I am a common cation that is found in the alkali group. I am found in salt. I have a +1 charge. What am I?
Answers
Answer:sodium
Explanation:
Sodium
Related Questions
ASAP IXL Question
A producer is the organism at the base, or beginning, of a food chain. Many producers use photosynthesis to make their own food. This food becomes the matter that moves up the food chain.
algae, zooplankton, shiner, largemouth and bass
Use the information in the food chain to fill in the blanks below.
algae zooplankton shiner largemouth bass
Matter begins to move through this food chain when the____ a producer is eaten by the____
, a primary consumer.
Answers
Answer:
algae, zooplankton
Explanation:
Algae photosynthesize to make their own food using sunlight. Algae produces energy and this energy is transferred when it is consumed by the zooplankton.
Iron is produced from its ore, iron oxide, by using a displacement reaction.
This is done in a blast furnace.
Which element is used to displace iron?
Answers
Answer:
its carbon
Explanation:
iron oxide + carbon = iron + carbon dioxide
as coke(mainly carbon) is a raw material gone into a blast furnace displaces iron.
What type of organism secretes calcium carbonate plates that overlap to form a spherical test?
A. Coccolithophores
B. Decomposers
C. Dinoflagellates
D. Diatoms
Answers
Answer:
A. Coccolithophores
Explanation:
Coccolithophores are organisms that are single-celled, and secret calcium carbonate plates. They produce calcium carbonate plates that is referred to as coccoliths.
These organisms absorb carbon dioxide during the production of their food. After its death, the calcium carbonate can be collected from the remains. Therefore causing an increase generally in the carbon deposits.
Answer:
A. Coccolithophores
Explanation:
Coccolithophores Create a calcium carbonate plating that protects the cell's surface. This action is called spherical coating.
pls help
Gold has a specific heat of 0.129 J/(g . C°). How much energy does a 0.700 gram piece of gold give off as it's temperature cools from 53.0°C to 22.0°C?
Answers
Answer:
2.8J
Explanation:
Given parameters:
Specific heat capacity = 0.129J/g°C
Mass = 0.7g
Initial temperature = 53°C
Final temperature = 22°C
Unknown:
Quantity of heat released = ?
Solution:
To solve this problem, we use the expression below;
H = m c Ф
H is the quantity of heat released
m is the mass
c is the specific heat capacity
Ф is the change in temperature
Now,
Insert the given parameters and solve;
H = 0.7 x 0.129 x (22 - 53) = -2.8J
The amount of heat released is 2.8J, the negative depicts the heat released.
Show that the formula, c4h6 is consistent with a lewis structure with two double bonds separated by a single bond. Explain/list all steps that would be followed in constructing the lewis structure for this molecule.
Answers
The formation of molecule can easily be shown by drawing Lewis dot structure. In given molecule, C\(_4\)H\(_6\), the carbon chain is like this C=C-C=C.
What is Lewis dot structure?Lewis dot structure is a way to represent the valence electron of an element in the form of dot. These are mainly beneficial in understanding the chemical formula of covalent compound.
In given molecule, C\(_4\)H\(_6\), the carbon chain is like this C=C-C=C. From this we can see that the corner carbon have two hydrogen attached to it using one electron of carbon. So, now corner carbon has two electrons left which will form double bond with the central carbon. The central carbons forms double bond with corner carbon using two electrons and one bond with hydrogen using one electron and one left electron is used in making bond with other central carbon.
Therefore, in given molecule, C\(_4\)H\(_6\), the carbon chain is like this C=C-C=C.
To learn more about Lewis dot structure, here:
https://brainly.com/question/20300458
#SPJ1
How does the octet rule apply to ionic and covalent bonds?
Select all that apply. A. Carbon with 4 valence electrons can satisfy the octet rule by gaining one electron from four different hydrogen atoms, each with 1 valence electron. B. Carbon with 4 valence electrons can satisfy the octet rule by sharing one electron with four different hydrogen atoms, each with 1 valence electron. C. Chlorine with 7 valence electrons can satisfy the octet rule by sharing one electron with a lithium atom with only 1 valence electron. D. Chlorine with 7 valence electrons can satisfy the octet rule by gaining one electron from a lithium atom with only 1 valence electron.
Answers
The octet rule is applicable to both ionic and covalent bonding. The appropriate responses to the above question are -
B. Carbon with 4 valence electrons can satisfy the octet rule by sharing one electron with four different hydrogen atoms, each with 1 valence electron.
D. Chlorine with 7 valence electrons can satisfy the octet rule by gaining one electron from a lithium atom with only 1 valence electron.
