if 1495 j of heat is needed to raise the temperature of a 347 g sample of a metal from 55.0°c to 66.0°c, what is the specific heat capacity of the metal?
Answers
The specific heat capacity of a metal can be calculated using the formula: q = m × c × ΔtWhere q is the amount of heat absorbed or released,
m is the mass of the substance, c is the specific heat capacity of the substance, and t is the change in temperature of the substance. We can solve for c by rearranging the formula as follows:
c = q / (m × Δt)Given: q = 1495 Jm = 347 gc = ?Δt = 66.0°C - 55.0°C = 11.0°CSubstituting the given values into the formula: c = q / (m × Δt)= 1495 J / (347 g × 11.0°C)= 0.39 J/(g·°C)Therefore, the specific heat capacity of the metal is 0.39 J/(g °C).
To know more about heat capacity refer to:
https://brainly.com/question/29792498
#SPJ11
Related Questions
How do heavy metals like cobalt, zinc, mercury, and lead get into the environment, and why are they harmful?
Answers
Answer:
How they get into the environment

decide whether these proposed lewis structures are reasonable. proposed lewis structure is the proposed lewis structure reasonable? yes. no, it has the wrong number of valence electrons. the correct number is: no, it has the right number of valence electrons but doesn't satisfy the octet rule. the symbols of the problem atoms are:
Answers
1. The C in the first structure should have four total valence electrons. To complete its octet, it thus requires 4 extra electrons. The molecule should thus have 8 + 2 = 10 valence electrons overall, yet 14 valence electrons are present in this configuration. Thus, this is not the proper Lewis structure.
2. While both elements' octets are full in the second form, the total valence electron is incorrect. Total valence electrons should be =7+6+1=14, with 7 coming from the 7 valence electrons of a Cl atom, 6 from the 6 valence electrons of O, and 1 from the negative charge. Yet, there are a total of 8 + 4 = 12 electrons in the structure, where 8 is for 4 lone pairs of electrons and 4 is from the double bond. Thus, it is likewise an improper Lewis structure.
3. The valence electron count in the N2 molecule, the third structure, is accurate. The N atom, however, has not yet reached octet. There are 6 electrons in each N. The two N atoms do not thus have an octet arrangement. Thus, it is not a valid Lewis structure.
Learn more about Lewis structure here:
https://brainly.com/question/31147973
#SPJ4

if the phases of matter are arranged in order of increase disorder, what would that arrangement be
Answers
The order of increasing disorder is solid, liquid, and gas. This arrangement is based on the increasing freedom of movement and randomness of the particles as we move from solid to liquid to gas.
Solid: Solids have a highly ordered arrangement of particles, with strong intermolecular forces and fixed positions of atoms or molecules.
Liquid: Liquids have less order compared to solids, as particles have more freedom of movement while still maintaining close proximity to one another.
Gas: Gases have the highest degree of disorder among the three phases. Gas particles are widely spaced, move freely, and lack a definite shape or volume.
To know more about Solid, here
brainly.com/question/20461295
#SPJ4
What is the mass of a dolphin swimming at 16 m/s with a momentum of 800 kg m/s?
Answers
Answer: 50 kg
Explanation: formula is
m= p/v
m=800 kg m/s : 16m/s
m= 50kg
idk tho hope this helps
Both suspensions and colloids are heterogeneous mixtures. Define and characterize a suspension, listing similarities and differences to a colloid. Give several examples of suspensions.
Answers
Suspensions are similar to colloids in that they are both heterogenous mixtures and are not true solutions.
Suspensions differ from colloids in that the particle sizes are larger and settle when left to stand.
Some examples of suspensions are smoke and dust particles in the air.
What are suspensions and colloids?Suspensions are a heterogeneous mixture of substances in which one substance is dispersed or distributed throughout a second phase. The particles of a suspension when left to stand will settles over time.
Some examples of suspension are dust particles dispersed in air, a gas.
Other suspensions may be solids dispersed in liquids such as in milk of magnesia.
Colloids or false solutions are a heterogeneous mixture of substances that are dispersed throughout a second medium and in which the particles do not settle when left to stand. The articles of a colloid can not be seen with the unaided eye.
Examples of colloids are gels, emulsions, and sols.
