if 22.5 l of nitrogen at 748 mm hg and 273 k are compressed to 725 mm hg and 50.0 degree c at constant moles, what is the new volume in liters? report to correct number of sig figs
Answers
The new volume of the nitrogen gas when compressed to the given conditions is 24.7 liters.
To solve this problem, we can use the combined gas law formula:
(P1 × V1) / T1 = (P2 × V2) / T2
Where P1, V1, and T1 represent the initial pressure, volume, and temperature, and P2, V2, and T2 represent the final pressure, volume, and temperature.
Given:
P1 = 748 mm Hg
V1 = 22.5 L
T1 = 273 K
P2 = 725 mm Hg
T2 = 50.0°C = 50.0 + 273.15 = 323.15 K
We need to find V2, therefore, rearrange the combined gas law formula for V2:
V2 = (P1 × V1 × T2) / (P2 × T1)
Now plug in the values:
V2 = (748 mm Hg × 22.5 L × 323.15 K) / (725 mm Hg × 273 K)
V2 = 24.7 L (rounded to correct number of significant figures)
To know something about the combined gas law, click below.
https://brainly.com/question/13538773
#SPJ11
Related Questions
how many types of kinetic energies are there? is it 4 or 5 types?
Answers
What percent of FeSO4 is Fe
Answers
Answer:
About 36%
Explanation:
How many moles of NaOH are needed to make with 45 120 mL of 15% (mass/volume) solution?
Answers
360 moles of NaOH are needed to make with 45 120 mL of 15% solution.
What is mole ?The term mole is defined as one mole of any substance is exactly equal to its atomic mass or molecular mass. It is expressed in grams.
The mole is also define as the amount of substance of a system which contains as many elementary entities. 1 mole = 6.023 X 10²³
Multiply the molarity by the volume of solution to get the number of moles of solute.
moles of solute = molarity × volume of solution
= 45 × 120 / 15
= 360 moles.
Thus, 360 moles of NaOH are needed to make with 45 120 mL of 15% solution.
To learn more about the mole, follow the link;
https://brainly.com/question/26416088
#SPJ1
Gallium is a metallic element in Group III. It has similar properties to aluminium.
(a) (i) Describe the structure and bonding in a metallic element.
Answers
Metallic elements exist in a solid-state and they are opaque, have a shiny surface, good conductors of electricity and heat, malleable and ductile, and are dense. The structure of metals is formed by atoms that are held together by metallic bonds. These atoms have loosely bound valence electrons that can be shared between the neighboring atoms.
Therefore, the outermost shells of these atoms are incomplete due to the sharing of valence electrons, forming a lattice structure known as a metallic bond.Metallic elements have a unique crystal structure that occurs in two forms. The most common type of metal crystal structure is the body-centered cubic structure where the atoms are arranged in a cube with one atom located at the center of the cube. The other type of metal crystal structure is the face-centered cubic structure, where each corner of the cube is an atom and there is an additional atom at the center of each face of the cube .Metallic bonding occurs due to the delocalized electrons that exist in the metal structure. The valence electrons from each atom are free to move throughout the entire metal lattice. Therefore, these electrons form a "sea of electrons" that is shared by all the atoms in the lattice. This results in the metal structure having high thermal and electrical conductivity.Metals are known for their ductility and malleability properties. These properties are due to the metallic bonding that exists in the metal structure. Since the valence electrons are shared, they can easily move past one another, allowing the metal to be hammered into different shapes without breaking.The properties of metals vary depending on their structure and bonding. Gallium, being a metallic element in Group III, has similar properties to aluminum. Therefore, it has a similar metallic bond structure with delocalized electrons that provide the metal with its unique properties.For such more question on valence electrons
https://brainly.com/question/371590
#SPJ8
Please select the word from the list that best fits the definition Composed of one type of compound molecule compound mixture chemical formula molecular formula pure substance
Answers
Answer:
Pure substance
Explanation:
Molecule: contains more atoms of different elements. An example is CO2 molecule. So this option is wrong.
Compound: All compounds are molecules so this option is also wrong.
Mixture: Refers to the union of more than one substances that can be separated.
Chemical and Molecular formular: Represents the constituents of a molecule. This is wrong.