The octet rule states that atoms tend to gain, lose, or share electrons in order to achieve a full outer shell of 8 electrons (or 2 electrons for hydrogen).
Option A is incorrect because it suggests that the carbon atom gains one electron from each of the four hydrogen atoms, which would result in the carbon atom having 8 electrons in its outer shell, violating the octet rule.
Option C is incorrect because it suggests that the chlorine atom shares one electron with the lithium atom, which would result in the chlorine atom having only 7 electrons in its outer shell, also violating the octet rule.
For such more questions on Covalent bonding.
https://brainly.com/question/12732708
#SPJ4
The Earth has always been a hospitable haven for life. ture or flase
Answers
Answer:
True and false at the same time
Explanation:
It is true that Earth is heaven for life but It's also hell for life because people suffer a lot and so does the Earth. Earth is a hell for life because of all the bullying, criticism, racism, sexism, and homophobic people. Life is a great thing to enjoy but with all the negative things that happened and keep happening in the world, it's a living hell for a life. The only good things are that you can explore and do things you never thought of doing before and you are ale=ways surrounded by people who love you and appreciate you for who you are but it's a 5-50 chance of you getting an opportunity like that.
I'm not a negative person it's just the ways that things are. I hope this answers your question!
Have a nice day! UwU
Consider the reaction Mg(s)+Fe2+(aq)→Mg2+(aq)+Fe(s) at 89 ∘C , where [Fe2+]= 3.80 M and [Mg2+]= 0.210 M .
Part A What is the value for the reaction quotient, Q, for the cell?
Part B What is the value for the temperature, T, in kelvins.
Part C What is the value for n?
Part D Calculate the standard cell potential for
Mg(s)+Fe2+(aq)→Mg2+(aq)+Fe(s)
Express the standard potential numerically in volts.
Answers
The value for the reaction quotient is 0.0553, the value for the temperature is 362.15 K, the value for n = 2, and the standard cell potential for Mg(s) + Fe²⁺(aq) → Mg²⁺(aq) + Fe(s) is -3.14 V.
The reaction quotient, Q, for the cell is given by;
Q = [Mg²⁺][Fe(s)]/[Mg(s)][Fe²⁺]
Substituting the given values;
Q = (0.210)(1)/1(3.80) = 0.0553
The temperature, T, in Celsius is given as 89°C. To convert to kelvins, we add 273.15 to get;
T = (89 + 273.15) K = 362.15 K
The balanced equation for the reaction is;
Mg(s) + Fe²⁺(aq) → Mg²⁺(aq) + Fe(s)
The number of electrons transferred in the reaction is 2
So n = 2.
The standard cell potential, E°cell, can be calculated using the formula:
E°cell = E°cathode - E°anode
where E°cathode is the standard reduction potential for the cathode (Mg²⁺ + 2e⁻ → Mg) and E°anode is the standard oxidation potential for the anode (Fe²⁺ → Fe + 2e⁻).
The standard reduction potential for Mg²⁺ + 2e⁻ → Mg is -2.37 V, and the standard oxidation potential for Fe²⁺ → Fe + 2e⁻ is +0.77 V. Substituting these values, we get:
E°cell = (-2.37) - (+0.77) = -3.14 V
Therefore, the standard cell potential for Mg(s) + Fe²⁺(aq) → Mg²⁺(aq) + Fe(s) is -3.14 V.
To know more about standard cell potential here
https://brainly.com/question/14240930
#SPJ4
Part A: To find the reaction quotient, Q, use the formula:
Q = [Mg2+]/[Fe2+]
Given the concentrations: [Fe2+] = 3.80 M and [Mg2+] = 0.210 M, plug these values into the equation:
Q = (0.210)/(3.80) = 0.0553
Part B: To convert the temperature from Celsius to Kelvin, use the formula:
T(K) = T(°C) + 273.15
Given the temperature: 89°C, plug the value into the equation:
T = 89 + 273.15 = 362.15 K
Part C: The value of n represents the number of electrons transferred in the redox reaction. In this case, both Mg and Fe undergo a change of 2 in their oxidation states (Mg goes from 0 to +2, and Fe goes from +2 to 0). So, n = 2.
Part D: To calculate the standard cell potential (E°), use the standard reduction potentials for the half-reactions. The standard reduction potential for Mg2+/Mg is -2.37 V, and for Fe2+/Fe is -0.44 V. Since Mg is being oxidized, reverse the sign of its potential:
E° = E°(cathode) - E°(anode) = (-0.44) - (-2.37) = 1.93 V
So, your answers are:
Part A: Q = 0.0553
Part B: T = 362.15 K
Part C: n = 2
Part D: E° = 1.93 V
Learn more about redox reaction here:
https://brainly.com/question/28300253
#SPJ11
What eventually happens to a gas if its pressure is increased?
