shadow
Cumulative Exam Active
41 42 43 144
The electron configuration of nitrogen (N) is
O 1s²2s²2p³
O 1s²2s²2p4
O 1s²2s²2p5
O 1s²2s²2p6
Answers
The answer is: The electronic configuration of Nitrogen is \(1s^22s^22p^3\).
Electronic configuration: The electronic configuration is defined as the distribution of electrons of an atom in the atomic or molecular orbitals and is written using the labels for the subshell.
How to decide which orbital is filled first?
The order in which electrons are filled in atomic orbitals as:(Shown in image)
Just follow the arrows to select the orbitals, s orbital can have 2 electrons, p can have 6 electrons, d can have 10 electrons and f can 14 electrons.The electronic configuration in which the outer shell is completely filled is known as noble-gas configuration as they are similar to electronic configurations of noble gases.Now, the given element is nitrogen (\(N\)). The atomic number of Nitrogen is 7. Thus, these 7 electrons are filled as-\(1s^22s^22p^3\)
Therefore, the electronic configuration of Nitrogen is \(1s^22s^22p^3\).To learn more about the electronic configuration, visit:
https://brainly.com/question/21977349
#SPJ4

Nitrogen's complete electron configuration is 12s2s22p3.
The shorthand electron configuration for noble gases is [He] 2s22p3. Nitrogen has an atomic number of 7. The nitrogen atoms' nucleus contain this many protons. An atom that is neutral has an equal number of protons and electrons. Thus, the ground state electron configuration will consist of 7 electrons in the suitable s and p orbitals (state of lowest energy). For nitrogen, the entire electron configuration is 1s22s22p. Scientists may easily express and explain how the electrons are organized around the nitrogen atom's nucleus by using the configuration notation for nitrogen (N). As a result, it is simpler to comprehend and forecast how atoms will cooperate to form chemical bonds.
Learn more about electronic configuration here-
https://brainly.com/question/11309892
#SPJ9
what is electronegativity and how can it be used in determining the polarity of nonpolar
Answers
Answer:
See explanation
Explanation:
Electro negativity refers to the ability of an atom in a molecule to attract the shared electrons of a bond towards itself.
Electro negativity is a property of elements. It increases across the period and decreases down the group in the periodic table.
When two elements with differing electronegativities form a bond, the atom that has a greater electro negativity tends to pull the shared electrons of the bond towards itself. By so doing, there is greater electron density around that atom compared to the other atom. The more electronegative atom therefore acquires a partial negative charge while the other atom acquires a partial positive charge. This leads to a dipole in the molecule.
A molecule is said to be polar when it possess a dipole, that is, a positive end and a negative end. This phenomenon in turn arises when there is a significant difference in electro negativity between two atoms that compose the bond.
For instance, consider the formation of HF. Hydrogen has an electro negativity of 2.2 while fluorine has an electro negativity of 3.98. The difference in electro negativity between the both atoms is about 1.78. The molecule is polar with the negative end of the dipole at fluorine and the positive end at hydrogen.
The spoon's broken appearance is caused by light waves that are
A
Reflected by the glass and then absorbed the water
B
Refracted by the water
С
Absorbed by the metal spoon
D
Reflected by the metal spoon and the water
Answers
Answer:
its b
Explanation: