Answers
Regarding the types of bonds, we can conclude that:
K(s) is an example of a metallic bond because potassium is a metal.HCl(l) is an example of a covalent bond because both hydrogen and chlorine are nonmetals.SrBr₂(s) is an example of an ionic bond because strontium is a metal and bromine is a nonmetalKinds of chemical bondsThere are 3 kinds of chemical bonds.
Metallic bonds: are formed between metals and the electrons are delocalized.Ionic bonds: are formed between metals, that lose electrons, and nonmetals that gain them.Covalent bonds: are formed between nonmetals that share electrons.Let's identify the type of bond in each of the following molecules.
K(s) is an example of a metallic bond because potassium is a metal.HCl(l) is an example of a covalent bond because both hydrogen and chlorine are nonmetals.SrBr₂(s) is an example of an ionic bond because strontium is a metal and bromine is a nonmetal.Learn more about bonds here: https://brainly.com/question/25385832
Related Questions
A ball is rolled at a velocity of 3 m/s and rolls for 5 seconds. How far does
the ball roll? *
Answers
Answer 15m
Explanation: Distance = Speed x Time
3 x 5 =15
Predict the ground-state electron configuration of each ion. Use the abbreviated noble gas notation.
Cu²+
Co³ +
I got answers of:
Cu - [Ar]4s^2 3d^7 ; [Ar]3d^7 ; [Ar]3d^10 ; [Ar]4s^1 3d^10
Co - [Ar]4s^2 3d^4 ; [Ar]3d^4
And these were all wrong, please help
Answers
The electron configuration of Cu²+ ion - \(1s^2 2s^2 2p^6 3s^2 3p^6 3d^9\)
The electron configuration of Co³ + ion - \(1s^2 2s^2 2p^6 3s^2 3p^6 3d^6\)
What is the electron configuration?When we are talking about the electron configuration, we are talking about the way that the electron is arranged around the nucleus of the atom. We know that electrons in the atom can be arranged in energy levels in the atom of the element.
We are asked to write here the electron configuration of the copper II ion and that of the cobalt III ion. In either case, we have to know that electrons have been removed from the neutral atom. In the first case, there are two electrons that have been removed while in the second case, there are three electrons that have been removed.
Thus in writing the electron configuration of the specie we have to take into account the number of the electrons that have been lost due to ion formation. Recall that the ground state is the lowest energy state of the chemical specie.
Learn more about electron configuration:https://brainly.com/question/14283892
#SPJ1
Carbon-14 has a half-life of 5730 years. If an original sample was 100g of C¹4 and it is now 0.781g of C14, how old is your sample?
Answers
Answer:
40,113 years
Explanation:
To find the age of the sample, you need to use the half-life formula:
\(N(t)=N_0(\frac{1}{2})^{t/h\)
In this formula:
------> N(t) = current mass (g)
------> N₀ = initial mass (g)
------> t = time passed (yrs)
------> h = half-life (yrs)
You can plug the given values into the equation and rearrange the formula to find "t".
N(t) = 0.781 g t = ? yrs
N₀ = 100 g h = 5730 yrs
\(N(t)=N_0(\frac{1}{2})^{t/h\) <----- Half-life formula
\(0.781=100(\frac{1}{2})^{t/5730}\) <----- Insert values
\(0.00781=(\frac{1}{2})^{t/5730}\) <----- Divide both sides by 100
\(log_{1/2}(0.00781)=log_{1/2}((\frac{1}{2})^ {t/5730})\) <----- Take \(log_{1/2}\) of both sides
\(7.00 = \frac{t}{5730}\) <----- Solve \(log_{1/2}\)
\(40,113 = t\) <----- Multiply both sides by 5730
The given sample is 40,113 years .
What do you mean by half-life ?Half-life, in radioactivity, is the interval of time required for one-half of the atomic nuclei of a radioactive sample to decay.