Pure substance: This is the correct option. For an object to be considered pure, it must be composed entirely of one compound.
starting with lead(II)oxide describe how you would prepare a solid sample of lead(II)Carbonate
Answers
The reaction involved is the reaction of PbO with sodium carbonate (Na2CO3) to produce lead(II) carbonate (PbCO3) and sodium oxide (Na2O).
To prepare a solid sample of lead(II) carbonate, we can start with lead(II) oxide (PbO) as the starting material. The chemical equation for the reaction is:
PbO + Na2CO3 → PbCO3 + Na2O
To carry out the reaction, we first need to weigh out the required amount of PbO and Na2CO3 based on the stoichiometry of the reaction. The PbO and Na2CO3 are then mixed thoroughly and placed in a crucible. The mixture is heated in a furnace at a temperature of around 600-700°C for a few hours until the reaction is complete and the mixture has turned into a solid mass.
Once the reaction is complete, the crucible is removed from the furnace and allowed to cool to room temperature. The solid mass of PbCO3 is then carefully removed from the crucible, crushed to a fine powder, and stored in an airtight container for further use. This method is a simple and efficient way to prepare a solid sample of lead(II) carbonate from lead(II) oxide.
For more such questions on reaction
https://brainly.com/question/29470602
#SPJ11
2 moles of NO, was placed in an empty I dm' bottle and allowed to reach equilibrium according to the equation:
At equilibrium, 1.2 moles of N,O, dissociated. Calculate the value of the equilibrium constant for the reaction at that
temperature.
Answers
2NO(g) ⇌ N2(g) + O2(g)
According to the problem statement, 2 moles of NO were placed in a 1 dm^3 bottle and allowed to reach equilibrium, and at equilibrium, 1.2 moles of NO had dissociated. This means that the initial concentration of NO was:
[NO]initial = 2 mol / 1 dm^3 = 2 M
And the concentration of NO at equilibrium is:
[NO]equilibrium = (2 - 1.2) mol / 1 dm^3 = 0.8 M
Since the stoichiometry of the balanced equation is 2:1:1 for NO, N2, and O2, respectively, the equilibrium concentrations of N2 and O2 will also be 0.6 M.
The equilibrium constant (Kc) can be calculated using the equilibrium concentrations of the reactants and products, raised to the power of their stoichiometric coefficients. Therefore:
Kc = ([N2][O2]) / ([NO]^2)
Substituting the equilibrium concentrations into the equation, we get:
Kc = (0.6 M x 0.6 M) / (0.8 M x 0.8 M)
Kc = 0.5625
Therefore, the value of the equilibrium constant for the reaction at that temperature is 0.5625. Note that the units of Kc depend on the stoichiometry of the balanced equation. Since the stoichiometric coefficients are all 1, the units of Kc in this case are M^-1
Most cells in the body of a fruit fly contain eight chromosomes. In some cells, only four chromosomes are present present, a condition, which is a direct result of
Answers
The condition where only four chromosomes are present in some cells of a fruit fly is known as haploidy.
Haploidy can occur due to a variety of reasons, including errors during cell division, such as nondisjunction, which is the failure of chromosomes to separate properly during meiosis. In nondisjunction, the chromosomes fail to divide equally, resulting in daughter cells with abnormal numbers of chromosomes. In the case of the fruit fly, this can result in cells with only four chromosomes instead of the normal eight.
Haploidy can also occur naturally in certain stages of development, such as during gamete formation, where cells undergo meiosis to produce haploid gametes with half the number of chromosomes as the parent cell.
To learn more about chromosomes refer to:
brainly.com/question/29514935
#SPJ4
How do you think weather satellites gather information about the weather?
Answers
The data collected by these satellites is critical in helping meteorologists and other weather experts make accurate predictions about the weather.
Weather satellites are responsible for gathering information about the weather. These satellites provide images of the Earth's atmosphere and its surface. The data gathered by these satellites is critical in studying and forecasting weather patterns. The instruments used on these satellites can measure a variety of things, such as temperature, humidity, pressure, and wind speed and direction.The first thing to understand about weather satellites is that they are in space. They are typically placed in geostationary orbit, which means they are positioned in such a way that they always remain over the same spot on the Earth's surface. This gives them a consistent view of the weather patterns in that particular area.The satellites have a variety of sensors and instruments on board that gather information about the weather. These instruments are capable of measuring a variety of things, such as temperature, humidity, pressure, and wind speed and direction. They also have cameras that take images of the Earth's surface and atmosphere.