Learn more about suspensions and colloids at: https://brainly.com/question/18276053
#SPJ1
What does a control group show in an experiment investigation
Answers
Control group is used to establish causality by isolating the effect of an independent variable.
What is Control group?Control group can be defined as any group used as a control in a statistical experiment, extrasensory perception a group of patients who receive either a placebo or a standard drug during an investigation of the effects of another drug on other patients.
Therefore control groups are an important aspect of true experimental designs. The presence of control groups allows researchers to confirm that study results are due to the manipulation of independent variables (IVs) rather than extraneous variables
Learn more about Control group here: brainly.com/question/26323529
#SPJ1
The ionic size of fluoride (F^-1)
Answers
Answer:lonic
Explanation:A neutral atom has an equal number of electrons and protons. When an electron is lost, the atom becomes positively charged and is called a cation. On the other hand, when an electron is gained by an atom, it becomes negatively charged and is called an anion. The distance of the nucleus of an ion and the valence electron is referred to as the ionic radius.
List all possible values of the angular momentum quantum number l for an electron in the L(n=2) shell of an atom.
Answers
In quantum mechanics, the angular momentum quantum number "l" defines the shape of the atomic orbital. The l value is an integer ranging from 0 to (n-1) where n is the principal quantum number.
Therefore, for an electron in the L(n=2) shell of an atom, the possible values of the angular momentum quantum number l would range from 0 to 1, since n=2.
This is because the L shell is the second shell, which has n=2. Therefore, it can have subshells with l=0 and l=1, also known as the s and p subshells respectively.
The angular momentum quantum number also has an effect on the energy of the electron, with higher l values having higher energy.
Thus, the possible values of the angular momentum quantum number l for an electron in the L(n=2) shell of an atom are l=0 and l=1.
To know more about quantum mechanics refer here: https://brainly.com/question/23780112#
#SPJ11
you have one mole of each of these atoms: carbon-12, oxygen-16, and uranium-235. Which substance has more atoms
Answers
One mole of each element contains Avogadro's number of atoms, which is 6.022 x 10^23 atoms. Therefore, all three substances have the same number of atoms, which is 6.022 x 10^23 atoms.
Please, someone help me.
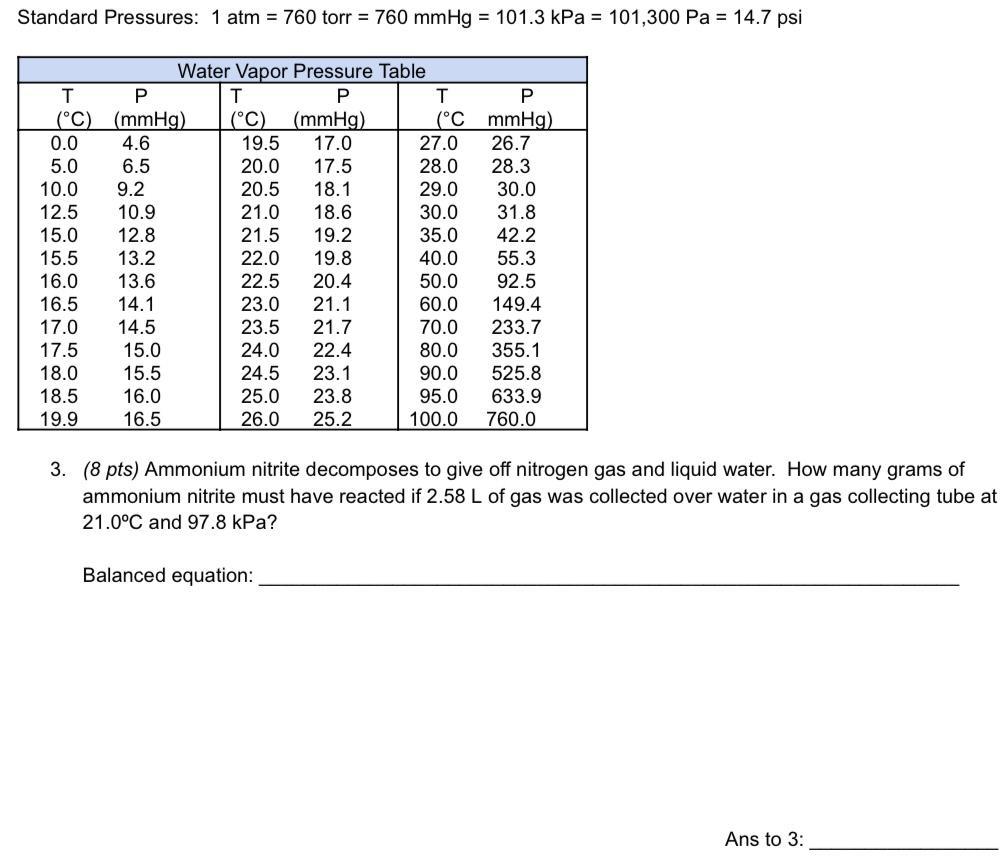
Answers
The mass of the ammonium nitrite that is needed is 6.4 g
What is the stoichiometry?Stoichiometry is based on the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction. Therefore, the total mass of the reactants must be equal to the total mass of the products.
