If 400 mL of a 40 %w/v solution is diluted to 1000 mL, what will be the percentage strength of the resulting solution(%w/v)?
Answers
If 400 mL of a 40% w/v solution is diluted to 1000 mL, the percentage strength of the resulting solution will be 16% w/v.
To find the percentage strength of the resulting solution, we need to use the formula:
C1V1 = C2V2
where C1 is the concentration of the initial solution, V1 is the volume of the initial solution, C2 is the concentration of the resulting solution, and V2 is the volume of the resulting solution.
We can plug in the given values and solve for C2:
(40%w/v) x 400mL = C2 x 1000mL
16000 = C2 x 1000
C2 = 16%w/v
Therefore, the percentage strength of the resulting solution is 16%w/v.
To find the percentage strength of the resulting solution after diluting 400 mL of a 40% w/v solution to 1000 mL, follow these steps:
Step 1: Calculate the amount of solute in the initial solution.
Since the initial solution is 40% w/v, it contains 40 g of solute per 100 mL of solution. To find the amount of solute in 400 mL, multiply the percentage by the volume:
Amount of solute = (40 g/100 mL) × 400 mL = 160 g
Step 2: Calculate the percentage strength of the resulting solution.
Now that you have 160 g of solute in the 1000 mL diluted solution, divide the amount of solute by the total volume and multiply by 100 to get the percentage:
Percentage strength (%w/v) = (160 g / 1000 mL) × 100 = 16%
If 400 mL of a 40% w/v solution is diluted to 1000 mL, the percentage strength of the resulting solution will be 16% w/v.
To know more about percentage, visit
https://brainly.com/question/29306119
#SPJ11
Related Questions
what is the molar concentration of cl- when 0.20 mol of zncl2 are dissolved in enough water to prepare 3.0 l of solution
Answers
The molar concentration of Cl- when 0.20 mol of ZnCl₂ are dissolved in enough water to prepare 3.0 l of solution is 0.13 M
What is it Chloride ion What is it?The chloride ion is the anion Cl. It is created when a molecule, such as hydrogen chloride, dissolves in water or other polar solvents, or when the element chlorine gets an electron. Sodium chloride and other chloride salts are usually highly soluble in water.
It is an electrolyte that is present in all biological fluids and is in charge of regulating the flow of liquid into and out of cells, conveying nerve impulses, and keeping the acid/base balance.
Less frequently, the "common" name of a chemical compound with one or more chlorine atoms covalently bound may contain the term chloride.
Given the molecular formula, we have two chlorine atoms for every mole of ZnCl₂
0.2 mol ZnCl₂ x 2 mole Cl⁻/ 1 mole ZnCl₂ = 0.4 mol Cl⁻
Now we will divide this by the volume of the solution as liters
Concentration = 0.4 mol Cl⁻ / 3 L = 0.13 M
Learn more about ZnCl at https://brainly.com/question/11408001
#SPJ4
An isotope of nitrogen experiences the weak force which transforms one of its
protons to a neutron. How would this change the atom?
Select one:
O a. It would have no effect because nitrogen still has the same mass
number.
O b. It would transform the nitrogen atom to a carbon atom.
It would make the atom transform it into a heavier isotope of nitrogen.
O d. It would transform nitrogen to an oxygen atom.
Check
Answers
Answer:
It would transform the nitrogen atom to a carbon atom
Explanation:
If you have a liquid that you suspect might be a mixture explain what you might do to find out if it is.
Answers
Answer:
add a substace devider
Explanation:
i forgot what it was called but there is a chemical that allows the substances to seperste and than you can test the substaces ph and what it is
why was it necessary to titrate the cold solution in the ice bath, but not necessary to titrate the hot solution in the hot bath?
Answers
The solubility of the Ca(OH)2 would be greatly increased since the lower temperatures would result in a slower titration and the solution would have time to warm up.
What is titration?