Answers
Answer:
According to Boyle's law pressure of a particular amount of gas is inversely proportional to the volume of the gas.So, when we increase the pressure of the gas the volume of the gas decrease and the gas start to condense.Thus if the pressure of a gas increases it starts to condense.Explanation:
Hope It Helps!!!!
Answer:
See belwo
Explanation:
First it becomes a compressed gas.... further pressure will cause it to condense into a liquid.
At height of 10 000 m, the outside air pressure is around 0. 27 atm. Inside an aircraft flying at this altitude, the air pressure is normally maintained at around 0. 78 atm. Assuming that the temperature inside and outside of the plane is the same, if a balloon was blown inside the aircraft until it contained a volume of 700 mL of gas, what volume would it be, in mL, if it was placed outside the aircraft?
Answers
The volume of the balloon outside the aircraft would be approximately 2037 mL.
We can solve this problem using the combined gas law, which relates the pressure, volume, and temperature of a gas. The combined gas law states that:
(P₁ × V₁) / T₁ = (P₂ × V₂) / T₂
where P1 and P2 are the initial and final pressures, V₁ and V₂ are the initial and final volumes, and T₁ and T₂ are the initial and final temperatures.
We can use this formula to solve for V₂, the final volume of the balloon outside the aircraft:
(0.78 atm × 700 mL) / T = (0.27 atm × V₂) / T
0.78 atm × 700 mL = 0.27 atm × V₂
V2 = (0.78 atm × 700 mL) / 0.27 atm
V2 ≈ 2037 mL
To know more about combined gas law here
https://brainly.com/question/29419201
#SPJ4
What is the chemical name for 1 nitrogen atom,
2 hydrogen atom?
Answers
NH3 has 1 Nitrogen atom and 3 hydrogen atoms.
Explain the work energy theorem including the direction energy moves and how it relates to positive and negative work
Answers
Answer and Explanation:
The working-energy theorem defines work as the energy change brought on by the velocity change. The environment has acted on the object and it increases the energy of the object. When an object's velocity decreases, the object has worked on the world, and the object's energy decreases.
Unless the displacement is in the reverse direction and the force applied, then the work performed is negative. If the displacement is in the same direction and the force applied, then the work performed is positive.
Answer:
Work is the change in energy brought about by a change in velocity.
When the velocity of an object increases, the environment has done work on the object and the energy of that object is increased.
When the velocity of an object decreases, the object has done work on the environment and the energy of the object is decreased.
Explanation:
which of the following molecules would have at least one atom that violates the octet rule? a. O-C1-O
b. F-Xe-F
c. both of these
d, none of these
Answers
This molecule violates the octet rule because xenon, the central atom, has 8 valence electrons surrounding it (6 from the fluorine atoms and 2 from its own), which is less than the typical octet of 8 electrons. The correct answer is b. F-Xe-F.
This is known as an expanded octet. O-C1-O and all other non-halogen compounds typically follow the octet rule. Therefore, the answer is not c. both of these, and it is not d. none of these.
The core atom, xenon, has 8 valence electrons surrounding it (6 from the fluorine atoms and 2 from its own), which is less than the normal octet of 8 electrons. As a result, this molecule breaks the octet rule. It's called an expanded octet. All non-halogen compounds generally adhere to the octet rule, including O-C1-O. Therefore, neither c. both of these nor d. none of these are the correct answers.
To know more about octet rule click here:
https://brainly.com/question/865531
#SPJ11
Señala en cuál de los siguientes sistemas puede haber un equilibrio físico dinámico o un equilibrio químico. a. Cristalización y disolución del cloruro de sodio. b. Conversión de oxígeno gaseoso en ozono. c. Condensación y evaporación de un líquido. d. Reacción entre H2 y l2 para producir Hl. e. Una solución saturada de azúcar. por favor, solo me falta esa pregunta.
Answers
Answer:
Ver explicacion
Explanation:
Cristalización y disolución de cloruro de sodio: equilibrio físico dinámico
Conversión de oxígeno gaseoso en ozono - equilibrio químico
Condensación y evaporación de un líquido - equilibrio físico dinámico
Reacción entre H2 y 12 para producir Hl - equilibrio químico
Una solución saturada de azúcar - equilibrio físico dinámico
El equilibrio alcanzado en los procesos físicos se llama procesos físicos. El equilibrio físico se llama. Ejemplos de tales procesos físicos incluyen; condensación y evaporación, cristalización y disolución, etc.
