Answers
Answer:
H₂(g) + Cu²⁺(aq) → 2H⁺(aq) + Cu(s)
Explanation:
In a redox reaction, one half-reaction is the oxidation (where the atom loss electrons) whereas the other reaction is the reduction (Where the atom is gaining electrons.
In the reactions:
H₂(g) → 2H⁺(aq) + 2e⁻ oxidation
Here, the reaction is written as the oxidation because the hydrogen H₂ is in oxidation state 0 and H⁺ in +1. That means each atom is loosing one electron.
Cu²⁺(aq) + 2e⁻ → Cu(s) reduction
And here, the Cu²⁺ is in +2 oxidation state and after the reaction is in Cu(s) 0 state. Thus, each atom is gaining 2 electrons.
The sum of both reactions is:
H₂(g) + Cu²⁺(aq) + 2e⁻ → 2H⁺(aq) + 2e⁻ + Cu(s)
Subtracting the electrons in both sides of the reaction:
H₂(g) + Cu²⁺(aq) → 2H⁺(aq) + Cu(s)Related Questions
In which way are photosynthesis and cellular respiration different?
A.
Cellular respiration stores ATP, while photosynthesis releases ATP.
B.
Cellular respiration produces oxygen, while photosynthesis uses oxygen.
C.
Photosynthesis releases energy, while cellular respiration stores energy.
D.
Photosynthesis used carbon dioxide, while cellular respiration produces carbon dioxide.
Answers
Answer:
D. Photosynthesis uses carbon dioxide while cellular produces carbon dioxide
1. HCI (aq) + NaOH (aq) → NaCl (aq) + H₂O (1)
a. What are the reactants?
b. What are the products?
Answers
In the given reaction, the reactants are hydrochloride acid (HCI) and sodium hydroxide (NaOH). The products are sodium chloride (NaCl) and water.
The chemical equation provided represents a neutralization reaction between hydrochloric acid (HCI) and sodium hydroxide (NaOH) in aqueous solution.
A neutralization reaction is a type of double displacement reaction in which an acid and a base react to form salt and water.
The reactants in this equation are hydrochloric acid (HCI) and sodium hydroxide (NaOH). Hydrochloric acid is a strong acid that dissociates in water to form hydrogen ions (H+) and chloride ions (Cl-). Sodium hydroxide, on the other hand, is a strong base that dissociates in water to form sodium ions (Na+) and hydroxide ions (OH-).
To learn more about reactants, follow the link:
https://brainly.com/question/29816521
#SPJ1
g Calculate the energy of a photon that has a wavelength of 965 nm. 2.06x10-33 J 2.06x10-19 J 3.78x10-28 J 3.78x10-29 J None of these
Answers
Answer: The energy of a photon that has a wavelength of 965 nm is \(2.06\times 10^{-16}J\)
Explanation:
The relation between energy and wavelength of light is given by Planck's equation, which is:
\(E=\frac{hc}{\lambda}\)
where,
E = energy of the photon =
h = Planck's constant = \(6.626\times 10^{-34}Js\)
c = speed of light = \(3\times 10^8m/s\)
\(\lambda\) = wavelength of light = 965 nm = \(965\times 10^{-9}m\) \((1nm=10^{-9}m)\)
Putting in the values we get:
\(E=\frac{6.626\times 10^{-34}\times 3\times 10^8m/s}{965\times 10^{-9}m}=2.06\times 10^{-16}J\)
Thus the energy of a photon that has a wavelength of 965 nm is \(2.06\times 10^{-16}J\)
name two physical properties that could be used to distinguish between the material in each pair 1,cork and lead 2,copper and silver 3,water and benzene 4,sulphur and iron?
Answers
The physical properties of each material can be used to tell each apart. One example of these properties is their respective densities and polarities, among others.
In the case of Cork and Lead, we can name differences such as:
Densities, since lead has a very high density when compared to the low density of corkColors, given that lead is a blueish silver color and cork is not. Melting points are another way to tell them apart since lead melts at 330 degrees while cork begins to melt at 200.As for copper and silver, though not identical in numbers, both elements share a wide range of properties. One physical property to be used when telling them apart is color, as silver has its own aptly named color whilst copper shines in a bright brown-red coloration.
As opposed to the previous group, benzene and water can often be physically identical. However, this does not mean that they share all physical properties. One example of this and a property that can tell them apart is their polarity, given that water is highly polar and benzene is not polar at all.