When a reaction reaches neutralisation, which is sometimes signalled by a colour change, titration is the steady addition of one solution with a known concentration (referred to as a titrant) to a known volume of another solution with an unknown concentration. To be a primary or secondary standard, the so-called titrant must meet the appropriate criteria. Titration is a method for figuring out a solution's concentration in a wide sense.
One of the processes in the manufacture of silver nanoparticles involves gently combining sodium borohydride with silver nitrate in an ice bath to create yellow colloidal silver.
Since the titration would proceed more slowly at the lower temperatures and the solubility of the Ca(OH)2 would increase dramatically as the solution warmed, it was necessary to titrate the cold solution in the ice bath.
Learn more about titration from given link
https://brainly.com/question/13307013
#SPJ4
PLZ HELP ASAP IM TIMED!
Which of the following statements is true?
a.) Sound vibrations are changed into electrical impulses by the eardrum.
b.) It is dangerous to listen to sounds over 160 dBs.
c.) High-pitched sounds have low frequencies.
d.) All of the above.
THERE IS A QUESTION LIKE THIS ON BRAINLY BUT ITS VOTED 1.5 STARS PLZ HELP ASAP PLZ ILL GIVE 25 POINTS PLZ
Answers
Which term best characterizes the relation of hydrogen to deuterium?
(A) allotropes (B) somers (C) isotopes (D) polymers
Answers
The term that best characterizes the relation of hydrogen to deuterium is isotopes. The correct option is C.
Isotopes are elements that have the same number of protons but different numbers of neutrons in their nuclei, resulting in different atomic masses. Hydrogen and deuterium are isotopes of each other because they both have one proton in their nucleus but hydrogen has no neutron while deuterium has one neutron.
This difference in atomic mass has important implications in the physical and chemical properties of the two isotopes. For example, deuterium is twice as heavy as hydrogen, which affects its behavior in reactions and its use in nuclear applications. Moreover, the fact that hydrogen and deuterium are isotopes of each other allows for a variety of studies on isotopic effects, such as kinetic isotope effects, which can reveal details about reaction mechanisms and molecular dynamics.
In summary, the relation of hydrogen to deuterium is best described as isotopes, a term that refers to elements with the same number of protons but different numbers of neutrons in their nuclei, resulting in different atomic masses and physical and chemical properties.
The correct option is C.
To know more about isotopes, refer to the link below:
https://brainly.com/question/27475737#
#SPJ11
Study the image.
Which phrase describes this plate boundary?
does not occur in oceans
may form rift valleys
is a type of convergent boundary
is a region where earthquakes occur
Answers
The phrase that describes the plate boundary shown is D. is a region where earthquakes occur .
What is the plate boundary shown ?A transform boundary is the name given to this tectonic plate boundary. Two or more plates slip past one another in such a border. This shift is typically accompanied by some highly active earthquakes.
At a transform plate border, the grinding motion between the plates causes shallow earthquakes, significant lateral rock displacement, and a wide zone of crustal deformation. Essentially While no new crust is produced, subducted, or formed, and no volcanoes are formed, the fault produces earthquakes.
Find out more on plate boundaries at https://brainly.com/question/22005072
#SPJ1
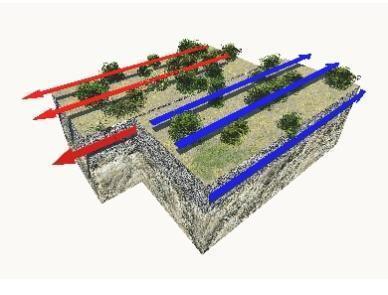
5. did the ph meter, ph paper, and universal indicator give similar ph values (agree) when evaluating the pond water, the tap water, the baking soda solution, and the soil sample? did the ph meter give one value, and the ph paper or universal indicator give an entirely different value for that sample? was this expected? why or why not?
Answers
No. they can't give the same pH value because pH meter will give only the single value and pH paper and indicator can give slightly more or less value compared to pH meter.