Un equilibrio dinámico ocurre en un sistema químico cuando la reacción directa y la reacción inversa se desarrollan a la misma velocidad.
I need help, plz help me with this problem

Answers
Answer:
It's b
Explanation:
I had the same exact question
During an action potential, Na
+
ions move into the cell at a rate of about 5×10
−7
mol/m
2
⋅s. - Part A How much power must be produced by the "active Na
+
pumping" system to produce this flow against a +25mV potential difference? Assume that the axon is 10 cm long and 20μm in diameter. Express your answer using one significant figure.
Answers
The power required by the "active Na⁺ pumping" system to produce this flow against the +25 mV potential difference is approximately 4 × 10⁻¹⁷ W.
To calculate the power required by the "active Na⁺ pumping" system, we need to consider the current (rate of ion movement) and the potential difference across the axon. Power is given by the equation:
Power = Current × Voltage
Given:
Current (I) = 5 × 10⁻⁷ mol/(m²·s)
Voltage (V) = +25 mV = +25 × 10⁻³ V (since 1 mV = 10⁻³ V)
To determine the power, we need to convert the current to amperes (A) and multiply it by the voltage:
I (in A) = Current × elementary charge (e)
e = 1.6 × 10⁻¹⁹ C (charge of an electron)
Now we can calculate the power:
Power = I × V
First, let's convert the current from mol/(m²·s) to A/m²:
I (in A/m²) = Current (in mol/(m²·s)) × Avogadro's number (Nₐ) / time (s)
Nₐ = 6.022 × 10²³ mol⁻¹ (Avogadro's number)
Now, we can calculate the power:
Power = I (in A/m²) × V (in V)
Note: We assume the axon is a cylinder with a circular cross-section.
Given:
Length of axon (L) = 10 cm = 0.1 m
Diameter of axon (d) = 20 μm = 20 × 10⁻⁶ m
To calculate the cross-sectional area (A) of the axon, we use the formula for the area of a circle:
A = π × (d/2)²
Now, we can calculate the power:
Power = I (in A/m²) × V (in V) × A (in m²)
Substituting the given values:
A = π × (20 × 10⁻⁶ / 2)² = π × 100 × 10⁻¹² m²
Power = (5 × 10⁻⁷ A/m²) × (25 × 10⁻³ V) × (π × 100 × 10⁻¹² m²)
Simplifying the expression:
Power ≈ 4 × 10⁻¹⁷ W
Rounding to one significant figure, the power required by the "active Na⁺ pumping" system to produce this flow against the +25 mV potential difference is approximately 4 × 10⁻¹⁷ W.
To know more about potential difference , visit:
https://brainly.com/question/23716417
#SPJ11
What volume of 0. 455 m koh solution is needed to neutralize 82. 0 ml of 0. 150 m solution of nitrous acid?.
Answers
A total volume of 27.03 ml of KOH is required to neutralize 82 ml of 0.150M Nitrous acid.
Let us first write the balanced chemical equation of reaction of KOH and HNO₂,
KOH + HNO2 → KNO₂ + H₂O
From the reaction, we can see,
One mole of KOH reacts completely with one mole HNO₂ to form KNO₂ and H₂O.
So,
One mole KOH = One mole HNO₂
We also know,
Molarity = moles/volume of solution
So,
Moles = molarity × volume of solution
Moles of KOH = 0.455 × V
Moles of HNO₂ = 0.150 × 82
So,
0.455 × V = 0.150 × 82
V = 27.03ml
So, the volume of KOH required is 27.03 ml.
To know more about Molarity, visit,
https://brainly.com/question/26873446
#SPJ4
which of the following is the weakest? hydrogen bonds dipole forces dispersion forces ionic bonds
Answers
Among hydrogen bonds, dipole forces, dispersion forces, and ionic bonds, the weakest bond is: A. hydrogen bonds.
A chemical bond is defined as the forces of attraction that are existing between ions, crystals, atoms or molecules and they are mainly responsible for the formation of chemical compounds.
Hence, a chemical bond refers to a force holding atoms together and binding ions, crystals, or molecules together, in order to form a chemical compound.
Typically, there are three main types of chemical bonds and these are:
Hydrogen bonds.Covalent bonds.Ionic bonds.Hydrogen bond can be defined as a weak chemical bond existing between a partially positively charged hydrogen atom and an electronegative chemical atom.
A hydrogen bond has the strongest intermolecular force of attractions, but it is weaker than an ionic bond or covalent bond.
