Hydrogen (H2) gas and oxygen (02) gas react to form water (H20) vapor. Suppose you have 1.0 mol of H, and 13.0 mol of O, in a reactor. What would be the limiting reactant? Enter its chemical formula below
Answers
From the given situation, the limiting reactant would be hydrogen (H₂).
In this reaction, hydrogen (H₂) gas and oxygen (O₂) gas react to form water (H₂O) vapor. The balanced chemical equation for this reaction is:
2H₂ + O₂ → 2H₂O
To determine the limiting reactant, we can compare the mole ratio of the reactants provided. You have 1.0 mol of H₂ and 13.0 mol of O₂. The stoichiometric ratio of H₂ to O₂ is 2:1. Therefore, you would need 0.5 mol of O₂ for every 1.0 mol of H₂.
Since you have 13.0 mol of O₂, it can react with (13.0 mol / 0.5 mol) = 26.0 mol of H₂. However, you only have 1.0 mol of H₂ available.
Therefore, the limiting reactant is hydrogen (H₂).
Learn more about limiting reactant here: https://brainly.com/question/26905271
#SPJ11
Related Questions
Mg reacts with HCL to produce H2 and MgCl2. Calculate the number of grams of magnesium necessary to produce .00300 moles of hydrogen gas.
Answers
The reaction between Mg (Magnesium) and HCL (Hydrochloric acid) is shown below;Mg + 2HCl → MgCl2 + H2Molar mass of Mg = 24 g/mole.
Number of moles of H2 = 0.00300 moles (Given)The balanced equation for the reaction shows that for every 1 mole of Mg, 1 mole of H2 is produced.Therefore;Number of moles of Mg = Number of moles of H2 = 0.00300 moles Mass of Mg needed = Number of moles of Mg × Molar mass of Mg= 0.00300 moles × 24 g/mole= 0.072 g Therefore, 0.072 g of magnesium is necessary to produce 0.00300 moles of hydrogen gas.
The formula for calculating the number of grams of magnesium necessary to produce .00300 moles of hydrogen gas can be found by the following steps:Write the balanced equation for the reaction between Mg and HCL.Find the molar mass of Mg.Calculate the number of moles of H2 from the given data (0.00300 moles).Use the balanced equation to equate the number of moles of Mg to the number of moles of H2.Calculate the mass of Mg from the number of moles of Mg and the molar mass of Mg.
To know more about moles visit:-
https://brainly.com/question/15209553
#SPJ11
49 grams of sulfuric acid, H2SO4, is dissolved in 1 liter of solution. Determine the molarity (M).
Answers
Answer: .5m
Explanation:
Calculate the molar mass of :
a) NH⁴
b) LiBr
Answers
The molar mass of NH₄ is 18 g/mole while that of compound of lithium bromide is 86.84 g/mole.
What is molar mass?Molar mass of a compound or a molecule is defined as the mass of the elements which are present in it.The molar mass is considered to be a bulk quantity not a molecular quantity. It is often an average of the of the masses at many instances.
The molecular mass and formula mass are used as synonym for the molar mass.It does not depend on the amount of substance which is present in the sample.It has units of gram/mole.
Molar mass of NH₄= 14+ 4= 18 g/mole while that of lithium bromide =6.941+ 79.904=86.84 g/mole.
Learn more about molar mass,here:
https://brainly.com/question/12127540
#SPJ1
chemicals like bacterial toxins that poison cells are described as being
Answers
Chemicals like bacterial toxins that poison cells are commonly described as being toxic or poisonous. These substances have the ability to disrupt normal cellular functions and processes, leading to harmful effects on the cells and organisms they come into contact with.
Toxins can have various mechanisms of action. Some toxins interfere with essential biochemical pathways, disrupt cellular membranes, or inhibit vital enzymes, while others may directly damage DNA or disrupt cellular signaling. Regardless of their specific mode of action, toxins are designed to have a detrimental impact on cellular function and can cause a wide range of adverse effects, from mild symptoms to severe illness or even death.
The toxicity of a substance is often determined by its concentration, exposure duration, and the specific vulnerability of the target cells or organisms. Toxins produced by bacteria can be classified into exotoxins, which are secreted by bacteria and released into the surrounding environment, or endotoxins, which are part of the bacterial cell wall and are released upon cell death or lysis.
Chemicals like bacterial toxins are referred to as toxic or poisonous due to their ability to disrupt cellular function and cause harm to cells and organisms.