Half-life formula,
\(\rm N(t)\;=N_0(\dfrac{1}{2})^\frac{t}{t1/2}\) .......(1)
where,
N(t)=current mass
N₀=initial mass
t=time period
h=half -life
Given,
N(t)=0.781g, t=? yrs, N₀=100g, h=5730 years
\(\rm N(t)\;=N_0(\dfrac{1}{2})^\frac{t}{t1/2}\)
put the values, in ......(1)
0.781=100(1/2) \(t/5730\\\)
log₁/₂(0.00781)=log₁/₂ ( 1/2)\(t/5730\)
7=t/5730
40,113=t
Hence, the given sample is 40,113 years .
Learn more about half-life ,here:
https://brainly.com/question/16387602
#SPJ1
Given a 64.2-g
sample of this substance with a specific heat of 50.6 J/(kg·°C),
how much heat is required to change its temperature from 180.0 °C
to 244.0 °C?
Answers
The total amount of heat generated is 207.5 J, under the condition that the given sample possess 64.2-g sample of this substance with a specific heat of 50.6 J/(kg•°C).
The heat needed to change the temperature of a substance can be evaluated applying the given formula
q = m × c × ΔT
Here
q = energy added,
m = mass of the substance,
c = specific heat capacity of the substance
ΔT = change in temperature.
Then we can proceed by calculating the heat required to change its temperature from 180.0 °C to 244.0 °C by converting the mass of the substance from grams to kilograms
m = 64.2 g = 0.0642 kg
Then, we can evaluate the change in temperature
ΔT = (244.0 °C - 180.0 °C)
= 64.0 °C
Lastly, we can apply the formula above to evaluate the heat required
q = m × c × ΔT
= (0.0642 kg) × (50.6 J/(kg•°C)) × (64.0 °C)
= 207.5 J
Hence, 207.5 J of heat is required to change the temperature of this substance from 180.0 °C to 244.0 °C.
To learn more about specific heat capacity
https://brainly.com/question/27991746
#SPJ1
Plzzzzzzzzz help
It's your 16th birthday. You've been given 1 penny every second since you were born. You want to use all that money to buy gold to make rings for your classmates. How many rings can you make? Useful information: 1 ring = 1.3 mL of gold, 1 gram of gold = $58, density of gold is 19.3 grams/mL
Answers
Explanation:
Since you're 16, you have to calculate how many seconds you've lived.
16 years × 31,536,000= 504,576,000 pennies.
504,576,000÷58 (for the amount of gold) = 8699586.2069 grams
Then go on from there. Hope that helped!
What food color mix to make yellow?
Answers
Answer:
read and green
Explanation:
By convention, the three primary colors in additive mixing are red, green, and blue. In the absence of light of any color, the result is black. If all three primary colors of light are mixed in equal proportions, the result is neutral (gray or white). When the red and green lights mix, the result is yellow.
Answer:
ground tumeric
Explanation:
Mix 1/4 cup water and 1/2 teaspoon of ground tumeric
light energy travels in
Answers
Light energy travels in the form of waves.
Which of the following locations is the most likely source of young sand with little quartz, that is dark in color, and that does not bubble when acid is added to it? (a) Great Salt Lake in Utah (b) a North American beach with pounding waves (c) a volcanic island (d) a beach near a coral reef
Answers
The location that is the most likely source of young sand with little quartz, that is dark in color, and that does not bubble when acid is added to it is a volcanic island (C)
The presence of either black basalt or green olivine gives volcanic sand its characteristically dark appearance. Volcanic sand is relatively young and, with the exception of obsidian particles, contains very little or any quartz at all. The majority of volcanic oceanic sands can be found on volcanic islands. Sand grains are created when dissolved mineral matter, primarily calcium carbonate, undergoes the process of precipitation in water, this results in the formation of precipitate sand.
To learn more about the volcanic sand, click here:
https://brainly.com/question/1156480
#SPJ4
The image "Sodium Chloride, Water, Carbon Dioxide, and Hydrogen Peroxide" illustrates the chemical
structure of water, which is a(n):
H
Water, H₂O
Sodium chloride, NaCl
H
Carbon dioxide, CO₂ Hydrogen peroxide, H₂O₂
Oelemental molecule.