This information is sent back to Earth where it can be analyzed by meteorologists and other weather experts.There are two main types of weather satellites: polar orbiting and geostationary. Polar orbiting satellites travel in a north-south orbit over the Earth's poles. They are able to gather more detailed information about the weather, but they have limited coverage and can only see a particular area of the Earth for a short period of time. Geostationary satellites, on the other hand, remain in a fixed position above the Earth's equator. They are able to provide continuous coverage of a particular area of the Earth, but they are not able to gather as much detailed information about the weather as polar orbiting satellites do.In conclusion, weather satellites gather information about the weather by using a variety of sensors and instruments on board. These instruments measure temperature, humidity, pressure, wind speed and direction, and other factors that are important in studying and forecasting weather patterns.
for such more questions on satellites
https://brainly.com/question/31451650
#SPJ8
name the following compound Mol2
Answers
Answer:
you cant answer this
Explanation:
you left something out of the question
Calculate the percent mass of a solution of 5 g NaOH in 95 g of H2O.
Answers
Answer:
=50g NaOH) then,no.of moles of the NaOH solution=50g/40g=1.25 moles/litre
Explanation:
answer=50g NaOH
Which is an empirical formula?
1 P2O5
2 P4O6
3 C2H4
4 C3H6
Answers
P₂O₅ is an empirical formula
Further explanationGiven
compounds
Required
an empirical formula
Solution
The empirical formula is the smallest comparison of the atoms forming the compound.
Molecular formulas are formulas that show the number of atomic elements that make up a compound.
(empirical formula) n = molecular formula
1. P₂O₅ : the ratio of compounds cannot be further reduced to whole numbers⇒empirical formula
2. P₄O₆ : molecular formula
(P₂O₃)₂=P₄O₆
P₂O₃ : empirical formula
3.C₂H₄ : molecular formula
(CH₂)₂=C₂H₄
CH₂ : empirical formula
4. C₃H₆ : molecular formula
(CH₂)₃=C₃H₆
CH₂ : empirical formula
Answer:
P₂O₅
Explanation:
Which is the correctly balanced chemical equation for the reaction of KOH and H2SO4?
A,B,C,or D?

Answers
Answer:
B
Explanation:
Potassium has a density of 0.856 g/cm3 and crystallizes with a body-centered cubic unit cell structure. what is the atomic radius of the atom?
Answers
To find the atomic radius of a potassium atom with a body-centered cubic (BCC) unit cell structure and a density of 0.856 g/cm³, you can follow these steps:
1. Determine the number of atoms in a BCC unit cell: There are 2 atoms per BCC unit cell.
2. Obtain the atomic weight of potassium: The atomic weight of potassium (K) is approximately 39.1 g/mol.
3. Apply the BCC unit cell density formula: Density = (2 x atomic weight) / (a³ x Avogadro's number), where 'a' is the edge length of the unit cell and Avogadro's number is approximately 6.022 x 10²³ atoms/mol.
4. Solve for the edge length 'a': Using the given density of 0.856 g/cm³, you can rearrange the formula to find the edge length of the unit cell.
5. Calculate the atomic radius: For a BCC unit cell, the diagonal of the cube can be represented as 4r = √3a, where 'r' is the atomic radius. Solve for the atomic radius using the edge length obtained in step 4.
By following these steps, you can find the atomic radius of a potassium atom with a BCC unit cell structure and a density of 0.856 g/cm³.
Learn more about Potassium:
https://brainly.com/question/24527005
#SPJ11
The atomic radius of a potassium atom in a body-centered cubic unit cell can be calculated by utilizing the given density and atomic weight with the formula r = ∛(3*m / 4π * ρ*N), which accounts for the two atoms in each unit cell.
Explanation:To calculate the atomic radius of potassium with a body-centered cubic (BCC) lattice structure, we first need to define the unit cell. In a BCC lattice, the unit cell is a cube defined by the centers of eight atoms and contains one atom at each of the eight corners, plus one atom from the center. This means that there are 2 atoms contained within this cube (0.125 * 8 corners = 1 atom from the corners + 1 atom from the center).