To know more about Toxins, visit:
brainly.com/question/9348025
#SPJ11
how deficiency and excess of chromium
Answers
Answer:
The chromium found in foods will not hurt you. But taking excessive chromium supplements can lead to stomach problems and low blood sugar (hypoglycemia). Too much chromium from supplements can also damage the liver, kidneys, and nerves, and it may cause irregular heart rhythm.
What are the coefficients when this equation is balanced explain
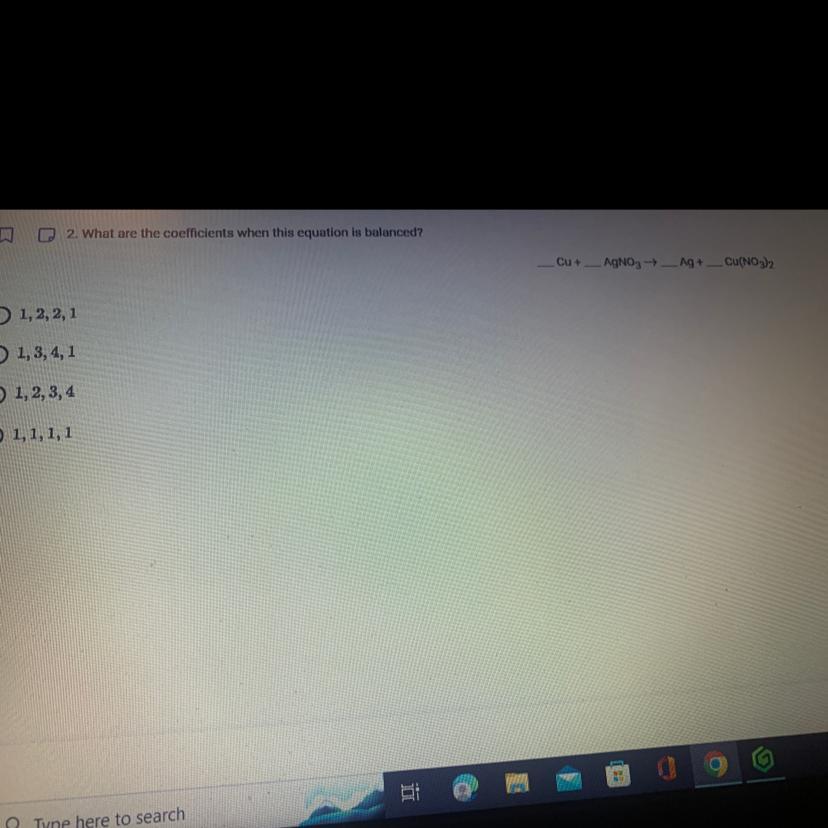
Answers
The balanced equation will be:
Cu + 2 AgNO3 ----> 2Ag + Cu(NO3)2
The balancing of equation is done to satisfy the law of conservation of mass. This law states - "mass can neither be created, nor be destroyed". Thus, the mass of the element in the equation cannot be created or destroyed, it is just shifted.
The answer is 1,2,2,1; the first option.
(Help!) What is the mass of hydrogen atoms that is measured at 72 u?
Answers
Answer:
=71.439911 u
Explanation:
We know that 1 mass of hydrogen atom = 1.00784 u
If it's measured at 72u: 72/1.00784=71.439911 u
Mass of 1 mol hydrogen atoms =1.00784u
Total mass in 72u
72/1.0078471.43uName the four types of friction. Provide one example not used in your lesson for each type.
Answers
Molecules with identical chemical formulas but with atoms differing in three-dimensional orientations are called:_____
Answers
The term for molecules with identical chemical formulas but with atoms differing in three-dimensional orientations is isomers.
Isomers are compounds that have the same molecular formula, but their atoms are arranged differently in space. There are two main types of isomers: structural isomers and stereoisomers. Structural isomers have their atoms connected in a different order, while stereoisomers have the same connectivity but differ in the arrangement of their atoms in three-dimensional space.
Stereoisomers can be further classified into enantiomers, which are mirror images of each other, and diastereomers, which are not mirror images. Understanding isomers is essential in chemistry, as different isomers can have distinct physical, chemical, and biological properties.
Learn more about stereoisomers here:
https://brainly.com/question/30406911
#SPJ11
N2(g) + 3H2 (g)→2NH3(g) The reaction rate is measured as 0.032 M NH3/s. Determine the rate of disappearance of N2 and the rate of disappearance H2. Explain how you arrived at your answers.