Of the groups listed, the grouping of sulfur and iron is the easiest to differentiate given that they possess distinct:
ColorMelting pointsBoiling pointsOdorsConductivitiesand so on.
These are some properties that are different between each grouped elements and can be used by scientists or students to differentiate the substances from one another.
To learn more visit:
https://brainly.com/question/18327661?referrer=searchResults
Write the chemical formula for and name the following compounds.10) CH3-CH2-CH2-CH2-CH2-CH2-CH311) CH3-CH2-CH2-CH312) CH3-CH2-CH2-CH2-CH3
Answers
1) To write the chemical formula you need to count the number of each element and put it in the following order: CHONPS
10) CH3-CH2-CH2-CH2-CH2-CH2-CH3
There is seven carbons and 16 hydrogen atoms. So the chemical formula is C₇H₁₆.
It's an alkane, so the name is prefixed with the carbon number and ending with ane.
The name of the compound is heptane.
11) CH3-CH2-CH2-CH3
There is four carbons and 10 hydrogen atoms. So the chemical formula is C₄H₁₀.
It's also an alkane, so the name is prefixed with the carbon number and ending with ane.
The name of the compound is butane.
12) CH3-CH2-CH2-CH2-CH3
There is five carbons and 12 hydrogen atoms. The chemical formula is C₅H₁₂.
It's also an alkane, so the name is prefixed with the carbon number and ending with ane.
The name of the compound is pentane.
What is the wavelength of an electromagnetic wave that has a frequency of
2,45 x 10° Hz in a vacuum? (The speed of light in a vacuum is 3,00 x 108
m/a.)
O A. 8.17 m
OB, 1.22 x 10 m
c. 7,35% 1017 m
O
D. 4.08 * 10-10 m

Answers
Answer:
B
Explanation:
To calculate questions like this, you need to make use of the equation below:
ν = c / λ
ν is the frequency, c is the speed of light, and λ is the wavelength
ν = c / λ
2.45 • 10^9 Hz = (3•10^8 m/s)/(λ)
λ = 0.122 m = B
List the following compounds in order of elution from a gc column that separates by boiling point only: acetic acid, diethyl ether, ethanol, hexane, octane, pyridine Explain the relative boiling points in terms of the structure and intermolecular forces in the molecules.
Answers
The order of elution from a GC column based on boiling point only would be: hexane, octane, ethanol, diethyl ether, acetic acid, pyridine.
The boiling points of these compounds are related to the intermolecular forces present in the molecules. Hexane and octane are both alkanes with nonpolar C-H bonds and weak Van der Waals forces. The weak intermolecular forces between nonpolar molecules cause these compounds to have relatively low boiling points. Ethanol, diethyl ether and acetic acid all contain O-H bonds which are polar, leading to stronger intermolecular forces and higher boiling points. Pyridine has a particularly strong intermolecular force, hydrogen bonding, due to its nitrogen-containing heterocyclic ring, which leads to its highest boiling point of the compounds.
To know more about boiling point click on below link :
https://brainly.com/question/2153588#
#SPJ11
Help! Can someone please explain and break down the answers to both? I’m so confused!
1) The moles of H2O that f am be obtained from 15.0mL of 0.250 M HCl
2) the volume of 0.150 M KMnO4 needed to replaced 1.85 mol MnCl2

Answers

How can the third digit of the VSPER number be determined if only 1st two VSEPR number are known
Answers
The VSEPR (Valence Shell Electron Pair Repulsion) theory describes the arrangement of electron pairs around a central atom in a molecule, and the VSEPR number represents the total number of electron pairs around the central atom, including both bonding pairs and lone pairs.The VSEPR number can be determined from the Lewis structure of the molecule, which shows the arrangement of atoms and lone pairs around the central atom. The first two digits of the VSEPR number correspond to the number of bonding pairs and lone pairs, respectively, around the central atom.To determine the third digit of the VSEPR number, you need to consider the shape of the molecule. The shape is determined by the repulsion between electron pairs, which is strongest between lone pairs and decreases in the order lone pair-lone pair > lone pair-bonding pair > bonding pair-bonding pair.The third digit of the VSEPR number indicates the shape of the molecule according to the following scheme:1: linear shape2: trigonal planar shape3: tetrahedral shape4: trigonal bipyramidal shape5: octahedral shapeThus, to determine the third digit of the VSEPR number, you need to determine the shape of the molecule based on the number of electron pairs and their relative positions. This can be done by applying the VSEPR theory and considering the repulsion between electron pairs. Alternatively, you can consult a table or chart that lists the shapes associated with different VSEPR numbers.