Because we know that a corner atom is shared by 8 unit cells and a center atom is completely within the unit cell, this equates to 2 atoms per unit cell. To calculate the radius, the equation r = ∛(3*m / 4π * ρ*N) can be used, where m is the atomic weight of potassium (39.10 g/mol), ρ is the density (0.856 g/cm³), and N is Avogadro's number(6.022 x 10^23 mol⁻¹).
Learn more about Atomic Radius of Potassium here:https://brainly.com/question/21251053
#SPJ12
What is the ΔG of the following hypothetical reaction? 2A(s) + B2(g) → 2AB(g)
Given: A(s) + B2(g) → AB2(g) ΔG = -215.6 kJ 2AB(g) + B2(g) → 2AB2(g) ΔG = -672.7 kJ
Enter your answer in decimal notation with four significant figures.
Answers
The ΔG value for the hypothetical reaction \(2A(s) + B_2(g)\) → \(2AB(g)\) can be calculated by summing the individual ΔG values of the given reactions.
To find the overall ΔG for the reaction, we need to combine the two given reactions and cancel out the common intermediate species, AB(g). We can achieve this by the second reaction and multiplying the first reaction by 2.
The first reaction, \(A(s) + B_2(g)\) → \(AB_2(g)\), has a ΔG of -215.6 kJ. By reversing the second reaction, \(2AB(g) + B_2(g)\)→ \(2AB_2(g)\), the ΔG becomes +672.7 kJ.
Now, we can add the two reactions together:
\(2A(s) + B_2(g)\)→ 2AB(g) (ΔG = -215.6 kJ)
\(2AB(g) + B_2(g)\) → \(2AB_2(g)\) (ΔG = +672.7 kJ)
By summing the ΔG values, we have -215.6 kJ + 672.7 kJ = 457.1 kJ.
Thus, the ΔG for the hypothetical reaction \(2A(s) + B_2(g)\)→ 2AB(g) is 457.1 kJ.
Learn more about hypothetical reaction here:
https://brainly.com/question/31975534
#SPJ11
imagine that a classmate draws an electron dot diagram for a helium atom with two dots. he or she tells you that these dots mean helium atom has two unpaired electrons to have four pairs of valence electrons and become stable. What is wrong with your classmate's argument?
Answers
Answer:
The helium atom is already stable and doesn't need to bond with other atoms or share electrons. Helium's lowest energy level can only hold two electrons, which are paired.
Imagine that a classmate draws an electron dot diagram for a helium atom with two dots. He or she tells you that these dots mean helium atom has two unpaired electrons to have four pairs of valence electrons and become stable. If it loses 2 electrons then it becomes double charged helium and helium has no unpaired electron.
HeliumHelium is a noble gas. The symbol of Helium is He. Helium has two valence electrons. Helium atom has no unpaired electrons. Helium has two electrons in the outermost shell.
Thus, from the above conclusion we can say that If helium loses 2 electrons then it becomes double charged helium and helium has no unpaired electron.
Learn more about Valence electrons here: https://brainly.com/question/371590
#SPJ2

Calculate the new volume if 12.78 L of a gas at -50*C is heated to a temperature of 28*C
Answers
Explanation:
V1/T1 = V2/T2 T must be in Kelvin
12.78 / (-50 + 273.15) = V2 / ( 28+ 273.15)
V2 = 17.25 L
A balloon inflated in a room at 24 °C has a volume of 2.00 L. The balloon is then heated to a temperature of 88 °C. What is the volume of the balloon assuming the pressure remains constant?
Answers
Therefore, the volume of the balloon when heated to 88°C is 2.432 L.
What is volume?In the case of a gas, the volume is the amount of space that the gas occupies in a container. The volume of a gas can be affected by changes in pressure and temperature, as described by the ideal gas law. In this case, the volume is often expressed in terms of standard temperature and pressure (STP), which are defined as a temperature of 0°C and a pressure of 1 atmosphere (atm). In chemistry, volume is an important property for understanding the behavior of substances in chemical reactions. The volume of reactants and products can be used to determine the stoichiometry of a reaction, which is the relationship between the amounts of reactants and products in a chemical equation.