A pH paper is defined as a piece of paper used to find out if a solution is basic, acidic or neutral. The pH is determined by dipping part of the paper into a solution and observing the color changes. This changes color in different solutions due to the chemical flavin. A universal indicator is defined as a pH indicator composed of a solution of several compounds that exhibits several smooth color changes over a pH value range from 0 to 14 to indicate the acidity or alkalinity of solutions, where 7 indicates neutral. If we evaluating the pond water, the tap water, the baking soda solution, and the soil sample, pH meter, pH paper and universal indicator doesn't give the same value. pH meter will give only single pH value. but if we use pH paper and universal indicator they will give range of pH value not single value so paper and indicator can give slightly more or less value compared to pH meter.
To learn more about pH value
https://brainly.com/question/1810086
#SPJ4
Would a rollercoaster have the greatest kinetic energy at the top of the highest hill or at the bottom on the highest hill
Answers
Answer:
The rollercoaster has the lowest kinetic energy at the top of the hill.
The rollercaoster has the highest kinetic energy at the bottom of the hill.
Explanation:
The kinetic energy of any solid body in motion is usually computed using this formula:
K.E = \(\frac{1}{2}mv^2\)
From this, we can see that it varies based on two major parameters - The mass of the moving object and the velocity of the moving object.
We can assume that the mass of the rollercoaster is constant since no one gets off and it does not shrink in its size during the ride.
This means that the variations in the K.E are mainly coming from its velocity.
At the top of the hill, the rollercoaster is moving at its slowest pace. hence, it has the lowest kinetic energy at the top of the hill.
However, at the bottom of the hill, the rollercoaster is moving its fastest, hence it has the highest kinetic energy at the bottom of the hill.
(c) 10g of HBr reacted with 5g of Al. Calculate the: (i) mass of Aluminium bromide produced. (ii) number of bromide ions formed. (iii) percentage yield if 0.55 g of aluminum bromide is actually yielded. [Al-27, Br-80, H=1, L-6.022×10²3]
Answers
6HBr+2Al → 2AlBr3+3H2
now, as the reaction is showing
6 moles of HBr reactes with 2 moles of Aluminium gives us 2 moles of Aluminium bromide and 3 moles of hydrogen gas
as the question says 10g of HBr reacted with 5g of Al.
(i) mass of Aluminium bromide produced.
moles = given mass/molecular mass
moles = 5g/54g
moles 0.0925 mole
(ii) number of bromide ions formed
6 moles of bromide ions formed in this reaction
now 1 moles = 6.022×10^23
6 moles = 6 ×6.022×10^23
= 36.132×10^23ions
(iii) percentage yield if 0.55 g of aluminum bromide is actually yielded
percentage yield= moles×100
percentage yield= 0.55×100÷107
percentage yield=0.514%
To know more about moles visit : https://brainly.com/question/21323029
#SPJ9
Explain how natural resources are identified and why natural recourses are in evenly distributed.
Answers
Answer:
Natural resources are not evenly distributed all over the world. Some places are more endowed that others — for instance, some regions have lots of water (and access to ocean and seas). Others have lots of minerals and forestlands. Others have metallic rocks, wildlife, fossil fuels and so on.
Explanation:
What is the oxidation state of iron inK3[Fe(C2O4)3]3H2O? Justify your answerDraw clearly and describe in words the structure of[Fe(C2O4)3]3- in thecoordination complexK3[Fe(C2O4)3]3H2O
Answers
The overall structure of the coordination complex K₃[Fe(C₂O₄)3]3H₂O can be represented as a distorted octahedron, with the six C₂O₄₄₋groups surrounding the iron atom at the center. The oxidation state of iron in K₃[Fe(C₂O₄)3]3H₂O is +3.
In the coordination complex K₃[Fe(C₂O₄)3]3H₂O, iron is in the +3 oxidation state, which means that it has gained three electrons in its outermost energy level.