O compound molecule.
atom.
state of matter.
Answers
The image features a molecular diagram of each of these substances, with the molecules displayed in a 3D structure. The diagram shows the chemical bonds that link the atoms of the molecules together.
Relative reactivity of moleculeThe reactivity of each molecule depends on the type of reaction being carried out and the functional groups present on each molecule. Generally, molecules with more electronegative or polar functional groups are more reactive than those without.
For example, molecules with carbonyl, amine, or hydroxyl groups tend to be more reactive than those without. Additionally, molecules with strained bonds, such as cyclic compounds, tend to be more reactive than linear molecules.
Additionally, molecules of higher molecular weight tend to be less reactive than those of lower molecular weight. Finally, the presence of electron-withdrawing groups, such as nitro or halogens, can increase the reactivity of a molecule.
In summary, the reactivity of a molecule is determined by a variety of factors, including the type of reaction, the functional groups present, the strain of the molecule, the molecular weight, and the presence of electron-withdrawing groups.
To learn more about reactivity of molecule refer to:
https://brainly.com/question/28383861
#SPJ1
The combustion of octane, C8H18, proceeds according to the reaction shown.
2C8H18(l)+25O2(g)⟶16CO2(g)+18H2O(l)
If 354 mol of octane combusts, what volume of carbon dioxide is produced at 15.0 ∘C
and 0.995 atm?
Answers
The concept ideal gas equation is used here to determine the volume of the carbondioxide. Combustion reactions are generally highly exothermic reactions. The volume of CO₂ is
A combustion is a chemical reaction in which a fuel undergoes oxidation as a result of the reaction with an oxidizing agent which causes the release of energy in the form of heat.
15.0 °C = 288 K
The ideal gas equation is:
PV = nRT
V = nRT / P
V = 354 × 0.0821 × 288 / 0.995 = 8412.3 L
To know more about combustion, visit;
https://brainly.com/question/14335621
#SPJ1
What is true of spontaneous reactions?
O They are indicated by a negative change in Gibbs free energy.
O They have a positive value of AS.
O They are instantaneous.
O They always release heat.
Help 20pts
Answers
Explanation: Spontaneous reactions are those that occur without any external input of energy. A negative change in Gibbs free energy (ΔG) indicates that a reaction is spontaneous. The other options do not always hold true for spontaneous reactions. The value of entropy change (ΔS) can be positive or negative, spontaneous reactions are not necessarily instantaneous, and they do not always release heat.
The formula of a salt is XCl2. The X ion in this salt has 20 electrons. The metal X is ________.
Answers
Answer:
titanium
Explanation:
The ion has a charge of +2 since each of the chrlorine atoms have a charge of -1. if x has 20 electrons, the it must have 22 protons to get a charge of +2. The proton number defines the element, so the element with 22 protons is titanium.
Select the atomic models that belong to the same element.