Here,
To solve this problem, we can use the ideal gas law, which states that:
PV = nRT
where P is the pressure of the gas, V is its volume, n is the number of moles of gas, R is the universal gas constant, and T is the temperature in Kelvin.
Since the pressure of the gas is assumed to remain constant, we can rearrange the ideal gas law to solve for V:
V1/T1 = V2/T2
where V1 is the initial volume of the balloon, T1 is the initial temperature in Kelvin (24°C = 297 K), V2 is the final volume we want to find, and T2 is the final temperature in Kelvin (88°C = 361 K).
Using this equation, we can plug in the given values and solve for V2:
V1/T1 = V2/T2
2.00 L / 297 K = V2 / 361 K
Multiplying both sides by 361 K, we get:
V2 = 2.00 L x (361 K / 297 K)
V2 = 2.00 L x 1.2162
V2 = 2.432 L
To know more about volume,
https://brainly.com/question/25252629
#SPJ1
Thermal windows often have all the air pumped out between the panes and a gas pumped in that is not harmful and won't react with other things. What gas would be used?
Answers
Answer:
Argon gas
Explanation:
Argon is a chemical element. Its symbol is Ar. It is the noble gas which is odorless and colorless. It is one of the most abundant gas on the earth after oxygen and nitrogen.
Argon gas is most commonly used in the panes of the thermal windows. It has 34 percent lower thermal conductivity as compared to the air and so it is more comfortable to used instead of the air. It is an inert gas and does not react with other things. It is not harmful.
A short chain of monomer liquid not long enough to be considered a polymer is called a(n)?
a. methacrylate
b. acrylate
c. urethane
d. oligomer
Answers
Answer:
d. oligomer it is the correct answer also if you would just search that up it pops up
HD:pSun =rhoMan =pTue =pWed =pThu =pFri =pSat =71 Ha : Not all proportions are equal. HD: Not all proportions are equal. Ha:pSun =pMon =pTue =rhoWed =pThu =rhoFri =rhoSat =71 HD: Not all proportions are equal. Ha:pSun =pMon =pTue =pWed =pThu =rhoFri =pSat =71 HD:pSun =pMon =pTue =pWod =pThu =pFri =pSat =71 Ha : All proportions are equal. Find the value of the test statistic. (Round your answer to three d: Find the p-value. (Round your answer to four decimal places.) p-value = State your conclusion. Do not reject H0−. We conclude that the proportion of traffic Reject HD. We conclude that the proportion of traffic acciden Reject HD. We conclude that the proportion of traffic acciden Do not reject H0−We conclude that the proportion of traffic Compute the percentaqe of traffic accidents occurring on each day What day has the highest percentage of traffic accidents? Sunday Monday Tuesday Wednesday Thursday Friday Saturday Based on 2017 sales, the six top-selling compact showed the following number of vehicles sold. Use a goodness of fit test to determine if the sample data indicate that the market shares for compact cars in the city are different than the market shares suggested by nationwide 2017 sales. Use a 0.05 level of significance. State the null and alternative hypothesis. Ha : The market shares for the compact cars in the city are different for at least one of the nationwide market shares listed. o: The market shares for the compact cars in the city do not differ from market shares nationwide. : The market shares for the compact cars in the city are different from at least one of the nationwide market shares listed. Ha : The market shares for the compact cars in the city are not different from any of the natione Ha : The market shares for the compact cars in the city do not differ from market shares nationwide. "the test statistic.(Round your answer to two decimal places.) d the rho-value. (Round your answer to four decimal places.) Reject H0. We cannot conclude that market shares for the compact cars in the city differ from the nationwide market shares. Do not reject H0. We conclude that market shares for the compact cars in the city differ from the nationwide market shares. Do not reject H0. We cannot conclude that market shares for the compact cars in the city differ from the nationwide market shares.
Answers
The p-value is greater than the significance level of 0.05, we do not reject the null hypothesis and conclude that all proportions are equal.
Firstly, let us conduct a Chi-square test of independence of categorical variables based on the information given above. We have three different cases of hypothesis testing that we have to solve one by one.
Case 1: HD:pSun =rhoMan =pTue =pWed =pThu =pFri =pSat =71
Ha : Not all proportions are equal.