Answers
The rate at which N\(_{2}\) disappears is 0.016 M/s, while the rate at which H\(_{2}\) disappears is 0.0213 M/s.
In the balanced chemical equation N\(_{2}\)(g) + 3 H\(_{2}\) (g) → 2NH\(_{3}\)(g), the stoichiometric coefficients represent the mole ratios between the reactants and products.
Since the reaction rate is given for NH\(_{3}\), we can determine the rates of disappearance of N\(_{2}\) and H\(_{2}\) by comparing their stoichiometric ratios in the reaction.
The stoichiometric ratio between N\(_{2}\) and NH\(_{3}\) is 1:2, meaning for every mole of N\(_{2}\) consumed, 2 moles of NH\(_{3}\) are produced. Therefore, the rate of disappearance of N\(_{2}\) is half of the rate of formation of NH\(_{3}\).
Similarly, the stoichiometric ratio between H\(_{2}\) and NH\(_{3}\) is 3:2. This means that for every 3 moles of H\(_{2}\) consumed, 2 moles of NH\(_{3}\) are produced. Therefore, the rate of disappearance of H\(_{2}\) is (2/3) times the rate of formation of NH\(_{3}\).
Given the rate of formation of NH\(_{3}\) as 0.032 M/s, the rate of disappearance of N\(_{2}\) would be 0.016 M/s (0.032 M/s ÷ 2), and the rate of disappearance of H\(_{2}\) would be approximately 0.0213 M/s (0.032 M/s × 2/3).
Therefore, the rate of disappearance of N\(_{2}\) is 0.016 M/s, and the rate of disappearance of H\(_{2}\) is 0.0213 M/s.
You can learn more about chemical equation at
brainly.com/question/4425414
#SPJ11
if the maximum temperature for a particular day is 20°c and the minimum temperature is 10°c, the daily mean would be:
Answers
The daily mean, given the data from the question is 15 °C
Data obtained from the questionMaximum temperature = 20 °CMinimum temperature = 10 °CMean = ?How to determine the daily meanMean = Sumation of data / Number of data
Mean = (20 + 10) / 2
Mean = 30 / 2
Mean = 15 °C
Learn more about statistics:
https://brainly.com/question/8974461
#SPJ12
Aluminum metal reacts with chlorine gas. Determine the mass of chlorine to react completely with 5.0
moles of aluminum.
Answers
Answer:
The mass of chlorine to react completely with 5.0 moles of aluminum is 531.75 grams.
Explanation:
Aluminum reacts with chlorine gas to form aluminum chloride through the following reaction, which is balanced (In a chemical equation, the number of atoms of each element in the reactants must be equal to the number of atoms of each element in the products In order to comply with the Law of Conservation of Mass, that is, in a chemical reaction the mass remains constant, that is, the mass that is consumed from the reactants is the same as that obtained from the products of the reaction):
2 Al + 3 Cl₂ ⇒ 2 AlCl₃
The rule of three is a way of solving problems of proportionality between three known values and one unknown, establishing a proportionality relationship between all of them. A proportionality is direct when one magnitude increases the other also does it, and if the magnitude decreases the other in the same way. In this case, the rule of three, knowing a, b and c and with x being the unknown, is applied as follows:
a ⇒ b
c ⇒ x
So \(x=\frac{c*b}{a}\)
In this case the rule of three can be applied as follows: if by stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction) 2 moles of aluminum react with 3 moles of chlorine, 5 moles of aluminum with how many moles of chlorine do they react?
\(moles of chlorine=\frac{5 moles of aluminum*3 moles of chlorine}{2 moles of aluminum }\)
moles of chlorine= 7.5
Since the molar mass of the gas Cl2 is 70.9 grams/mole, you can apply the following rule of three: if 1 mole has 70.9 grams, 7.5 moles, how much mass does it have?
\(mass of chlorine=\frac{70.9 grams*7.5 moles}{1 mole}\)
mass of chlorine= 531.75 grams
The mass of chlorine to react completely with 5.0 moles of aluminum is 531.75 grams.
Which describes the difference between a claim and a scientific claim?
Claims are based more on truth than scientific claims are.
Anyone can make a claim, but a scientific claim is backed by experimental evidence.
Claims are based on evidence and scientific claims are made by scientists.
Controlled experiments are used in claims, while scientific claims use multiple trials.