The equation of the reaction is;
[NH4]NO2 → N2 + 2H2O
We have the pressure is;
97.8 kPa - 2.5 Kpa = 95.3 kPa or 0.94 atm
PV = nRT
n = PV/RT
n = 0.94 * 2.58/0.082 * 294
n = 0.1 moles
If the reaction 1:1, mass of the ammonium nitrite is;
0.1 moles * 64 g/mol
= 6.4 g
Learn more about stoichiometry:https://brainly.com/question/28780091
#SPJ1
In the modern periodic table, which of the following represents the last pair of elects for which Newlands' Law of Octaves would hold true?
1. AI and GA
2. AI and SI
3. NE and AR
4. MG and CA
Answers
Answer: Mg and Ca
Explanation:
In the modern periodic table, Mg and Ca are the last pair of elects for which Newlands' Law of Octaves would hold true.
Law of Octaves is the generalization that was made by Newlands. It states that when chemical elements are arranged based on their increasing atomic weight, then the ones that have the same chemical and physical properties will occur after an interval of seven elements.
In such a case, every eighth element will have identical properties when the elements in the periodic table are arranged based on their atomic masses.
Based on the above information, Magnesium (Mg) has an atomic number of 12 while Calcium (Ca) has an atomic number of 20. Therefore, the elements follow the law of Octaves as they have a difference of 8 in their atomic numbers.
Read related link on:
https://brainly.com/question/16430105
Lab titration data table!!! PLS HELP
Answers
A lab titration data table is a table that is used to record the results of a titration experiment in a laboratory. A titration is a chemical process that involves the gradual addition of one solution to another until a chemical reaction occurs, which can be used to determine the concentration of one solution.
In order to record the results of a titration experiment, a data table is used to record the volume of the titrant (the solution being added), the volume of the analyte (the solution being tested), and the equivalence point (the point at which the reaction is complete).
The lab titration data table is an essential tool in any titration experiment because it provides a way to record and organize data in a clear and concise manner. This makes it easier to analyze the data, draw conclusions, and make accurate calculations. When creating a lab titration data table, it is important to include all relevant information and ensure that the data is accurate and complete. This includes recording any errors or deviations from expected results, as well as any observations or notes that may be relevant to the experiment.
In conclusion, the lab titration data table is a crucial component of any titration experiment, as it provides a clear and organized record of the results of the experiment. By carefully recording and analyzing the data in a data table, researchers can draw meaningful conclusions and make accurate calculations, which can be used to further scientific research and understanding.
To know more about Lab Titration visit:
https://brainly.com/question/29122258
#SPJ11
what is the formula for chlorine
Answers
How would you use volume (Potentiometer )to perform the measurements?
Answers
Answer:
oj simpson
Explanation:
Which best completes the analogy?
B Cells : Produce Antibodies :: T Cells : ___________________
A. Get rid of good cells that have been infected
B. Tell B cells to start making antibodies
C. Tell killer cells to attack
D. remember antigens that have attacked the body
Answers
Answer:
d
Explanation:
d
3. According to Newton's First Law of Motion, what does an object at rest do?
stays at rest
begins to move
cannot be moved
continues to move

Answers
Answer:
stays at rest
Explanation:
Answer:
stays at rest
Explanation:
25.00 g of the compound is composed of 6.77 g of tin and 18.23 g of bromine. What is the percent by mass of tin in the compound? Show work.