Read more: https://brainly.com/question/24212500
A sample of an unknown alkaline earth metal hydroxide is dissolved in 100.0 mL of water. The resulting solution is titrated to an indicator endpoint with 1.008 M aqueous hydrochloric acid. The indicator changes color after 17.00 mL of the acid has been added.
Write the generic equation.
If the sample amount is 1.042 g, what is the molar mass of the unknown metal hydroxide?
What is the identity of the metal?
Answers
The generic equation is M(OH)2 (s) + 2HCl (aq) → 2H2O (l) + MCl2 (aq), the molar mass of the unknown metal hydroxide is 121.4 g/mol and the identity of the metal is strontium (Sr).
The generic equation for the reaction between an alkaline earth metal hydroxide and hydrochloric acid is:
M(OH)2 (s) + 2HCl (aq) → 2H2O (l) + MCl2 (aq)
From the balanced equation, we can see that the stoichiometry of the reaction is 1:2 between the metal hydroxide and the hydrochloric acid. This means that for every mole of metal hydroxide reacted, 2 moles of hydrochloric acid are required.
To find the molar mass of the unknown metal hydroxide, we first need to calculate the number of moles of hydrochloric acid used in the titration:
Number of moles of HCl = 1.008 M × 0.01700 L = 0.01714 mol
Since 2 moles of HCl react with 1 mole of the metal hydroxide, the number of moles of metal hydroxide in the sample is:
Number of moles of metal hydroxide = 0.01714 mol / 2 = 0.00857 mol
We can now calculate the molar mass of the unknown metal hydroxide by dividing the mass of the sample by the number of moles:
Molar mass of metal hydroxide = 1.042 g / 0.00857 mol = 121.4 g/mol
To identify the metal, we can compare the molar mass of the unknown metal hydroxide (121.4 g/mol) to the molar masses of known alkaline earth metal hydroxides. The possible metals are beryllium, magnesium, calcium, strontium, and barium.
Beryllium hydroxide (Be(OH)2) has a molar mass of 43.03 g/mol, which is much lower than the molar mass of the unknown metal hydroxide. Magnesium hydroxide (Mg(OH)2) has a molar mass of 58.32 g/mol, which is also lower than the unknown metal hydroxide. Calcium hydroxide (Ca(OH)2) has a molar mass of 74.09 g/mol, which is still lower than the unknown metal hydroxide. Strontium hydroxide (Sr(OH)2) has a molar mass of 121.63 g/mol, which is very close to the molar mass of the unknown metal hydroxide. Finally, barium hydroxide (Ba(OH)2) has a molar mass of 171.34 g/mol, which is higher than the molar mass of the unknown metal hydroxide.
Based on the molar mass, the identity of the unknown metal is most likely strontium (Sr).
Know more about alkaline earth metal here:
https://brainly.com/question/27786158
#SPJ11
Directions: Answer the following questions in your own words using complete sentences. Do not copy and paste from the lesson or the internet.
1. How does the condition of the soil impact all aspects of the environment?
2. Conduct research on an extinct species. Identify the species, discuss the reasons for extinction, and how the extinction may have impacted the environment.
3. Conduct research on a threatened or endangered species. Identify the species, discuss the threats to the species, and any attempts to save the species. The species may be plant or animal.
4. Locate a park or other natural space near your home. Explain what type of natural space it is, when and how it was established, and the major purpose of the space.
5. What impact does it have on the environment if one type of biome is damaged or under threat?
Answers
Answer:
This took forever T-T
Explanation:
1. The condition of the soil has a big impact on the environment. Good soil helps plants grow, supports different kinds of life, and prevents erosion. It also keeps nutrients in balance and affects the quality of water and air. If the soil is unhealthy or polluted, it can harm plants, animals, and the overall ecosystem.
2. The dodo bird is an example of a species that no longer exists. It used to live on an island called Mauritius. Sadly, people hunted the dodo bird for food and destroyed its habitat. They also introduced other animals that harmed the dodo bird's population. Because of these reasons, the dodo bird became extinct. This affected the environment because the dodo bird played a role in spreading seeds and helping plants grow.
3. The Sumatran orangutan is a species in danger of disappearing. Its biggest threats are losing its home due to forests being cut down for palm oil, illegal hunting, and being taken as pets. People are working to protect the orangutans by preserving their habitat, rescuing and rehabilitating them, and educating communities about their importance.
4. Central Park in New York City is a natural area created in 1857. It was made for people to enjoy nature in the middle of the city. People can do many outdoor activities there like walking, picnicking, and playing sports. The park is also home to various birds and animals, which adds to the city's biodiversity.