Lewis Structure for NO3-
Answers
Answer::
Explanation::
What are five constitutional isomers of hydroxymethylcyclohexane that contain the cyclohexane ring?
Answers
The five constitutional isomers of hydroxymethylcyclohexane that contain the cyclohexane ring are:
hydroxymethylcyclohexanemethyl cyclohexanol2-methyl cyclohexanol3-methyl cyclohexanol4-methyl cyclohexanolHydroxymethylcyclohexane is an alcohol that contains a cyclohexane ring, as shown in the attached picture.
Constitutional or structural isomers are compounds with the same molecular formula but different structural formulas.
We can find the five constitutional isomers of hydroxymethylcyclohexane that contain the cyclohexane ring by attaching the hydroxyl group to the cyclohexane ring and varying the positions of the methyl group, as shown in the picture.
The five constitutional isomers of hydroxymethylcyclohexane that contain the cyclohexane ring are:
hydroxymethylcyclohexanemethyl cyclohexanol2-methyl cyclohexanol3-methyl cyclohexanol4-methyl cyclohexanolLearn more: https://brainly.com/question/14391080

What is the mass of a sample of copper with a volume of 4.5 cubic centimeters?
Answers
The mass of a sample of copper with a volume of 4.5 cubic centimeters is 40.32 grams.
A substance's density is defined as its mass per unit of volume. Density is most frequently represented by the symbol, however Latin letter D may also be used. Mass divided by volume is the formula for density in mathematics: ρ = m / v , where ρ is the density, m is the mass, and V is the volume.
So, ρ = m/v
8.96 = m / 4.5
40.32 g = mass
Therefore, the mass of a sample of copper with a volume of 4.5 cubic centimeters is 40.32 grams.
Learn more about density here;
https://brainly.com/question/15164682
#SPJ9
When zinc reacts with copper sulfate solution, zinc sulfate solution and copper are formed.(i) An experiment was carried out to measure the temperature change when zinc powder reactswith copper sulfate solution.initial temperature of copper sulfate solution = 20 °Cfinal temperature of mixture after the reaction = 46 °CExplain what the temperature readings show about the type of heat change that occurs duringthis reaction.
Answers
The temperature increase from 20 °C to 46 °C indicates that the reaction between zinc and copper sulfate solution is exothermic, with heat being released into the surroundings.
In the given reaction between zinc and copper sulfate solution, the temperature change can provide insights into the type of heat change occurring during the reaction. Based on the provided information, the initial temperature of the copper sulfate solution was 20 °C, and the final temperature of the mixture after the reaction was 46 °C.
The temperature increase observed in this reaction indicates an exothermic heat change. An exothermic reaction releases heat energy into the surroundings, resulting in a temperature rise. In this case, the reaction between zinc and copper sulfate solution is exothermic because the final temperature is higher than the initial temperature.
During the reaction, zinc displaces copper from copper sulfate to form zinc sulfate and copper metal. This displacement reaction is known as a single displacement or redox reaction. Zinc is more reactive than copper and therefore replaces copper in the compound.
The formation of new chemical bonds during the reaction releases energy in the form of heat. This energy is transferred to the surroundings, leading to an increase in temperature. The heat released is greater than the heat absorbed, resulting in a net increase in temperature.
The exothermic nature of this reaction can be explained by the difference in bond energies between the reactants and products. The breaking of bonds in the reactants requires energy input, while the formation of new bonds in the products releases energy.
In this case, the energy released during the formation of zinc sulfate and copper metal is greater than the energy required to break the bonds in copper sulfate and zinc.