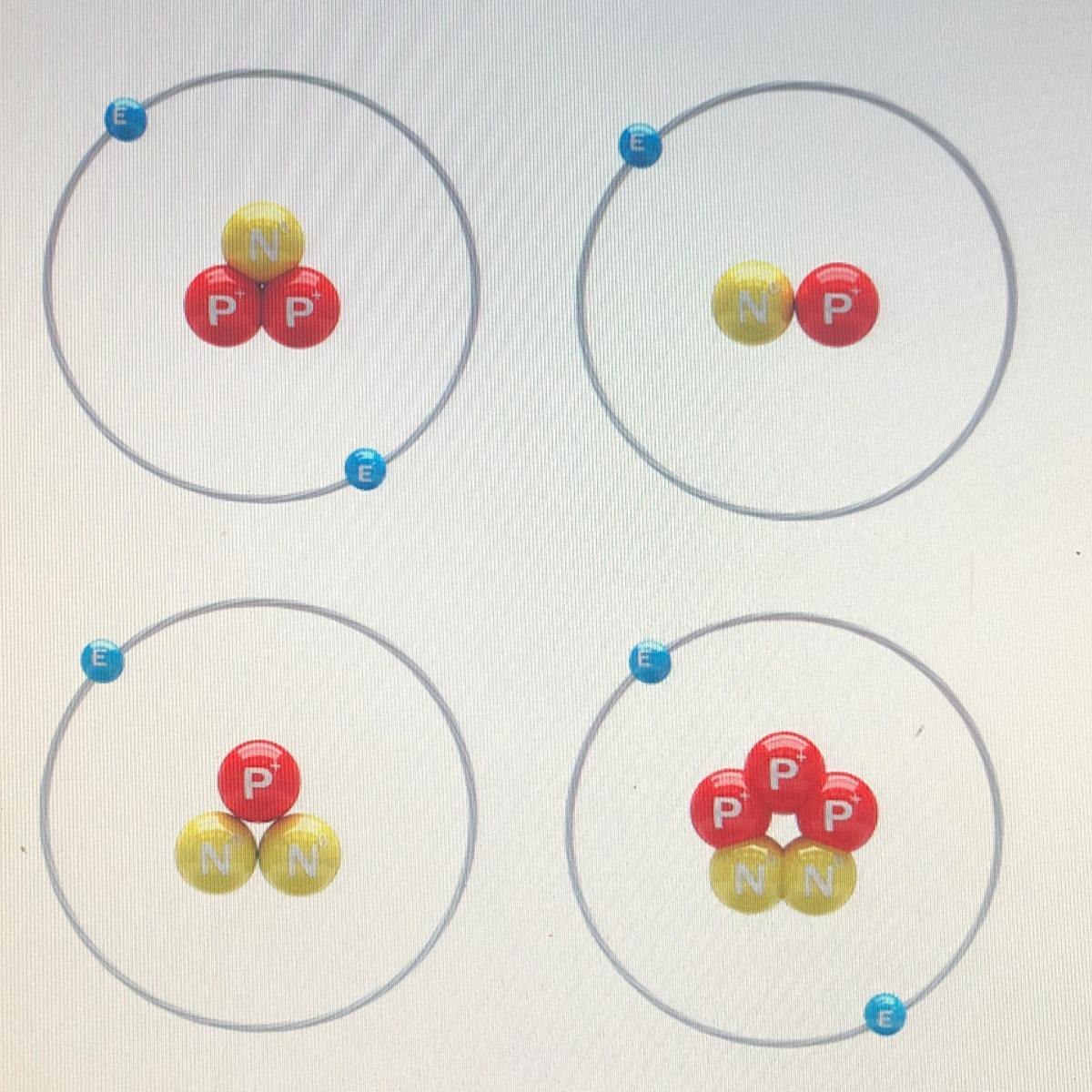
Answers
Answer:
top right and bottom left
Explanation:
just took test plato/edmentum
Titration of 25.0 mL of an HCl solution of unknown concentration requires 14.8 mL of 0.100 M NaOH. What is the molar concentration of the HCl solution
Answers
M1 = 0.100 M NaOH
V1 = 14.8 mL NaOH
M2 = ?
V2 = 25.0 mL HCl
(0.100 M)(14.8 mL) = M2(25.0 mL)
M2 = 0.0592 M HCl
Fossilized remains of similar plant species were found in all four layers of the rock in the diagram above. Which of the following species would be MOST closely related?
A. species found in layer A and species found in layer D
B. species found in layer B and species found in layer D
C. species found in layer C and species found in layer D
D. species found in layer A and species found in layer C

Answers
Answer:
A and C
Explanation:
so D
AP chem. Sublevels!!!!!!!!!!!

Answers
Answer:
2d
Explanation:
The lowest d is 3d.
Hope this helps!