Answers
Answer:
B. Anyone can make a claim, but a scientific claim is backed by experimental evidence.
<3 Have a nice day!!
Answer:
the awnser is B i got it right
Explanation:
what is the molarity ofa solution containing 8.0 g of naoh in 400. ml of naoh solution?
Answers
The molarity of the solution containing 8.0 g of NaOH in 400 mL of NaOH solution is 0.5 M (moles per liter).
To find the molarity of a solution containing 8.0 g of NaOH in 400 mL of NaOH solution, you will need to follow these steps:
Step 1: Determine the molecular weight of NaOH.
NaOH (sodium hydroxide) has a molecular weight of 40 g/mol. This is calculated by adding the atomic weights of sodium (Na, 22.99 g/mol), oxygen (O, 16.00 g/mol), and hydrogen (H, 1.01 g/mol).
Step 2: Calculate the moles of NaOH in the solution.
To find the moles of NaOH, you'll need to use the mass of NaOH and its molecular weight. The formula is:
moles of NaOH = (mass of NaOH) / (molecular weight of NaOH)
moles of NaOH = (8.0 g) / (40 g/mol) = 0.2 moles
Step 3: Convert the volume of the solution to liters.
Molarity is defined as moles of solute per liter of solution. Therefore, you need to convert the volume of the solution from milliliters (mL) to liters (L). To do this, use the conversion factor:
1 L = 1000 mL
Volume in liters = (400 mL) * (1 L / 1000 mL) = 0.4 L
Step 4: Calculate the molarity of the solution.
Now that you have the moles of NaOH and the volume of the solution in liters, you can calculate the molarity of the solution using the formula:
Molarity = (moles of solute) / (volume of solution in liters)
Molarity = (0.2 moles) / (0.4 L) = 0.5 M
So, the molarity of the solution containing 8.0 g of NaOH in 400 mL of NaOH solution is 0.5 M (moles per liter).
To know more about molarity of the solution refer here: https://brainly.com/question/20054062#
#SPJ11
The reaction between sodium and chlorine that forms table salt is shown
_Na(s) + Cl2 (g) → _NaCl (3)
What coefficient should be added to the blanks to balance the equation?
А.
2
В.
1
2.
2
Answers
Answer: 2 Na (s) + Cl(g) -> 2 NaCl (s)
Explanation:
What would happen to the biodiversity index of an ecosystem if a change in the ecosystem caused the number of species to stay the same and the total number of individuals to increase?
1-The biodiversity index would stay the same
2-The biodiversity index would decrease
3-The biodiversity would increase, and then decrease
4-The biodiversity index would increase
Answers
If a change in the ecosystem caused the number of species to stay the same and the total number of individuals to increase then The biodiversity index would increase".
An ecosystem's efficiency (the quantity of food energy transformed into biomass) and the quality of its services (which frequently include preserving the soil, cleaning the water that runs through it, as well as providing food and shade, etc.) both decrease with declining biodiversity.
An ecosystem's efficiency (the quantity of food energy transformed into biomass) as well as the quality of its services (which frequently include preserving the soil, cleaning the water which flows through it, as well as providing food as well as shade, etc.) both decrease with declining biodiversity.
Therefore, the correct option will be (4)
To know more about biodiversity index
https://brainly.com/question/14437032
#SPJ1
How many miles are there in 2345 feet? (1 mile=5280 ft)
2 points
Answers
Answer:
0.444 mile
Explanation:
If 12.3 grams of CCl4 are produced from the reaction of 18.0 g of carbon disulphide with 22.0 grams of Cl2, what is the percent yield of CCl4???