5. When a certain environment, like a forest or a desert, is damaged or in danger, it has a big impact on the whole ecosystem. Many different plants and animals depend on each other in these environments. If something harms or destroys their homes, it can lead to the loss of species, disruption of food chains, and less diversity. It can also affect important processes like water and carbon cycles, and even influence the climate. People who rely on these environments for resources and livelihoods are also affected. That's why it's important to protect and take care of these natural areas.
Zinc oxide + carbon =
Answers
Answer:
carbondioxe and zinc as solid
what volume of water has the same mass as 100 cm3 of gold?
Answers
The volume of water that has the same mass as 100 cm³ of gold is 1,932 cm³.
To find the volume of water which has the same mass as 100 cm³ of gold, the volume of the water and the mass of the gold needs to be taken into consideration. A gold of 100 cm³ has a certain mass and to find the volume of water which has the same mass as the gold, the following formula can be used;
Density = Mass ÷ Volume
Density is equal to the mass of the object divided by the volume of the object. In other words, it tells us how tightly matter is packed together. By rearranging the formula, Volume can be calculated;
Volume = Mass ÷ Density
Density of gold = 19,320 kg/m³
Therefore, the mass of 100 cm³ of gold can be converted to volume of water which has the same mass as gold using the formula:
Volume = Mass of Gold ÷ Density of Water
Here, the volume of gold is 100 cm³.
1 cm³ = 0.000001 m³
Since mass of gold is not given, let's assume that the gold is of a certain mass of 19,320 kg/m³.
Hence,
Mass of Gold = 19,320 x (100 x 10^-6)
Mass of Gold = 1.932 kg/m³
Volume of Water = Mass of Gold ÷ Density of Water
The density of water is approximately equal to 1,000 kg/m³
Therefore,
Volume of Water = Mass of Gold ÷ Density of Water
Volume of Water = 1.932 ÷ 1,000
Volume of Water = 0.001932 m³
To convert 0.001932 m³ into cm³,
it can be multiplied by 1,000,000.
Hence, Volume of Water = 1,932 cm³
Therefore, the volume of water that has the same mass as 100 cm³ of gold is 1,932 cm³.
Learn more about the density from the given link-
https://brainly.com/question/26364788
#SPJ11
What size volumetric flask would you use to create a 1.25 M solution using 29.75 g of MgCl2?
Answers
Answer:
250 ml
Explanation: I got it right on the lab.
The size of the volumetric flask that would you use to create a 1.25 M solution using 29.75 g of MgCl₂ is 250mL.
How do we calculate volume of the solution?
Volume (V) in liter of the solution will be calculated by using the molarity (M) formula as:
M = n/V, where
n is the moles of the solute and it will be calculated by using mass as:
n = W/M, where
W = given mass
M = molar mass
Moles of 29.75g of MgCl₂ = 29.75g / 95g/mol = 0.313 mol
Given that molarity of solution = 1.25M
On putting values on the equation of molarity we will get the value of volume as:
V = 0.313 / 1.25 = 0.2504 L = 250mL
For the preparation of 250 mL of MgCl₂ solution we required the volumetric flask of 250 mL.
Hence volumetric flask of 250mL is required.
To know more about volumetric flask, visit the below link:
https://brainly.com/question/24196698
#SPJ2
the temperature will decrease the rate of reactions.
Answers
Answer:
true?
Explanation:
a 172.0-mci sample of a radioactive isotope is purchased by a medical supply house. if the sample has a half-life of 14.0 days, how long will it be before its activity is reduced to 16.0 mci?
Answers
Before its activity is lowered to 16.0 mci, it will take 47 days. A medical supply store buys a radioactive isotope sample with a 172.0-mci sample. The term "radioisotope" refers to an element's radioactive form.
which consists of atoms with unstable nuclei that undergo radioactive decay to stable forms, producing distinctive alpha, beta, or gamma radiation. Radioisotopes are an element's radioactive isotopes. They are the atoms with unstable neutron-proton combinations or excess energy in their nuclei. During those processes, the radionuclide is said to experience radioactive decay, albeit the surplus energy may be put to use in any number of ways. one of two or more atoms with a nuclear makeup that has a different amount of neutrons than protons but the same number of protons overall.
t = - ln(R/Ro)/lamda
t = -14 (ln(16/172)/ln2
t = 47 days.