Test Statistic
For this hypothesis, we need to compute the test statistic that is given as:
\($$\chi^2=\sum_{i=1}^{k}\frac{(O_i-E_i)^2}{E_i}$$\) where k is the number of groups/categories. Since we have 7 days of the week, \(k = 7. $O_i$ and $E_i$\) are the observed and expected frequencies respectively.
Here, we have equal proportions of 71 for each day of the week.
Therefore, the expected frequencies are also equal to 71.
\($$E_i = 71, i=1,2,3,4,5,6,7.$$\)
We also have to use the given information to compute the observed frequencies,
\($O_i$.$$O_1 = 90, O_2 = 99, O_3 = 122, O_4 = 123, O_5 = 130, O_6 = 160, O_7 = 126$$\)
Therefore, the test statistic can be computed as \($$\chi^2=\frac{(90-71)^2}{71} + \frac{(99-71)^2}{71} + \frac{(122-71)^2}{71} + \frac{(123-71)^2}{71} + \frac{(130-71)^2}{71} + \frac{(160-71)^2}{71} + \frac{(126-71)^2}{71}$$$$\chi^2=180.14\)
Now we have to find the p-value of this test. Since the number of degrees of freedom is k - 1 = 7 - 1 = 6, the p-value can be found using the chi-square distribution table with 6 degrees of freedom at the 0.05 significance level. The p-value is 0.000014. ConclusionSince the p-value is less than the significance level of 0.05, we reject the null hypothesis and conclude that not all proportions are equal.
The total number of accidents is \($$90+99+122+123+130+160+126=850$$\)
The percentage of accidents occurring on each day of the week can be found as follows:
\($$Sunday: $$\frac{90}{850}\times 100 = 10.59\%$$Monday: $$\frac{99}{850}\times 100 = 11.65\%$$Tuesday: $$\frac{122}{850}\times 100 = 14.35\%$$Wednesday: $$\frac{123}{850}\times 100 = 14.47\%$$Thursday: $$\frac{130}{850}\times 100 = 15.29\%$$Friday: $$\frac{160}{850}\times 100 = 18.82\%$$Saturday: $$\frac{126}{850}\times 100 = 14.82\%$$\)
From the above percentages, we can see that Friday has the highest percentage of traffic accidents.
Case 2:
HD: Not all proportions are equal.
Ha:pSun =pMon =pTue =rhoWed =pThu =rhoFri =rhoSat =71
Test Statistic
\($$E_1 = 78.57, E_2 = 86.57, E_3 = 106.86, E_4 = 107.43, E_5 = 113.57, E_6 = 139.43, E_7 = 109.14$$\)
We already know the observed frequencies,
\($$O_1 = 90, O_2 = 99, O_3 = 122, O_4 = 123, O_5 = 130, O_6 = 160, O_7 = 126.$$\)
The test statistic can be computed as:
\($$\chi^2=\frac{(90-78.57)^2}{78.57} + \frac{(99-86.57)^2}{86.57} + \frac{(122-106.86)^2}{106.86}+ \frac{(123-107.43)^2}{107.43} + \frac{(130-113.57)^2}{113.57} + \frac{(160-139.43)^2}{139.43} + \frac{(126-109.14)^2}{109.14} $$$$ \implies \chi^2=34.98$$\)
The p-value is 0.000001.
Conclusion- Since the p-value is less than the significance level of 0.05, we reject the null hypothesis and conclude that not all proportions are equal.
Case 3:
All proportions are equal.
Test Statistic
The expected frequency for each group is
\(E = \frac{850}{7} = 121.43\)
We already know the observed frequencies,
\($$O_1 = 90, O_2 = 99, O_3 = 122, O_4 = 123, O_5 = 130, O_6 = 160, O_7 = 126.$$\)
The test statistic is,
\($$\chi^2=\frac{(90-121.43)^2}{121.43} + \frac{(99-121.43)^2}{121.43} + \frac{(122-121.43)^2}{121.43} + \frac{(123-121.43)^2}{121.43} + \frac{(130-121.43)^2}{121.43} + \frac{(160-121.43)^2}{121.43} + \frac{(126-121.43)^2}{121.43}} \\\implies \chi^2=9.17$$\)
The p-value is 0.1664.
Since the p-value is greater than the significance level of 0.05, we do not reject the null hypothesis and conclude that all proportions are equal.