Answers
The percent yield of CCl₄ from the reaction of 18.0 g of carbon disulphide with 22.0 grams of Cl₂ is 77.3%
Equation of reaction:
CS₂ + 3 Cl₂ ----> CCl₄ + S₂Cl₂
molar mass of CS₂ = 78 g/mol
molar mass of Cl₂ = 71 g/mol
molar mass of CCl₄ = 154 g/mol
1 mole of carbon disulphide reacts with 3 moles of chlorine gas78 g of CS₂ reacts with * 71 g of Cl₂
78 g of CS₂ reacts with 213 g of Cl₂
18 g of CS₂ reacts with 213 * 18/ 78 g of Cl₂
18 g of CS₂ requires 49.1 g of Cl₂
Therefore, Cl₂ is the limiting reactant3 moles of Cl₂ produces 1 mole of CCl₄
213 g of Cl₂ produces 154 g of CCl₄
22.0 g of Cl₂ will produce 154 * 22 / 213 g of CCl₄
22.0 g of Cl₂ will produce 15.9 g of CCl₄
Percent yield = (actual yield / expected yield) * 100%Actual yield = 12.3 g
Expected yield = 15.9 g
Percent yield = 12.3/15.9) * 100%
Percent yield = 77.3 %
The percent yield of CCl₄ from the reaction of 18.0 g of carbon disulphide with 22.0 grams of Cl₂ is 77.3%
Learn more about percent yield at: https://brainly.com/question/12890861
A person uses 720 kcal on a long hike. Calculate the energy used for the hike in each of the following energy units. A.) joules B.) kilojoules
Answers
A person uses 720 kcal on a long bike. A. The energy in joules is equal to 3221680 Joules. B. The energy in kilojoules is 3221.689 KJ.
What is energy ?
The term energy is defined as the capacity for doing work. It is exist in different types like potential, kinetic, thermal, electrical, chemical, nuclear, or other forms.
Given the energy used for the bike is 770 kcal.
We know that
1 cal = 4.184 J
The energy in Joules = 770 × 1000 × 4.184
= 3221680 J
The energy in kilojoules = 3221689 / 1000
= 3221.689 KJ
Thus, A. The energy in joules is equal to 3221680 Joules. B. The energy in kilojoules is 3221.689 KJ.
To learn more about the energy, follow the link;
https://brainly.com/question/1932868
#SPJ1
Compare the compressibility of solids and liquids. Support your answer by describing the arrangement of particles in solids and liquids.(Does,t need to be very long or detailed)
Answers
Liquids are more compressible than solids.
In liquids there is space between the molecules, not a lot, but there is enough space to offer some compressibility. Solids are arranged in regular patterns and their molecules are almost fixed close together.
7. Convert 8. How many milligrams of magnesium sulfate (MgSO, MW 120) should be added to a one liter IV solution to provide 10 mEq of the magnesium ion per liter? [Round to the nearest whole number] n
Answers
We should add approximately 600 mg of magnesium sulfate to the one-liter IV solution to achieve the desired concentration.
The first step to convert mEq to milligrams is to know the atomic weight of magnesium, which is 24.3. To get 10 mEq of magnesium ion per liter, we need to add 1,203 milligrams of magnesium sulfate (10 x 24.3 x 2 x 1000 / 1) to a one liter IV solution. Therefore, the answer is 1,203 milligrams of magnesium sulfate should be added to the IV solution. Remember to always round to the nearest whole number in this case, so the answer would be 1,203. The MEW of MgSO₄ is its molecular weight (120) divided by the valence of Mg²⁺ (2). Thus, MEW = 120 / 2 = 60. Next, multiply the desired milliequivalents (10 mEq) by the MEW (60) to obtain the required amount in milligrams: 10 mEq x 60 mg/mEq = 600 mg. Therefore, you should add approximately 600 mg of magnesium sulfate to the one-liter IV solution to achieve the desired concentration.
To know more about concentration visit:
https://brainly.com/question/3045247
#SPJ11
How is the kinetic energy of molecules changing when the molecules move faster?
The kinetic energy ...
a
is not changing
b
depends on the molecules.
С
is decreasing.'
d
is increasing
Answers
Answer:
I think it is increasing
If you prepared a solution by adding equal numbers of moles of sodium sulfite (Na2SO3 which is the A-) and sodium hydrogen sulfite (NaHSO3 which is the HA) to 50 mL of water, what would be the pH of the solution?
acid dissociation: HSO3- + H2O ---> H3O+ + SO3^2-
Answers
The pH of the solution is approximately 7.21.
The acid dissociation reaction for sodium hydrogen sulfite is:
\(HSO3- + H2O ⇌ H3O+ + SO32-\)
The acid dissociation constant (Ka) for this reaction is:
\(Ka = [H3O+][SO32-]/[HSO3-]\)
We can use the expression for the acid dissociation constant to determine the pH of the solution.
Since equal numbers of moles of Na2SO3 and NaHSO3 are added, we can assume that the concentrations of Na2SO3 and NaHSO3 are equal, and that the initial concentration of HSO3- is equal to the initial concentration of NaHSO3. Let x be the concentration of H3O+ and SO32- that is formed when the acid dissociation reaches equilibrium. Then, the initial concentration of \(HSO3-\) is also x.