Learn more about radioactive isotope here
https://brainly.com/question/1907960
#SPJ4
Answer question number 6 (only that one if you want to): answer for questions:4: T = 296 K5: 5 g more


Answers
Explanation:
Freezing point depression:
It's colligative property and it describes that when we add a solute to a solvent the freezing point of the solution goes down. The formula that describes this property is:
ΔTf = i * Kf * m
Where ΔTf is the freezing point depression, i is the Van't Hoff factor, Kf is the constant and m is the molality of the solution.
Molality is expressed as the moles of solute divided by the mass of solvent in kg.
molality = moles of solute/kg of solvent
According to the problem we are dilluting the solution adding 100 g of water. That means that the molality of the solution will decrease (we are dividing by a greater number). And if we look at the formula of the freezing point depression we will see that if the molality decreases the freezing point depression decreases. So, the freezing point of the solution will increase.
Boiling point elevation:
When we add a solute to a solvent the boiling point of the solution will be elevated.
ΔTb = i * Kb * m
Where ΔTb is the boiling point elevation, i is the Van't Hoff factor, Kb is the constant and m is the molality of the solution.
If we are dilluting the solution adding 100 g of water, the molality of the solution will decrease. If the molality decreases the boiling point elevation decreases. So, the boiling point of the solution will decrease.
Answer: The freezing point goes up or increases. The boiling point goes down or decreases.
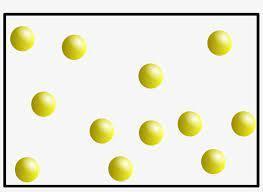
CCC Systems and System Models Liquid gallium changes to a gas at 2229°C.
Describe how a model of gaseous gallium would compare to the model of liquid
gallium shown in the picture.
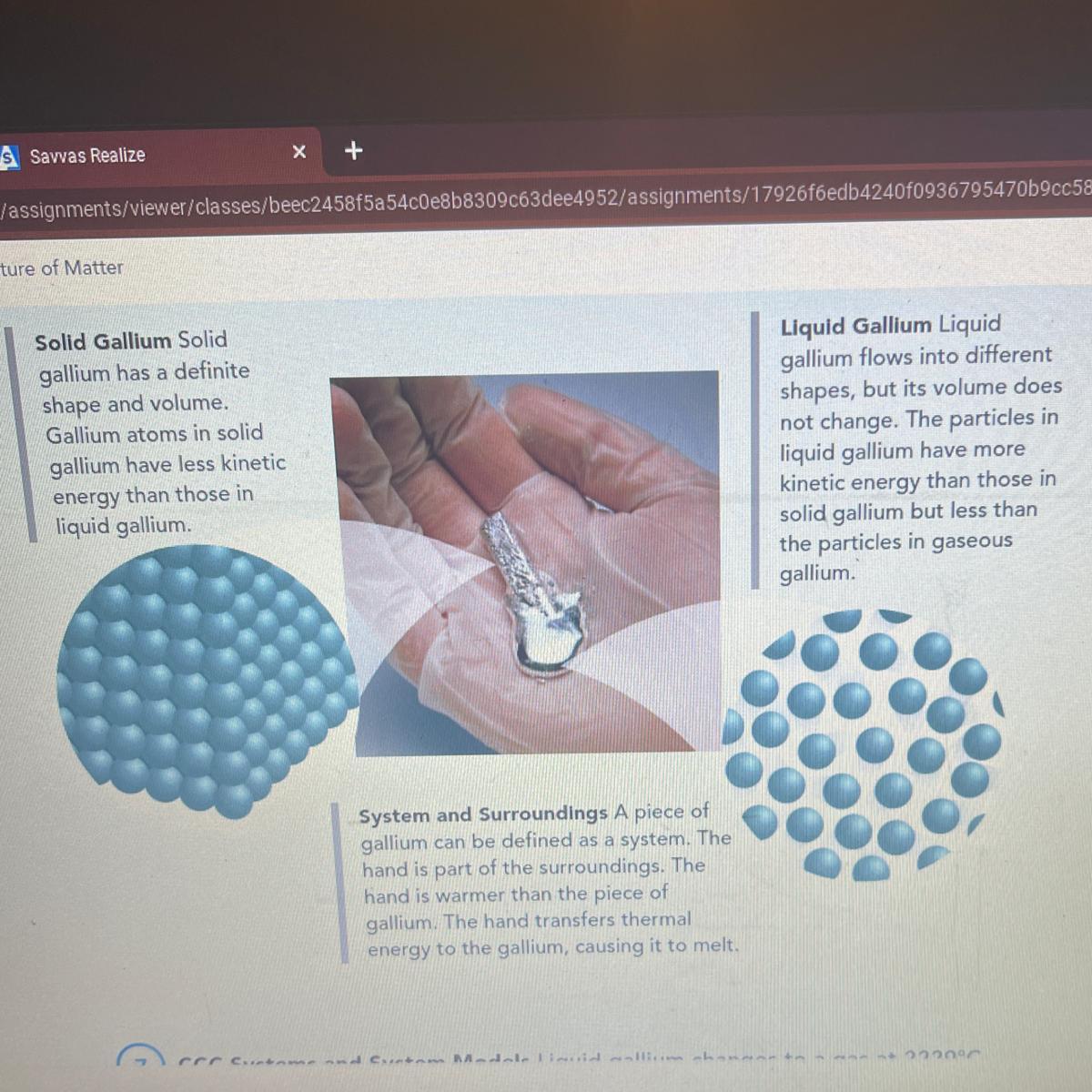
Answers
Gaseous gallium will consist of atoms separated from each other by a large distance and do not experience inter-particle attractive forces.