To know more about p-value visit:
https://brainly.com/question/30761573
#SPJ11
thermodynamic parameters are usually calculated for compounds under conditions, since values tend to vary somewhat with conditions. to be in this state, a gas must have a pressure of 1 , and a solution must have a concentration of 1 .
Answers
The state at which thermodynamic parameters are usually calculated for compounds is the standard state, which is defined as the state of a substance at a pressure of 1 bar (or 1 atm) and a temperature of 25°C (298.15 K), for gases, and a concentration of 1 M, for solutions.
The reason for defining a standard state is to allow for a consistent comparison of thermodynamic properties of different substances under the same conditions. By using a standardized state, it is possible to calculate and compare thermodynamic parameters such as enthalpy, entropy, and Gibbs free energy for different compounds, and to make predictions about the feasibility and spontaneity of chemical reactions.
It should be noted, however, that the actual values of thermodynamic parameters for a compound may vary somewhat with conditions such as temperature, pressure, and concentration. Thus, when performing actual experiments or calculations, it is important to take into account the specific conditions and adjust the parameters accordingly. Nonetheless, the standard state provides a useful reference point for comparing and understanding the thermodynamic properties of different compounds.
To learn more about thermodynamic parameters refer to:
brainly.com/question/28216413
#SPJ4
draw the structure of water. is water considered polar or nonpolar? explain why
Answers
Answer:
The structure of water consists of two hydrogen atoms bonded to one oxygen atom. The oxygen atom is located in the center, with the hydrogen atoms bonded to it at an angle of approximately 104.5 degrees. This arrangement gives water a bent or V-shaped molecular geometry.Water is considered a polar molecule. This is because the oxygen atom is more electronegative than the hydrogen atoms, meaning it has a stronger pull on the shared electrons in the covalent bonds. As a result, the oxygen atom gains a partial negative charge (δ-) while the hydrogen atoms acquire partial positive charges (δ+).The unequal distribution of charges in water creates a dipole moment, with the oxygen atom being the negative end and the hydrogen atoms being the positive ends. This polarity allows water molecules to form hydrogen bonds with each other and with other polar molecules or ions.The hydrogen bonds between water molecules are responsible for many of its unique properties, such as its high boiling point, surface tension, and ability to dissolve a wide range of substances. These hydrogen bonds also contribute to the cohesive and adhesive properties of water, allowing it to form droplets and capillary action.In summary, water is considered a polar molecule due to the unequal distribution of charges caused by the electronegativity difference between oxygen and hydrogen.Hey besties can y'all help me out
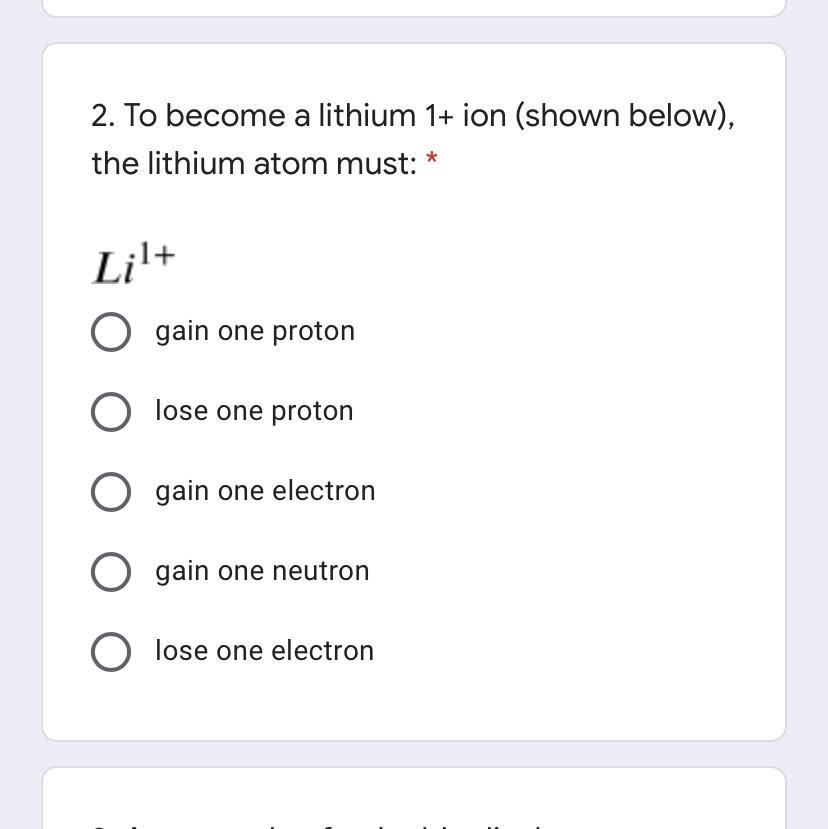
Answers
you need to lose one electron
how many protons are in each of the following

Answers
carbon has 6 protons and hydrogen has 1 proton
Question 2
5 pts
The air inside a beach ball is at a temperature of 25°C a pressure of 1.0 atm. The ball
contains 0.85 mol of air, what is its volume?