A student combined two solutions of clear liquids in a test tube, after one minute a solid substance appeared in the test tube. Based on their observations, can the students correctly conclude that a chemical reaction occurred?
Answers
Please help me answer this!!! 20 points
Metals lose electrons to form cations. Do the cations have a smaller or larger ionic radius than the neutral atom from which they were formed? Why?
Answers
Cations are formed through lose of electrons and thus, possess positive charge. Cations has smaller ionic radius than the neutral atom, because, they have are fewer electrons and thus experience more nuclear attractive pull.
What are cations?Cations are charged particles or ions formed by the lose of electrons from the neutral atom. Atoms acquires positive charge when they lose electrons and acquire negative charge by gaining electrons and the negative ions are called anions.
For example, Na metal loses one electrons to form the cation Na+. Similarly Mg loses two electrons, to form Mg²⁺ ion. When these atoms loses electrons, the outermost shell can be emptied and the atomic radius shrinks to the penultimate shell.
Similarly, as the number of electrons reduces, the screening of electron from neighboring electrons reduces results in greater nuclear attractive pull and thereby the atomic radius shrinks to smaller than the neutral atom.
To find more cations, refer here:
https://brainly.com/question/28710872
#SPJ1
It says express the numeral, I’ve never heard about this and I’m rlly bad at math..

Answers
a. 7.623 × 10⁵ nm is expressed as 762300nm
b. 5.34 × 10⁻³ L is expressed as 0.00534
Following numerals are explain below:
What are numerals ?A numeral is a name or symbol that represents a certain number. Examples include the numerals three, four, and twelve. So, the numeral is how we express the number, which is a concept. Reason: A numeral is a word that describes a number, whereas a number is conveyed via digits.
c. 5.453 × 10⁶ m is expressed as 5453000
d. 6.791 × 10⁻⁵pm is expressed as 6791m
e. 2.4 × 10⁹pg is expressed as 2400000000
f. 3.65 × 10⁵ is expressed as 365000
g. 3.4287 × 10⁻⁶L is expressed as 0.0000034287
h. 9.2 × 10²mL is expressed as 920L
i. 6.927 × 10⁴ is expressed as 69270m
j. 9.23 × 10⁻³ is expressed as 0.00923
Thus, a. 7.623 × 10⁵ nm is expressed as 762300nm.
To learn more about numeral, follow the link;
https://brainly.com/question/21597658
#SPJ9
can anyone please help with this

Answers
Answer:
I guess option \(b) \:CCl_4\) is the correct choice.
Explanation:
Carbon tetrachloride \((CCl_4)\) is a non-polar solvent. Therefore, the non-polar substance will be most soluble in \(CCl_4\).
Best Regards!
3. A Wilkinson’s catalyst is widely used in the hydrogenation of alkenes. Show a catalytic cycle, including: i. chemical structure of the catalyst, with complete stereochemistry ii. molecular geometry of catalyst iii. type of reactions involved iv. the appropriate starting material, reagent and solvent v. major and minor end-products vi. all intermediates, for each reaction stated in (iii)
Answers
We can see here that the catalytic cycle for the hydrogenation of alkenes using Wilkinson's catalyst involves several steps.
What are the steps involved?Here's an overview of the catalytic cycle, including the necessary details:
i. Chemical structure of the catalyst:
Wilkinson's catalyst, also known as chloridotris(triphenylphosphine)rhodium(I), has the following chemical structure: [RhCl(PPh3)3]
ii. Molecular geometry of the catalyst:
The Wilkinson's catalyst has a trigonal bipyramidal geometry around the rhodium center. The three triphenylphosphine (PPh3) ligands occupy equatorial positions, while the chloride (Cl) ligand occupies an axial position.