Substituting these concentrations into the expression for Ka, we get:
\(Ka = x^2 / x = x\)
Solving for x gives:
\(x = Ka = 6.2 × 10^-8\)
The pH of the solution can be calculated from the concentration of H3O+:
\(pH = -log[H3O+]\)
Since x = [H3O+], we have:
\(pH = -log(6.2 × 10^-8) ≈ 7.21\)
Therefore, the pH of the solution is approximately 7.21.
To know more about pH refer to-
https://brainly.com/question/491373
#SPJ11
If the external vapor pressure is 2.0 atm , what is the vapor pressure of the water at its boiling point?
Answers
Answer:
22
Explanation:
22
Pa help po thanks! I need the answer ASAP

Answers
Answer:
1.T
2.I
Explanation:
hope it help
#carry on learning
The object and description that matches is Object 2 and T.
Object 1 has no matching description.
Fish Aquarium FilterA Fish aquarium filter is a filter whose function is to clean the water of debris, removes the toxic buildup of ammonia and nitrates, and aerates the water so that fish can in a conducive environment and breathe properly
Engine Oil FilterAn engine oil filter is a filter whose function is to filter and remove contaminants that may be present in the engine oil, transmission oil, lubricating oil, or hydraulic oil in order for proper functioning of the engine.
Therefore, the object and description that matches is Object 2 and T.
Object 1 has no matching description.
Learn more about filters and their uses at: https://brainly.com/question/10719424
Which shows the correct order of steps during the formation of an ionic bond?
Answers
The correct Option is C. Electrons are transferred → Ions form → Ions are attracted to each other.
The right request of steps during the development of an ionic bond is:
C: Electrons are transferred → Ions form → Ions are attracted to each other
In ionic holding, at least one electrons are moved from a metal particle to a non-metal molecule, framing positive and negative particles, separately. The contrary charges of the particles draw in one another, subsequent in the arrangement of an ionic compound. Accordingly, the right request of steps in the development of an ionic bond is first, electrons are moved starting with one molecule then onto the next, then particles are framed, lastly, the oppositely charged particles are drawn to one another to shape an ionic compound.
To learn more about ionic bond formation, refer:
https://brainly.com/question/3211376
#SPJ4
The complete question is:
Which shows the correct order of steps during the formation of an ionic bond?
A: Ions are attracted to each other → Electrons are transferred → An ionic compound forms
B: An ionic compound forms → Ions are attracted to each other → Electrons are transferred
C: Electrons are transferred → Ions form → Ions are attracted to each other
D: Ions form → Electrons are transferred → Ions are attracted to each other
Answer: C
Explanation: JUST TOOK THE QUIZ HOPE THIS HELPED
Calculate the number of molecules of sulphur S8 in 8 gram of solid Sulphur
Answers
Answer:
12gram
Explanation:
test told me
A student measures the mass of an 8 cm^3 block of brown sugar to be 12.9 g. What is the density of
the brown sugar?
Answers
Answer:
1.6125 g/cm^3
Explanation:
Density= mass/volume
Question 3 of 25
What is meant by the rate of a reaction?
OA. How slow or fast a reaction progresses
B. How much energy the reaction requires
C. How far to completion the reaction goes
D. How concentrated the final products are
4
SUBMIT
Answers
Calculate how many atoms are in 98.6 g Carbon (C)?
Answers
Okay, lets see a breakdown of this. Let's say that you are given an amount of grams of a substance. For this case, lets say that that substance is Carbon (C). And, lets assume that you are given 4.01 g of Carbon, and you are tasked to find the number of atoms in that mass of Carbon. The breakdown would be as follows, with dimensional anaysis:
4.01
g Carbon
(
1
mol Carbon
12.01
g Carbon
)
(
6.022
⋅
10
23
a
t
m
s
C
a
r
b
o
n
1
mol Carbon
)
=
2.01
⋅
10
23
atms Carbon
Basically, I first wrote down the amount in grams, and I used the molar mass of Carbon (which can be found on the periodic table under Carbon) 12.01 g/mol to convert 4.01 g of Carbon to moles of Carbon. Then, I used "Avogadro's Number", or
6.022
⋅
10
23
atoms per mole
to convert the mole amount to atoms of Carbon.
The process should be very similar with other such atoms, just make sure to keep your periodic table and calculator handy.
I hope that helps!