For more such question on temperature visit:
https://brainly.com/question/4735135
#SPJ8
Fructose-1-phosphate can be hydrolyzed into fructose + inorganic phosphate (Pi) with a ΔG° of –16.0 kJ/mol. If ATP can be hydrolyzed into ADP + Pi with a ΔG° of –30.5 kJ/mol, what is the free energy change for the reaction of fructose + ATP → fructose 1-phospate + ADP
Answers
ΔG° (overall reaction) = ΔG° (sum of products) - ΔG° (sum of reactants)
Given:
ΔG° for the hydrolysis of fructose-1-phosphate = -16.0 kJ/mol
ΔG° for the hydrolysis of ATP = -30.5 kJ/mol
The reaction we want to calculate the ΔG° for is:
fructose + ATP → fructose 1-phosphate + ADP
From the given information, we can break down the reactants and products:
Sum of reactants:
fructose + ATP
Sum of products:
fructose 1-phosphate + ADP
Now, we can calculate the ΔG° for the overall reaction:
ΔG° (overall reaction) = ΔG° (sum of products) - ΔG° (sum of reactants)
ΔG° (overall reaction) = (-16.0 kJ/mol) + (-30.5 kJ/mol)
ΔG° (overall reaction) = -46.5 kJ/mol
Therefore, the free energy change (ΔG°) for the reaction of fructose + ATP → fructose 1-phosphate + ADP is -46.5 kJ/mol.
A rigid 3.80 L sealed vessel contains 0.650 mol Ne, 0.321 mol Kr, and 0.190 mol Xe. Find the density of the mixture in g/L.
Answers
Answer:
17.09g/L
Explanation:
Density = total mass of elements/ volume
We need to find the mass of each mixture constituents using their molar mass:
mole = mass/molar mass
For Neon (Ne) which contains 0.650mol;
0.650 = mass/20.18
mass = 0.650 × 20.18
mass = 13.12g
For Krypton (Kr) which contains 0.321mol;
0.321 = mass/83.79
mass = 0.321 × 83.79
mass = 26.89g
For Xenon (Xe) which contains 0.190mol;
0.190 = mass/131.3
mass = 0.190 × 131.3
mass = 24.95g
Total mass = 13.12g + 26.89g + 24.95g = 64.96g
Density = total mass / volume
Density = 64.96g / 3.80L
Density of the mixture = 17.09g/L
how many sulfur atoms are there in 5.5 moles?
Answers
Answer:
3.31x10^24 atoms
Explanation:
1 mole of S atom = 6.023^23 atoms
5.5 moles = 5.5 x 6.023^23
= 3.31x10^24 atoms
Given below are the nuclear symbols for three different types of nuclear particles: a proton (H+), a deuteron (2H+), and a helium-3 nucleus (3 He2+). Assuming all three particles have the same energy, arrange them in order from most penetrating to least penetrating.
1. = A 3 He2+
2. = B TH+
3. = C 2H+
Answers
Answer:
\(^1 H^+ < \ \ ^2H^+< \ \ ^3He^{2+}\)
Explanation:
Based on the given information, assuming that all three nuclear particles are isoelectronic, the greater the attraction from the nucleus, the greater the penetration. The greater the attraction to the nucleus, however, the less shielding there would be, resulting in a high effective nuclear charge.
Hence, in increasing of most penetrating to least penetrating, the arrangement is as follows.
\(^1 H^+ < \ \ ^2H^+< \ \ ^3He^{2+}\)
least most
penetrating penetrating
How many molecules are there in 3 moles of aluminum chloride ?
Answers
there are 4 atoms in aluminum chloride
The domain Archaea are unicellular prokaryotes and can be autotrophs or heterotrophs
true or false?
Answers
Answer:
I think its true I dont really know
Explanation:
true
What is the mass number for an atom of xenon containing 54 protons and 75 neutrons?
Answers
Answer:
Answer should 133
Explanation:
all you have to do is add the protons and neutrons
The mass number for an atom of xenon containing 54 protons and 75 neutrons is 129 amu.
What is an Atomic mass of an atom?The Atomic mass of any atom may be defined as the mass of an atom that is togetherly constituted by protons and neurons in the nucleus of an atom. It is approximately equivalent to the number of protons and neutrons in the atom (the mass number).
The mass number of Xenon is calculated by the following formula:
The Number of neutrons = Mass number - Atomic number.
where, number of neutrons = 75, and number of protons = 54.
75 = Mass number - 54 (number of protons = atomic number).
Mass number = 75 + 54 = 129 amu.
Therefore, the mass number for an atom of xenon containing 54 protons and 75 neutrons is 129 amu.
To learn more about the Mass number, refer to the link:
https://brainly.com/question/3187640
#SPJ2
How many ions are formed during the dissociation of 500 molecules of carbonic acid, if it dissociates in the first degree by 20%, and in the second degree by 1%? Explain your answer.
Answers
The dissociation of 500 molecules of carbonic acid would produce 200 + 15 = 215 ions.
What is Dissociation?