Answers
The percent by mass of Tin : = 27.08%
Further explanationGiven
25.00 g of the compound
6.77 g of Tin
18.23 g of Bromine
Required
The percent by mass of Tin
Solution
Proust stated the Comparative Law that compounds are formed from elements with the same Mass Comparison, so that compounds have a fixed composition of elements
%mass X = (mass X/mass sample) x 100%
So %mass of Tin :
= (6.77 g : 25 g) x 100%
= 27.08%
the structure of crestor (rosuvastatin), a medication used to reduce cholesterol, is shown. if the specific rotation for this compound is known to be 100, what would be the specific rotation for the stereoisomer shown at the right?
Answers
Rosuvastatin is a dihydroxymonocarboxylic acid that is used to treat (6E) -7-{4-(4-fluorophenyl)-2-[methyl(methylsulfonyl)amino] Two hydroxy substituents are present at positions 3 and 5 in the compound 6-(propan-2-yl)pyrimidin-5-yl hept-6-enoic acid (the 3R,5S-diastereomer).
What kind of medication is rosuvastatin?Since its introduction, rosuvastatin has lived up to its initial expectations as a very effective statin that has positive effects on HDL, demonstrates a decline or cessation in the atherosclerotic burden, and lowers cardiovascular events in reduced patients.
When should rosuvastatin be taken?Rosuvastatin is typically used once daily. Since you can use it whenever you choose, it's better to consume it at the exact hour each day. Taken with or without food, rosuvastatin typically won't make you feel sick.
To know more about rosuvastatin visit:
https://brainly.com/question/24891584
#SPJ4
What is the new concentration of a solution of CaSO3 if 10.0 mL of a 2.0 M CaSO3 solution is diluted to 100 ml?
Answers
Answer: The new concentration of a solution of \(CaSO_{3}\) is 0.2 M 10.0 mL of a 2.0 M \(CaSO_{3}\) solution is diluted to 100 mL.
Explanation:
Given: \(V_{1}\) = 10.0 mL, \(M_{1}\) = 2.0 M
\(V_{2}\) = 100 mL, \(M_{2}\) = ?
Formula used to calculate the new concentration is as follows.
\(M_{1}V_{1} = M_{2}V_{2}\)
Substitute the values into above formula as follows.
\(M_{1}V_{1} = M_{2}V_{2}\\10.0 mL \times 2.0 M = M_{2} \times 100 mL\\M_{2} = 0.2 M\)
Thus, we can conclude that the new concentration of a solution of \(CaSO_{3}\) is 0.2 M 10.0 mL of a 2.0 M \(CaSO_{3}\) solution is diluted to 100 mL.
Plz help! I don't want internet answers plz :D
1. What determines weight?
2. What does Homogeneous mean?
Answers
2. a particle that has the same properties at every point
Which of the following bonds is classified as a polar bond?
A K-CI
B Br-Br
C Si-F
D S-O
WILL MARK BRAINLIST
Answers
Answer:
the answer is b
Explanation:
the answer is b
Compound of KCl has a polar bond as there is a electronegativity difference between potassium and chlorine atoms.
What is a compound?
Compound is defined as a chemical substance made up of identical molecules containing atoms from more than one type of chemical element.
Molecule consisting atoms of only one element is not called compound.It is transformed into new substances during chemical reactions. There are four major types of compounds depending on chemical bonding present in them.They are:
1)Molecular compounds where in atoms are joined by covalent bonds.
2) ionic compounds where atoms are joined by ionic bond.
3)Inter-metallic compounds where atoms are held by metallic bonds
4) co-ordination complexes where atoms are held by co-ordinate bonds.
They have a unique chemical structure held together by chemical bonds Compounds have different properties as those of elements because when a compound is formed the properties of the substance are totally altered.
Learn more about compounds,here:
https://brainly.com/question/14658388
#SPJ6
It takes 49. 0 J to raise the temperature of an 8. 90 g piece of unknown metal from 13. 0 ∘C to 24. 0 ∘C. What is the specific heat for the metal?
Answers
The problem provides us with the values: q = 49.0 J, m = 8.90 g, and ΔT = 11.0 °C (from 13.0 °C to 24.0 °C). The specific heat of the unknown metal is approximately 0.615 J/g°C.