The +3 oxidation state of iron is consistent with the oxidation state of ferric ion, Fe3+, which is the dominant form of iron in this coordination complex. Ferric ion is formed by the removal of three electrons from the innermost shell of the iron atom.
The structure of the coordination complex K₃[Fe(C₂O₄)3]3H₂O can be described as a central iron atom surrounded by six C₂O₄₄₋ groups. The iron atom is bonded to each of the six C₂O₄₄₋ groups through a bidentate ligand, which is a ligand that binds to the iron atom through two of its atoms.
The C₂O₄₄₋ groups are coordinated to the iron atom through a pair of oxygen atoms and a pair of carbon atoms. The six C₂O₄₄₋groups are arranged around the iron atom in a hexagonal ring, with each group sharing a oxygen atom with the iron atom.
The overall structure of the coordination complex K₃[Fe(C₂O₄)3]3H₂O can be represented as a distorted octahedron, with the six C₂O₄₄₋groups surrounding the iron atom at the center.
Learn more about octahedron
https://brainly.com/question/18369918
#SPJ4
What are the protons neutrons and electrons of Vanadium-52 +3 charge
HELP
Answers
Answer:
Protons: 23
Neutrons: 28
Electrons: 23
I THINK
Explanation:
which 0.10 m solution will have the largest concentration of hydroxide ion? group of answer choices nh3 (kb of nh3
Answers
A 0.10 M solution of potassium hydroxide (KOH) will have the largest concentration of hydroxide ions.
To determine which 0.10 M solution will have the largest concentration of hydroxide ions, we need to compare concentrations of hydroxide ions (OH-)
For a generic ionic compound AOH, the equilibrium constant expression is:
AOH(s) ⇌ A+(aq) + OH-(aq)
Kb = [A+][OH-] / [AOH]
Since we are comparing solutions of different ionic compounds. The higher the Kb value, the stronger the base and the higher the concentration of hydroxide ions in the solution. Looking up the Kb values for various ionic compounds, we find that potassium hydroxide (KOH) has the highest Kb value: \((2.2 * 10^{-16})\), followed by sodium hydroxide: \((NaOH) (1.0 * 10^{-14})\),
and then calcium hydroxide \((Ca(OH)_2) (5.5 * 10^{-6})\).
To know more about potassium hydroxide, here
brainly.com/question/28457249
#SPJ1
--The complete Question is, which 0.10 m solutions will have the largest concentration of hydroxide ion? --
what is a mixture of elements and compounds

Answers
The substance in the image above would be classified as a mixture of elements (option E).
What is a compound and mixture?A compound is a substance formed by chemical bonding of two or more elements in definite proportions by weight.
On the other hand, a mixture is made when two or more substances are combined, but they are not combined chemically.
According to this question, an image is shown with two different substances or elements as distinguished by coloration (white and purple). These elements are combined but not chemically bonded, hence, is a mixture.
Learn more about mixture at: https://brainly.com/question/12160179
#SPJ1
How many moles of a gas at 100°C does it take to fill a 1.00 L flask to a pressure of 1.50 atm?
Answers
The number of moles of a gas at 100°C it takes to fill a 1.00 L flask to a pressure of 1.50 atm is 0.049 moles.
How to calculate number of moles?The number of moles of a substance can be calculated using the following formula (Avogadro's equation);
PV = nRT
Where;
P = pressureV = volumeT = temperaturen = no of molesR = gas law constantAccording to this question, a gas at 100°C is filled to 1.00 L flask at a pressure of 1.50 atm. The number of moles can be calculated as follows;
1.5 × 1 = n × 0.0821 × 373
1.5 = 30.6233n
n = 0.049 moles
Learn more about Avogadro's equation at: https://brainly.com/question/22651133
#SPJ1
How many joules are released when 1.70 mol of 239Pu decays if each nucleus releases 5.243 MeV? ___ x 10 ____
Answers
The amount of energy released when 1.70 mol of 239Pu decays is 1.46 x 10^14 joules.