There are three states of matter;
SolidLiquidGasMatter can exist in all these states of matter depending on the extent of inter-particle attractive forces in the substance.
Inter-particle attractive forces are strongest in solids hence they have a definite shape and volume and do not flow.
Liquids have weaker inter-particle attractive forces than solids hence liquid particles can flow. Liquids have a definite volume but not a definite shape.
Gases have almost no inter-particle attractive forces between particles hence gas particles are separated by large distances and move at very high velocities. A gas does not have definite shape or volume.
A model of gaseous gallium will show atoms of gallium separated by large distances and do not experience inter-particle attraction.
A model of gas particles is shown in the image attached to this answer.
Learn more: https://brainly.com/question/11067389
what is the name of the subatomic particles that can increase or reduce size & mass?
Answers
The size and mass of an atom are determined by the number of protons, neutrons, and electrons it contains and can increase or reduce size & mass.
Protons and neutrons are the subatomic particles responsible for most of the mass of an atom. Protons have a mass of approximately 1 atomic mass unit (amu), while neutrons have a slightly larger mass of approximately 1.008 amu. Electrons, on the other hand, have a much smaller mass of approximately 0.0005 amu.
The number of protons and neutrons in an atom determines its atomic mass, which is the sum of the masses of all its protons and neutrons. The number of electrons determines the chemical properties of an atom and how it interacts with other atoms.
In summary, subatomic particles, such as protons, neutrons, and electrons, do not have the ability to increase or decrease the size and mass of an atom. Rather, they determine the fundamental properties of an atom, including its size, mass, and chemical behaviour.
To learn more about subatomic particles Click here:
brainly.com/question/29765133
#SPJ4
According to its nutrition label, orange soda contains 49 g of sugar per 355-mL serving. If the density of the beverage is 1.043 g/mL, what is the percent sugar concentration in orange soda? (Hint: This is a two-step problem. First use the density to convert the 355-mL serving size to grams. Then calculate percent sugar in the beverage.)
Answers
The percent sugar concentration in orange soda is approximately 13.24%.
The volume of orange soda is given as 355 mL and its density is given as 1.043 g/mL. According to the nutrition label, there are 49 g of sugar in a 355 mL serving of orange soda.Using the density, we can convert the 355 mL volume into grams as follows:Volume = 355 mL; Density = 1.043 g/mL; Mass = ?To convert mL to g we need to multiply the volume with the density. Thus,Mass = Volume x Density= 355 x 1.043= 369.965 gThus, the mass of a 355 mL serving of orange soda is approximately 369.965 g.Next, we can calculate the percentage of sugar in the beverage as follows:Percent sugar concentration = (Mass of sugar / Total mass of beverage) x 100%Percent sugar concentration = (49 g / 369.965 g) x 100%Percent sugar concentration = 0.1324 x 100%Percent sugar concentration = 13.24%Therefore, the percent sugar concentration in orange soda is approximately 13.24%.
learn more about concentration
https://brainly.com/question/15306782
#SPJ11
3 reasons why aluminium is used in making of cooking vessels
Answers
Answer is : It is a good thermal and electrical conductor. -The main point to be noted is that aluminium is a highly reactive element and still it is used for making cooking utensils. The reason is that aluminium has a very high affinity for oxygen. So, it reacts with oxygen and forms a layer of aluminium oxide on its surface.
Answer:
This is because aluminium reacts with oxygen present in air to form a thin layer of aluminium oxide . This oxide layer is very stable and prevents further reaction of aluminium with oxygen . Also , it is light in weight and a good conductor of heat Hence , it is used to make cooking utensils .
Explanation:
Believe it