Dissociation is a chemical process in which a compound breaks down into two or more simpler components, usually ions, when it is exposed to a suitable solvent or energy source such as heat or light. In other words, it is the separation of a molecule or compound into smaller particles such as atoms, ions, or radicals.
The dissociation of carbonic acid (H2CO3) in the first degree produces two ions (H+ and HCO3-) per molecule, while the dissociation in the second degree produces three ions (H+, CO32-, and HCO3-) per molecule.
If 500 molecules of carbonic acid dissociate in the first degree by 20%, then 20% of the molecules (0.2 x 500 = 100) will dissociate, producing 100 x 2 = 200 ions (H+ and HCO3-).
If 500 molecules of carbonic acid dissociate in the second degree by 1%, then 1% of the molecules (0.01 x 500 = 5) will dissociate, producing 5 x 3 = 15 ions (H+, CO32-, and HCO3-).
Learn more about Dissociation from the given link
https://brainly.com/question/16818822
#SPJ1
At 20C what is the molar mass of a gas with a denisty of 1.02g/L at 2.13atm
Answers
The molar mass of a gas with a density of 1.02 g/L at 2.13 atm and a temperature of 20°C is 47.9 g/mol.The molar mass of an element or compound is the mass of one mole of that substance. A mole is the SI unit for the amount of a substance.
It's defined as the amount of a substance that contains the same number of entities as there are atoms in 12 grams of carbon-12.Molar mass (M) = mass (m) ÷ amount of substance (n)So, M = m/n
Where m is the mass in grams and n is the number of moles. The unit of molar mass is grams per mole (g/mol).
The ideal gas law is used to calculate the molar mass of a gas. The ideal gas law is:P V = n R T,Where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature.
Convert the density to grams per liter: 1.02 g/L.
The density is mass/volume, thus 1.02 g/L means that 1 liter of the gas weighs 1.02 g.
This means that 1 mole of gas will occupy 22.4 L (at standard temperature and pressure, STP).Calculate the number of moles of gas using PV = nRT.P = 2.13 atmV = 22.4 L (at STP)R = 0.0821 L·atm/K·molT = 273.15 K + 20 K = 293.15 K
Thus, n = PV/RT = (2.13 atm × 22.4 L)/(0.0821 L·atm/K·mol × 293.15 K) = 0.973 mol
Calculate the molar mass (M) using M = m/n.m = density × volume = 1.02 g/L × 22.4 L = 22.848 gM = m/n = 22.848 g/0.973 mol = 23.5 g/mol Convert to units of grams per mole: 23.5 g/mol
The molar mass of a gas with a density of 1.02 g/L at 2.13 atm and a temperature of 20°C is 47.9 g/mol.
For more question on compound
https://brainly.com/question/12651906
#SPJ8
Balance the redox reaction by inserting the appropriate coefficients.
redox reaction:
Fe^{3 + } + NO_{2}^{-} + H_{2}O -> Fe^{2 + } + H^{ + } + NO_{3}^{-}
Fe3++NO−2+H2O⟶Fe2++H++NO−3
Answers
The balanced redox reaction is \(Fe^{3+}+NO^{2-}+H_{2}O- > Fe^{2+}+H^{+}+NO^{3-}+H_{2}O\)
To balance the redox reaction: \(Fe^{3+}+NO^{2-}+H_{2}O- > Fe^{2+}+H^{+}+NO^{3-}\), we need to ensure that the number of atoms and charges are balanced on both sides of the equation.
First, let's balance the atoms. We have one Fe atom on both sides, so it's already balanced. Next, we have two oxygen atoms on the reactant side (from \(NO^{2-}\) and \(H_{2}O\)) and three on the product side (from \(NO^{3-}\)). To balance oxygen, we can add an \(H_{2}O\) molecule to the reactant side:
\(Fe^{3+}+NO^{2-}+H_{2}O- > Fe^{2+}+H^{+}+NO^{3-}+H_{2}O\)
Now, let's balance the charges. On the reactant side, the total charge is 3+ (from \(Fe^{3+}\) ) + 1- (from \(NO^{2-}\)) = 2+. On the product side, the total charge is 2+ (from \(Fe^{2+}\)) + 1+ (from \(H^{+}\)) + 1- (from \(NO^{3-}\)) = 2+. The charges are already balanced.