To find the amount of energy released, we need to multiply the number of nuclei by the energy released per nucleus. First, we need to convert the given amount of moles to the number of nuclei by using Avogadro's number (6.02 x 10^23 nuclei/mol).
1.70 mol x 6.02 x 10^23 nuclei/mol = 1.0244 x 10^24 nuclei
Next, we convert the energy released per nucleus from MeV to joules by using the conversion factor 1.602 x 10^-13 joules/MeV.
5.243 MeV x 1.602 x 10^-13 joules/MeV = 8.39 x 10^-13 joules
Finally, we multiply the number of nuclei by the energy released per nucleus.
1.0244 x 10^24 nuclei x 8.39 x 10^-13 joules/nucleus = 1.46 x 10^14 joules
Therefore, the amount of energy released when 1.70 mol of 239Pu decays is 1.46 x 10^14 joules.
Learn more about energy here:
https://brainly.com/question/31081047
#SPJ11
A blu-ray laser has a wavelength of 405nm. In what region of the electromagnetic spectrum is this radiation? What is its frequency?
Answers
Answer:
It lies in in the visible region.
Frequency: is 7.407 × 10^-14 Hz
\({ \bf{V = f \lambda}} \\ { \tt{3.0 \times {10}^{8} = f \times (405 \times {10}^{ - 9}) }} \\ { \tt{f = 7.407 \times {10}^{14} \: hertz}}\)
Explanation:
V is speed of light.
f is frequency
lambda is the wavelength
Can someone answer the bottom question (22-25)

Answers
23) Neon with a +2 charge
24) Fluorine with a -1 charge
25) Lithium with a +1 charge
PLEASEEEEEEEEE HELP MEEEE AND EXPLAINNNNN

Answers
to what volume must 1.0 l of a 6.0 m solution of hcl be diluted in order to prepare a 0.2 m solution? select one: a. 30 l b. 20 l c. 10 l d. 40 l
Answers
Answer:
A
Explanation:
To determine the volume required to dilute a 1.0 L solution of 6.0 M HCl to a 0.2 M solution, we can use the equation for dilution:
M1V1 = M2V2
Where:
M1 = Initial concentration of the solution (6.0 M)
V1 = Initial volume of the solution (1.0 L)
M2 = Final concentration of the solution (0.2 M)
V2 = Final volume of the solution (unknown)
Rearranging the equation, we have:
V2 = (M1/M2) * V1
Plugging in the values:
V2 = (6.0 M / 0.2 M) * 1.0 L
V2 = 30 L
Therefore, the volume required to dilute the 1.0 L solution of 6.0 M HCl to a 0.2 M solution is 30 liters (option a).
Which of the following represents a molecule
A Ca
B Co
C CO
D Cu
Answers
Answer:
C. CO
Explanation:
A molecule is a substance that is a combination of two or more atoms through a chemical bond while an element is a substance containing only one type of atom. However, some elements are also considered molecules because of having diatoms such as: Hydrogen, Oxygen, Chlorine, Bromine, Fluorine, Nitrogen and Iodine.
Among the choices above, it is only choice C (Carbon monoxide) that is a molecule. All the other options (Calcium, Cobalt and Copper) are elements. Carbon monoxide is a combination of one atom of carbon and another atom of oxygen.
The option that represents a molecule would be CO. That is option C.
What is a molecule?A molecule is defined as the substance that is made up of two or more elements that are chemically combined together.
From the options give above, CO which is carbon monoxide is diatomic. This shows that it contains two elements making it a molecule.
Learn more about molecules here;
https://brainly.com/question/26556885
#SPJ6
Please help! I upload a picture before but it was blank so I’m trying again :/ I’ll mark BRAINLIEST!

Answers
2:last one
3:third option
Hope this helps
Brainliest please
Answer:
for number one it is the first one. the second one it the third. and number 3 is the second one.