iii. Type of reactions involved:
The catalytic cycle involves several reactions, including:
Oxidative addition: The rhodium center undergoes oxidative addition, reacting with molecular hydrogen (H2) to form a dihydride intermediate.Alkene coordination: The alkene substrate coordinates to the rhodium center, forming a π-complex.Hydrogenation: The coordinated alkene undergoes hydrogenation, resulting in the addition of hydrogen atoms to the double bond and formation of a metal-alkyl intermediate.Reoxidation: The metal-alkyl intermediate reacts with a hydrogen molecule to regenerate the rhodium dihydride species.iv. Starting material, reagent, and solvent:
The starting material is an alkene, and the reagent is Wilkinson's catalyst ([RhCl(PPh3)3]). The reaction is typically carried out in a suitable solvent, such as dichloromethane (CH2Cl2) or tetrahydrofuran (THF).
v. Major and minor end-products:
The major end-product of the hydrogenation reaction is the fully saturated alkane, resulting from the addition of hydrogen across the double bond. The minor end-product may include cis- or trans-configured alkanes if the original alkene substrate possesses geometric isomers.
vi. Intermediates:
The intermediates in the catalytic cycle include:
Rhodium dihydride complex: [RhH2(PPh3)3]Alkene-Rhodium π-complex: [Rh(η2-alkene)(PPh3)3]Metal-alkyl intermediate: [Rh(alkyl)(PPh3)3]These intermediates play a crucial role in facilitating the hydrogenation reaction and enabling the catalytic cycle to proceed.
Learn more about Wilkinson’s catalyst on https://brainly.com/question/31972308
#SPJ1
Based on a Kc value of 0.250 and the given data table, what are the equilibrium concentrations of XY, X, and Y , respectively?
Answers
From the solution that we have in the question;
The concentration of X and Y is 0.28 MThe concentration of XY is 0.32 MWhat is the equilibrium constant?The equilibrium constant, denoted as K, is a value that quantitatively represents the ratio of the concentrations of products to reactants at equilibrium in a chemical reaction.
It is a fundamental concept in chemical equilibrium.
The value of the equilibrium constant provides valuable information about the position of equilibrium and the relative concentrations of species involved in a chemical reaction.
Kc = [X] [Y]/[XY]
\(0.25 = (0.1 + x)^2/(0.5 - x)\)
\(0.25(0.5 - x) = (0.1 + x)^2\)
\(0.125 - 0.25x =0.01 + 0.2x + x^2\\ x^2 + 0.45x - 0.115 = 0\)
x = 0.18 M
The equilibrium amount of X and Y= 0.28 M and the equilibrium concentration of XY = 0.32 M
Learn more about equilibrium constant:
https://brainly.com/question/29253884
#SPJ1
Based on the answer to the question that we have;
A 0.28 M concentration of X and Y exists at equilibriumXY's concentration at equilibrium is 0.32 M.The equilibrium constantThe ratio of the product to reactant concentrations in a chemical reaction at equilibrium is represented quantitatively by the equilibrium constant, abbreviated as K.
It is a cornerstone of the theory of chemical equilibrium.
A chemical reaction's equilibrium position and the relative concentrations of the species involved can both be learned from the equilibrium constant's value.
Kc = [X][Y]/[XY]
\(0.25 = (0.1 + x)^2/(0.5 - x)\\0.25(0.5 - x) = (0.1 +x)^2\\0.125 - 0.25x = 0.01 +0.2x +x^2\\= 0.18 M\)
The equilibrium concentration of;
XY =0.5 - 0.18
=0.32 M
Then the equilibrium amount of
X and Y is
0.1 + 0.18= 0.28 M.
Learn more about equilibrium:brainly.com/question/29253884
#SPJ1

star_____ has the greatest absolute brightness
Answers
Answer:
Star A would have the greater absolute brightness. This is because absolute brightness finds out the actual brightness of a star at a standard distance from Earth. If Star A is twice as far from Earth as Star B but they still both appear to have the same amount of brightness.
Which of the following claims is a correct statement based on the data in the Reason for Earth’s Seasons activity?
Question 3 options:
The seasons are caused by the distance of the Earth from the Sun because the Earth is farther away from the Sun in the winter and closer in the summer.