To determine the specific heat of the unknown metal, we can utilize the formula q = mcΔT, where q represents the heat energy, m is the mass of the metal, c is the specific heat, and ΔT is the change in temperature. The problem provides us with the values: q = 49.0 J, m = 8.90 g, and ΔT = 11.0 °C (from 13.0 °C to 24.0 °C).
Substituting these values into the formula, we obtain 49.0 J = (8.90 g) * c * 11.0 °C. Solving for c, we find c ≈ 0.615 J/g°C. Thus, the specific heat of the unknown metal is approximately 0.615 J/g°C. Specific heat is a measure of the amount of heat energy required to raise the temperature of a given mass of a substance by a certain amount.
Learn more about metal here:
https://brainly.com/question/4701542
#SPJ11
The combustion of methane, CH4(g), can be described by the following
CH4(g) + 2O2(g) → CO2(g) + 2H2O(g)
If 150 moles of carbon dioxide are produced, what mass, in grams, of methane is required?
Answers
A fuel (the reductant) and an oxidant, typically atmospheric oxygen and combustion.
It undergo a high-temperature exothermic redox chemical reaction that results in oxidized, frequently gaseous products and a mixture known as combustion.
Since a flame only appears when substances undergoing combustion evaporate, combustion does not always result in fire, but when it does, a flame is a distinctive sign of the event.
The heat from a flame may be enough energy to make the reaction self-sustaining, even though the activation energy must be overcome to initiate combustion (such as when using a lit match to start a fire).
A convoluted series of simple radical reactions frequently occurs during combustion.
Thus, A fuel (the reductant) and an oxidant, typically atmospheric oxygen and combustion.
Learn more about Combustion, refer to the link:
https://brainly.com/question/15117038
#SPJ1
10. Use the bonds below to characterize the following descriptions: i. ionic bond ii. polar covalent iii. non-polar covalent iv. hydrogen a. bond between an anion and a cation b. weak intramolecular b
Answers
i. Ionic bond: bond between an anion and a cation. ii. Polar covalent: bond between atoms of the same element but different electronegativities. iii. Non-polar covalent: weak intramolecular bond. iv. Hydrogen: bond between the hydrogen atom in one molecule and a more electronegative atom in another molecule
Based on their properties, chemical bonds are classified into four major types. These include Ionic bonds, covalent bonds, polar covalent bonds, and hydrogen bonds. Some characteristics of the four types of chemical bonds are as follows:
i. Ionic bond: An ionic bond is formed when electrons are transferred from one atom to another atom. The resulting ions are attracted to each other and form an ionic bond. Ionic bonds are typically between metals and nonmetals.
ii. Polar covalent bond: Polar covalent bonds occur when atoms of the same element but different electronegativities bond. The atoms share the electrons unequally in a polar covalent bond, creating a partial positive charge on the less electronegative atom and a partial negative charge on the more electronegative atom. Polar covalent bonds typically occur between nonmetals.
iii. Non-polar covalent bond: Non-polar covalent bonds occur between two atoms of the same element or between different elements with the same electronegativity. The sharing of electrons between the atoms in a nonpolar covalent bond is equal. As a result, there is no net charge distribution across the molecule, and the bond is nonpolar. Nonpolar covalent bonds typically occur between nonmetals.
iv. Hydrogen bond: Hydrogen bonds are weak intramolecular bonds that occur between the hydrogen atom in one molecule and a more electronegative atom in another molecule. Hydrogen bonds are important in the secondary and tertiary structures of proteins and the structure of water.
To know more about Ionic bond, refer
https://brainly.com/question/977324
#SPJ11
reasons why water is a good cooling agent in machines
Answers
Answer:
one reason is, Water is a very good liquid for cooling things down, for one thing there is plenty of it and it has also got a high specific heat capacity. This means that it can absorb a large amount of heat energy without getting too hot.
will not heat up or cool down very fast
Water has a high value of latent heat of vapourization so it has cooling properties.
hope this helps you. :)
Explanation:
The molar heats of fusion and vaporization for water are 6. 02 kJ/mol and 40. 6 kJ/mol, respectively, and the specific heat capacity of liquid water is 4. 18 J/g °C. What quantity of heat energy is required to melt 34. 3 g of ice at 0 °C? Heat = kJ What quantity of heat is required to vaporize 43. 3 g of liquid water at 100. °C? Heat = kJ What quantity of heat is required to warm 55. 1 g of liquid water from 0 °C to 100. °C? Heat = kJ
Answers
To find the volume of hydrogen gas, we can use the ideal gas law equation: PV = nRT. Rearranging the equation to solve for V (volume), we have V = (nRT) / P. Given that n = 28.3 moles, R is the ideal gas constant, T is 297 K, and P is 1.08 atm, we can substitute these values to find the volume.