21 L
27 L
0 171
06.11
< Previous
Nest

Answers
Answer: The volume of given ball is 21 L.
Explanation:
Given : Pressure = 1.0 atm, no. of moles = 0.85 mol
Temperature = \(25^{o}C = (25 + 273) K = 298 K\)
According to the ideal gas equation, formula used is as follows.
PV = nRT
where,
P = pressure
V = volume
n = no. of moles
R = gas constant = 0.0821 L atm/mol K
T = temperature
Substitute the values into above formula as follows.
\(PV = nRT\\1.0 atm \times V = 0.85 mol \times 0.0821 Latm/mol K \times 298 K\\V = 21 L\)
Thus, we can conclude that the volume is 21 L.
Which is MOST likely to be considered “newsworthy”?
a study on which foods contain the most antioxidants, done five years ago
a study on which foods contain the most antioxidants, done two years ago
a study on which foods contain the most antioxidants, done last year
a study on which foods contain the most antioxidants, done last month
Answers
Define photon as a unit of radiation energy.
Answers
Answer:
A photon is the smallest discrete amount or quantum of electromagnetic radiation. It is the basic unit of all light. As per Einstein's light quantum theory, photons have energy equal to their oscillation frequency times Planck's constant.
Explanation:
A photon is defined as the the smallest discrete amount or quantum of electromagnetic radiation. It can also be called as energy packets.
Photon is considered to be the basic unit of all light. As per Einstein's quantum theory, photons have energy equal to their oscillation frequency times Planck's constant.
The energy of photon equal to the difference in energy between the atom's excited state and its final state.
The photon energy of the emitted photon is equal to the energy difference between the two states. There are many possible electron transitions for each atom, and each transition has a specific energy difference.
When an atom in an excited state emits a photon of radiation, the energy of the photon is equal to the difference in energy between the atom's excited state and its final state.
To know more about photon here
https://brainly.com/question/30423305
#SPJ2
outline the sequence of steps needed to solve a typical stoichiometric problem
Answers
A typical stoichiometric problem involves writing the balanced chemical equation, identifying the known and unknown quantities, using stoichiometry to convert the known quantities to the required units for the unknown quantity, and calculating the unknown quantity based on the stoichiometric coefficients.
A stoichiometric problem involves calculating the amount of reactants and products involved in a chemical reaction. To solve a typical stoichiometric problem, the following sequence of steps is generally required:
1. Write the balanced chemical equation for the reaction.
2. Identify the known quantities, which may be given in units of mass, moles, or volume.
3. Determine the unknown quantity that needs to be calculated.
4. Use stoichiometry to convert the known quantities to the required units for the unknown quantity.
5. Calculate the amount of the unknown quantity based on the stoichiometric coefficients in the balanced equation.
6. Check the answer for accuracy and consistency with the laws of conservation of mass and energy.
To know more about stoichiometric coefficients visit:
https://brainly.com/question/32088573
#SPJ11
Charli is boiling a pot of water as she prepares dinner. The temperature at which the water boils can be explained by the external
pressure and
A
the amount of heat that is added to the pot of water.
B. the volume of the sample of water being heated.
C. the rate at which heat is added to the pot of water.
D
the strength of attractive forces among the water molecules.
PLEASE ANSWER QUICKLY THIS IS DUE IN 5 MINS!!
Answers
Answer:
ya tu sabes cómo se los aseguro