The seasons are caused by the tilt of Earth’s axis and how much solar radiation each area of the Earth receives.
Answers
Answer: the second one " the seasons are caused by the tilt of earth's axis and how much solar radiation each area of earth receives. "
Explanation:
i took the test on k12.
I WILL MARK YOU BRAINLIST PLEASE HELP

Answers
Answer: See below
Explanation: a. The mass of an element is composed of:
protons: 1 amu each
neutrons: 1 amu each
electrons: 0 amu each
Only the protons and neutrons are counted in the atomic mass of an element
b. Electrons are assigned a mass of 0. They do have a mass, but it is exceedingly small compared to the protons and neutrons, so they are left out of the calculation of an element's mass.
c. An element becomes unstable if the neutrons exceed the protons by a certain ratio, dependent on the element.
Why might helium be considered smaller than hydrogen?
Answers
The element helium is considered smaller than hydrogen because of the increase in the number of shells in the periodic table.
What is a periodic table?A periodic table is an arrangement for the elements to better learn and understand the properties and elements and gives information about the number of shells and effective nuclear charges on the atom.
Helium is present at the right in the periodic table and from moving hydrogen to helium the number of orbitals or inner shells increases which also increases the effective nuclear charge and size becomes small.
Therefore, because of the increase in the number of shells in the periodic table.The element helium is considered smaller than hydrogen.
Learn more about the periodic table, here;
https://brainly.com/question/11155928
#SPJ2
do this Q7 if someone do I will her or him brainliest

Answers
The average speed :
1. 10.44 m/s
2. 10.42 m/s
3. 9.26 m/s
The distance 100 m have the greatest average speed
Further explanationGiven
Distance and time of runner
Required
Average speed
Solution
Average speed : total distance : total time
1. d = 100 m, t = 9.58 s
Average speed : 100 : 9.58 = 10.44 m/s
2. d=200 m, t=19.19 s
Average speed : 200 : 19.19 = 10.42 m/s
3. d=400 m, t = 43.18 s
Average speed : 400 : 43.18 = 9.26 m/s
The distance 100 m have the greatest average speed
7. How many electrons can be held in the energy level n = 4?
Answers
32 electrons can be held in the energy level n = 4 as number of electrons is given as 2n²=2×(4)²=32.
What is an energy level?Electrons present in an atom revolve in different orbits which are stationary states and are also called as energy levels. The energy levels are numbered as integers which are also called as principal quantum numbers.
Energy of the stationary state is given as E= -R
1/n² where R
is the Rydberg's constant. When an electron is excited, and it moves from lower to higher energy levels there is absorption of energy, while when it moves from higher energy level to lower energy level it radiates or gives out energy in the form of radiation.
They can also be defined as the distances between electron and nucleus of an atom . Electrons present in K energy level have least energy .Energy level diagrams are studied to understand nature of bonding , placement of electrons in orbits and and elemental behavior under certain conditions.
Learn more about energy level,here:
https://brainly.com/question/17396431
#SPJ2
I'm not that smart :)
When a child slides down a slide (if there is no friction and all of the mechanical energy is conserved) what would happen?
Question 3 options:
A. Potential energy would turn to Kinetic energy
B. The energy is destroyed
C. Kinetic energy would turn in to Potential energy
D. All energy stays the same
Answers
the answer is C: Kinetic energy would turn into Potential energy.
complete the given table by mentioning the quantum numbers for each orbits
Quantum number orbital
2p 3d
azimuthal quantum number ? ?
magnetic quantum number ? ?
Answers
What are the quantum numbers?
The orbital's orientation in space is described by the magnetic quantum number (m). Any number between -l and +l may represent the value of m.
The electron's orbital form is determined by a quantum number called the azimuthal quantum number. Any integer between 0 and n-1 can be used to represent the value of l, and as it rises, the orbital's form becomes more complex.
The quantum numbers that are involved have been shown above.
Learn more about quantum numbers:https://brainly.com/question/16746749
#SPJ1