Therefore, the balanced redox reaction is:
\(Fe^{3+}+NO^{2-}+H_{2}O- > Fe^{2+}+H^{+}+NO^{3-}+H_{2}O\)
By adding an additional H2O molecule to the reactant side, we balanced both the atoms and charges in the equation.
Know more about atom here:
https://brainly.com/question/17545314
#SPJ8
A sample of an unknown compound is vaporized at 160 c . The gas produced has a volume of 2330 ml at a pressure of 1.00 atm ,and it weighs 2.10 g
Round answer to 3 significants digits

Answers
The molar mass is 3230.8 g/mol
How to determine the valueFirst, we need to know that the formula for the general gas law is represented as;
PV = nRT
such that the parameters are;
P is the pressureV is the volumen is the number of molesR is the gas constantT is the temperatureSubstitute the values
1 × 2.33 = n × 8.314 × 433.15
Multiply the values, we get;
n = 2.33/ 8.314 × 433.15
Divide the values
n = 6.5 × 10⁻⁴ moles
But, number of moles = mass/molar mass
Molar mass = 2.10/ 6.5 × 10⁻⁴
Molar mass = 3230.8 g/mol
Learn about ideal gas law at: https://brainly.com/question/25290815
#SPJ1
whats the energy in joules of one mole of photons of visible light having a wavelength of 4.11×10^2 nm?
Answers
The energy in joules of one mole of photons of visible light having a wavelength of 4.11×10^2 nm? can be expressed as 2.9*10^5 J
How can the energy be calculated?From the question we were told to find the energy and the parameters that is needed to calculate these are;
c=3*10^8
h= 6.626 * 10^-34 J.s
1 mol photons = 6.023x10^23 photon
λ = 4.11×10^2 nm = 4.11 × 10-7 meters
The parameters can be input as Energy of one mole photon (E) = ( 6.023x10^23 * 6.626 * 10^-34 * 3*10^8)/ (4.11 × 10^-7)
=291302
=2.9*10^5 J
Learn more about energy at:
https://brainly.com/question/13881533
#SPJ1
Which term refers to the process by which land is worn away by natural forces or human activity
I will mark brainliest
Answers
Answer:
The answer is A. Erosion
Explanation:
Took the quiz
Answer:
A- erosion
Explanation:
Erosion occurs when water, wind, or ice removes soil and rock and deposits it in a new area.
Occurs naturally, but is also impacted by human activity.
Caused by farming, deforestation, and building roads and cities.
"The pH of a solution of household ammonia, a 0.950-M solution of NH3, is 11.612. What is Kb" for NH3
Answers
Answer:
Kb = 1.77x10⁻⁵
Explanation:
When NH₃, a weak base, is in equilibrium with waterm the reaction that occurs is:
NH₃(aq) + H₂O(l) ⇄ NH₄⁺(aq) + OH⁻(aq)
And the dissociation constant, Kb, for this equilibrium is:
Kb = [NH₄⁺] [OH⁻] / [NH₃]
To find Kb you need to find the concentration of each species. The equilibrium concentrations are:
[NH₃] = 0.950M - X
[NH₄⁺] = X
[OH⁻] = X
Where X is reaction coordinate.
You can know [OH⁻] and, therefore, X, with pH of the solution, thus:
pH = -log [H⁺] = 11.612
[H⁺] = 2.4434x10⁻¹²
As 1x10⁻¹⁴ = [H⁺] [OH⁻]
1x10⁻¹⁴ / 2.4434x10⁻¹² = [OH⁻]
4.0926x10⁻³ = [OH⁻] = X
Replacing, concentrations of the species are:
[NH₃] = 0.950M - X
[NH₄⁺] = X
[OH⁻] = X
[NH₃] = 0.9459M
[NH₄⁺] = 4.0926x10⁻³M
[OH⁻] = 4.0926x10⁻³M
Replacing in Kb expression:
Kb = [NH₄⁺] [OH⁻] / [NH₃]
Kb = [4.0926x10⁻³M] [4.0926x10⁻³M] / [0.9459M]
Kb = 1.77x10⁻⁵The branch of science which deals with the chemical bond is called Chemistry.
The correct answer to the question is \(Kb = 1.77*10^{-5\)
Explanation:
When NH₃, is acts as a weak base it forms an equilibrium with water the reaction occurs is:
\(NH_3(aq) + H_2O(l) ---><NH_4^+(aq) + OH^-(aq)\)
The formula we gonna use is as follows:-
\(Kb = \frac{[NH_4^+] [OH^-]}{[NH_3]}\)
The data is given in the question is as follows:-
[NH₃] = 0.950M - X [NH₄⁺] = X [OH⁻] = X
Where X stands for reaction coordinate.