Explanation:
im just realy smart
1. Which of the following lists is correctly arranged in order of increasing size?
A. electron, neutron, nucleus, atom
B. electron, nucleus, neutron, atom
C.atom, electron, neutron, nucleus
D. neutron, electron, nucleus, atom
Answers
Answer:
C
Explanation:
The order of increasing size are electron, neutron, nucleus and atom.
The size of electron is the smallest among the given particles which followed by neutron that is bigger in size from electron but smaller than nucleus. Proton and neutron are located in the nucleus of an atom so it is bigger in size than neutron.
Atom is the largest among the given substances in which nucleus is present at the center and electrons are revolving around the nucleus in fixed orbits so we can conclude that electron is the smallest and atom is the largest particle.
Learn more: https://brainly.com/question/19353287
What is the electron configuration of titanium?
Answers
Answer:
i dont know why a, i here
Explanation:
why is it important to know gas properties at stp? select all that apply: because comparison of properties is possible only if the properties are reported against a standard temperature and pressure. gases can only react at stp. gases must be stored at stp. in order to know that exactly one mole of an ideal gas has a volume of 22.4l.
Answers
It is important to know gas properties at standard temperature and pressure since comparison of properties is possible only if the properties are reported against a STP and in order to know that exactly one mole of an ideal gas has a volume of 22.4 l.
Standard temperature and pressure are defined as a standard set of conditions required for experimental measurements which are established to allow comparison between different sets of data.
Standards which are commonly used are those International Union of pure and applied chemistry and national institute of standards and technology.It is useful in determination of one mole of a gas.
Learn more about standard temperature and pressure,here:
https://brainly.com/question/29129606
#SPJ4
ou are determining the bud for magic mouthwash, a simple non-sterile compound. magic mouthwash requires the mixing of three ingredients antacid liquid, lidocaine and diphenhydramine. each of these ingredients is a water containing oral formulation. what would be the bud for magic mouthwash?
Answers
The appropriate USP <795> BUD for Magic Mouthwash depends on whether it is compounded and dispensed as an oral formulation or as a mucosal formulation.
Oral formulations have a BUD of no more than 7 days and mucosal formulations have a BUD of no more than 14 days. The BUD should be determined based on the shortest expiry date of the three ingredients that make up Magic Mouthwash, as well as the conditions of storage.
Additionally, the specific conditions of storage such as temperature, humidity, and light can also affect the BUD of the mouthwash. It is recommended to consult the manufacturers of the active ingredients to determine the appropriate BUD for Magic Mouthwash.
Learn more about Magic Mouthwash:
https://brainly.com/question/25730862
#SPJ4
you are using food labels as a tool to help you avoid purchasing products with trans fatty acids. which of the following would you avoid, since it is the most likely to contain trans fatty acids?
Answers
Among the given options, the most likely to contain trans fatty acids would be partially hydrogenated vegetable oil.
Trans fatty acids are commonly found in processed foods that use partially hydrogenated oils as an ingredient. Partial hydrogenation is a process that converts liquid vegetable oils into solid or semi-solid fats, thereby increasing their shelf life and stability.
However, this process also generates trans fatty acids as a byproduct. Trans fats have been linked to various health issues, including heart disease. To make healthier choices, it is advisable to avoid products that list partially hydrogenated vegetable oil on their food labels.
For more information on fatty acids visit: brainly.com/question/28835959
#SPJ11
how much is 3.5 gallons in cups
Answers
Answer:
3.6 gallons are equal to 56 cups
Which organisms are producers?
Choose all correct answers.
tree
grass
monkey
mold
Answers
Answer: Tree and Grass are the two correct answers here
Explanation: Producers are photosynthesizing organisms. They are any kind of green plant that make their food by taking sunlight and using the energy to make sugar.
Answer:
Tree, grass
Explanation:
Producers make their own food. Trees and grass can make their own food using sunlight (photosynthesis). Monkey is consumer (eats other things like bananas- made by plants - for survival). Mold (like fungus) eat dead stuff and thus are decomposers (not producers).