To calculate the quantity of heat energy required for each process, we will use the following formulas:
Heat required to melt ice:
Heat = mass of ice * molar heat of fusion
Heat required to vaporize liquid water:
Heat = mass of liquid water * molar heat of vaporization
Heat required to warm liquid water:
Heat = mass of liquid water * specific heat capacity * temperature change
Now, let's calculate each quantity of heat energy:
Heat required to melt 34.3 g of ice at 0 °C:
Heat = 34.3 g * (6.02 kJ/mol / 18.02 g/mol) = 11.39 kJ
Heat required to vaporize 43.3 g of liquid water at 100 °C:
Heat = 43.3 g * (40.6 kJ/mol / 18.02 g/mol) = 96.07 kJ
Heat required to warm 55.1 g of liquid water from 0 °C to 100 °C:
Heat = 55.1 g * 4.18 J/g °C * (100 °C - 0 °C) / 1000 = 230.08 kJ
Therefore, the quantities of heat energy required for each process are:
To melt 34.3 g of ice: 11.39 kJ
To vaporize 43.3 g of liquid water: 96.07 kJ
To warm 55.1 g of liquid water: 230.08 kJ
To know more about heat energy click this link -
brainly.com/question/29210982
#SPJ11
7. A sample of helium gas occupies a volume of 24.6 L at 2.20 atm. What would its volume be at 3.50 atm?
Answer: 15.5L
Please show your work
Answers
so, here's your answer, 15.5L (appr)

the ph at one half the equivalence point in an acid-base titration was found to be 5.77. what is the value of ka for this unknown acid?
Answers
In order to determine the value of Ka for the unknown acid, we can use the given information about the pH at half the equivalence point in an acid-base titration. At half the equivalence point, the concentration of the weak acid ([HA]) and its conjugate base ([A-]) are equal.
At one half the equivalence point in an acid-base titration, the concentration of the acid is equal to the concentration of the conjugate base, and the pH is equal to the pKa of the acid. Therefore, we can write:
pH = pKa
Given that the pH is 5.77, we can substitute this value into the equation:
5.77 = pKa
Now, we can solve for pKa by taking the antilogarithm of both sides to get rid of the logarithm:
\(10^{5.77} = 10^{pka}\)
pKa = 5.77
So, the value of pKa for the unknown acid is 5.77. Please note that pKa and Ka are related by the equation Ka = 10^(-pKa), so we can calculate Ka as:
\(Ka = 10^{-5.77}\)
Using a calculator, we get:
\(Ka \approx 1.95 *10^{-6}\)
So, the value of Ka for the unknown acid is approximately 1.95 × 10^(-6).
Learn more about pH here:
https://brainly.com/question/31132561
#SPJ11
An object, such as a planet, circling another object, such as the Sun, at a constant
speed is said to be accelerating. Explain why this motion is an example of
acceleration.
Answers
Answer:
Because something that experiences change in magnitude or the direction of the velocity is said to be acceleration .
Explanation:
what is the correct chemical formula for diphosphorous pentoxide?
Answers
The correct chemical formula for diphosphorous pentoxide is P₄O₁₀.
This compound is formed from the reaction of two molecules of phosphorus trioxide (P₂O₃). The prefix "di" indicates that there are two phosphorus atoms, and the suffix "-pentoxide" indicates that there are five oxygen atoms. The formula can also be written as (P₂O₅)₂, which represents the two P₂O₅ units that combine to form the molecule.
Diphosphorous pentoxide is a white, crystalline solid that is used as a dehydrating agent and in the production of phosphoric acid and other phosphorus compounds. It reacts violently with water, producing phosphoric acid and releasing heat, so it must be handled with care.
To know more about dehydrating agent click on below link:
https://brainly.com/question/14125453#
#SPJ11