After solving the ph of the compound the value is as follows:-
[NH₃] = \(0.9459M\) [NH₄⁺] = \(4.0926*10^{-3}M\) [OH⁻] = \(4.0926*10^{-3}M\)
Putting the value in the formula.
\(Kb = \frac{[4.0926*10^{-3}M] [4.0926*10^{-3}M]}{[0.9459M]}\)
After solving the equation the value of Kb is \(1.77*10^{-5\)
Hence, the correct answer is \(1.77*10^{-5\)
For more information, refer to the link:-
https://brainly.com/question/25026730
If I have 7.0 x 10^24 formula units of magnesium chloride, how many grams of chlorine would I have?
Answers
Answer:
824 g
Explanation:
First we convert 7.0 x 10²⁴ formula units of magnesium chloride (MgCl₂) into moles, using Avogadro's number:
7.0 x 10²⁴ formula units ÷ 6.023x10²³ formula units/mol = 11.62 mol MgCl₂There are two Cl moles per MgCl₂ moles, meaning that we have (2 * 11.62) 23.24 moles of Cl
Finally we convert 23.24 moles of chlorine into grams, using chlorine's molar mass:
23.24 mol * 35.45 g/mol = 824 gcell and chromosome characteristics?
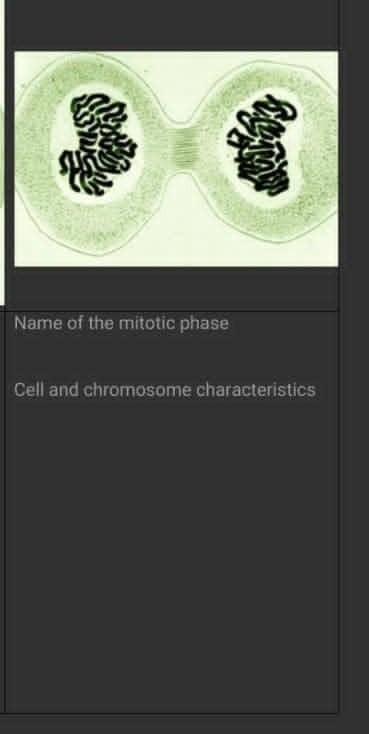
Answers
Answer:
hope the inserted image will help :)
Explanation:


Answer: Both are centered inside the nucleus.
Explanation: The characteristics between cells and chromosomes are that they both are centered inside the nucleus (which is a cell). This characteristic applies to both plant and animal cells.
Suppose that 25.0 mL of 0.440 M sodium chloride is added to 25.0 mL of 0.320 M silver nitrate. How many moles of silver chloride precipitate? What would be the concentrations of each of the ions in the reaction mixture after the reaction?
Answers
The number of moles of silver chloride that will precipitate is 0.008 mole
From the question,
We are to determine the number of moles of silver chloride that will precipitate
First,
We will write a balanced chemical equation for the reaction
The balanced chemical equation for the reaction is
NaCl + AgNO₃ → AgCl + NaNO₃
This means
1 mole of sodium chloride reacts with 1 mole of silver nitrate to produce 1 mole of silver chloride and 1 mole of sodium nitrate
Now, we will determine the number of moles of each reactant present
For sodium chloride (NaCl)Concentration = 0.440 M
Volume = 25.0 mL = 0.025 L
Using the formula
Number of moles = Concentration × Volume
∴ Number of moles of NaCl present = 0.440 × 0.025
Number of moles of NaCl present = 0.011 mole
For silver nitrate (NaNO₃)Concentration = 0.320 M
Volume = 25.0 mL = 0.025 L
∴ Number of moles of NaNO₃ present = 0.320 × 0.025
Number of moles of NaNO₃ present = 0.008 mole
From the balanced chemical equation,
1 mole of sodium chloride reacts with 1 mole of silver nitrate to produce 1 mole of silver chloride
Then,
0.008 mole of sodium chloride will react with the 0.008 mole of silver nitrate to produce 0.008 mole of silver chloride
∴ 0.008 mole of silver chloride will be produced
Hence, the number of moles of silver chloride that will precipitate is 0.008 mole
Learn more here: https://brainly.com/question/18434602