if a 10 ml solution of 0.5 m lead (ii) nitrate reacts with excess ki, how many gram of precipitate will form?
Answers
An inorganic substance having the chemical formula Pb(NO3)2 is lead(II) nitrate. In contrast to the majority of other lead(II) salts, it frequently appears as a colorless crystal or white powder and is soluble in water.
Pb(NO3)2 + 2KI → PbI2 + 2KNO3
moles KI=(0.5mol/L)(0.010L)=0.005mol
moles PbI2 = (0.005mol KI)(1 mol PbI2/2mol KI)=2.5×10^(-3)mol
mass PbI2 = (2.5×10^(-3)mol)(461.0g/mol)=1.1525g
Production of lead(II) nitrate from metallic lead or lead oxide in nitric acid was done on a modest scale and was used directly to create additional lead compounds, hence the name plumbum dulce since the Middle Ages. Commercial production of lead(II) nitrate started in Europe and the US in the nineteenth century. Historically, the primary usage was as a raw ingredient in the manufacture of lead paint pigments, but less hazardous paints based on titanium dioxide have replaced those lead paints. Heat stabilization in nylon and polyesters was another industrial application.
learn more about lead nitrate here:
https://brainly.com/question/29424558
#SPJ4
Related Questions
[20 points] What kind of bond is pictured in the diagram to the left? Describe the movement of the valence electrons in the bond above? (Hint: Sharing or giving up?)
![[20 points] What kind of bond is pictured in the diagram to the left? Describe the movement of the valence](https://brainimage.s3.us-east-005.backblazeb2.com/contents/attachments/UEEGfp0YkpKO4fapaR6yCm3W8W05PVmz.png)
Answers
Answer: The kind of bond is a covalent bond.
Explanation: The two atoms on top are separated or not together while the other is. the two atoms on top are negative while only one molecule on the bottom is negative. And, the top pair is atoms and the bottom pair is molecule
What is cr(no3)2 a lewis base ?
Answers
No, Cr(NO3)2 is not a Lewis base, it is a compound that acts as a Lewis acid.
A Lewis acid is a chemical species that can accept a pair of electrons (a Lewis base) to form a covalent bond. In Cr(NO3)2, the chromium ion (Cr2+) has an incomplete octet and can accept a pair of electrons from a Lewis base to form a coordinate covalent bond. Therefore, Cr(NO3)2 is a Lewis acid.
A Lewis base, on the other hand, is a chemical species that can donate a pair of electrons to form a covalent bond with a Lewis acid. Common examples of Lewis bases include molecules such as water, ammonia, and amines, which have lone pairs of electrons that can be donated to a Lewis acid.
For more similar questions on Lewis acid
brainly.com/question/28299444
#SPJ11
Using tabulated values from the textbook Resource section or the chemlibre links, calculate the standard reaction entropy of N2(g) + 3 H2(g) → 2 NH3(g). (3 sig figs, units of J/K/mol)
Answers
The standard reaction entropy of N2(g) + 3 H2(g) → 2 NH3(g) is -196.3 J/K·mol.
What do you mean by standard reaction entropy?
Standard reaction entropy (ΔS°) is a measure of the disorder or randomness of a chemical reaction at a constant temperature and pressure. It is defined as the change in the entropy of the system when a reaction occurs under standard conditions. The standard state for a substance is its pure form at a pressure of 1 bar and a temperature of 25°C (298 K).
The standard reaction entropy (ΔS°) of a reaction can be calculated using the standard molar entropies (S°) of the reactants and products. The formula to calculate the standard reaction entropy is:
ΔS° = ΣS° (products) - ΣS° (reactants)
To calculate the standard reaction entropy for the reaction: N2(g) + 3 H2(g) → 2 NH3(g)
S° (N2) = 191.8 J/K·mol
S° (H2) = 130.6 J/K·mol
S° (NH3) = 192.5 J/K·mol
ΔS° = [2(S° (NH3)) - (S° (N2) + 3(S° (H2))]
ΔS° = [2(192.5) - (191.8 + 3(130.6))] J/K·mol
ΔS° = -196.3 J/K·mol
Hence, the standard reaction entropy of N2(g) + 3 H2(g) → 2 NH3(g) is -196.3 J/K·mol.
To learn more about standard reaction entropy from the given link:
https://brainly.com/question/16795263
#SPJ4
figure 2. the concentration of nad (top) and lactic acid (bottom) in the blood of a representative treated individual (a) describe the pattern of inheritance that is most likely associated with a mutation in the mt -nd5 gene. explain why individuals are not typically heterozygous with respect to mitochondrial genes.
Answers
The pattern of inheritance most likely associated with a mutation in the mt-ND5 gene is maternal inheritance. This is because mitochondrial genes, including mt-ND5, are inherited exclusively from the mother.
The concentration of NAD (top) and lactic acid (bottom) in the blood of a representative treated individual can vary, but an mt-ND5 mutation may lead to abnormal concentrations due to its impact on cellular respiration. Individuals are not typically heterozygous with respect to mitochondrial genes because there is only one type of mitochondria in each cell, which comes from the mother.
This results in a homoplasmic condition, where all mitochondrial genes are identical, unlike nuclear genes where heterozygous conditions can occur due to contributions from both parents.
Learn more about inheritance at https://brainly.com/question/32099800
#SPJ11
a merchant has coffee worth $5 a pound that she wishes to mix with 60 pounds of coffee worth $9 a pound to get a mixture worth $6 a pound. How many pounds of the $5 coffee should be used?
Answers
The merchant should use 20 pounds of the $5 coffee.
To determine the number of pounds of the $5 coffee to be used, we can set up an equation based on the given information. Let's assume x represents the pounds of the $5 coffee.
The value of the $9 coffee (60 pounds) plus the value of the $5 coffee (x pounds) should equal the value of the resulting mixture (60 + x pounds) at $6 per pound. Mathematically, this can be expressed as:
$9(60) + $5(x) = $6(60 + x)
Simplifying the equation:
540 + 5x = 360 + 6x
Rearranging terms:
5x - 6x = 360 - 540
-x = -180
Dividing both sides by -1, we get:
x = 180
Therefore, the merchant should use 20 pounds of the $5 coffee to create the desired mixture.
Learn more about merchant
brainly.com/question/30724112
#SPJ11
The reaction R of the body to a dose M of medication is often represented by the general function R(M)=M^2(C/2−M/3where C is a constant. If the reaction is a change in blood pressure, R is measured in millimeters of mercury (mmHg). If the reaction is a change in temperature, Ris measured in degrees Fahrenheit ("F). The rate of change dR/dM is defined to
be the body's sensitivity to the medication. Find a formula for the sensitivity dR/dM=
Answers
A formula for the sensitivity dR/dM represents the sensitivity of the body's reaction to the medication. It shows how the reaction changes with respect to the dose of the medication, M. The term M*C represents the contribution of the constant C to the sensitivity, while the term \((2M^2)/3\) represents the contribution of the dose M itself.
To find a formula for the sensitivity, dR/dM, let's differentiate the given function R(M) with respect to M.
Step 1: Start with the function \(R(M) = M^2(C/2 - M/3).\)
Step 2: Apply the power rule of differentiation to differentiate M^2. The power rule states that if
\(f(x) = x^n, then f'(x) = n*x^(n-1). \\\)
n this case, n = 2.
\(dR/dM = 2M^(2-1)*(C/2 - M/3).\)
Simplifying, we have:
\(dR/dM = 2M*(C/2 - M/3).\)
Step 3: Distribute the 2M to each term inside the parentheses:
\(dR/dM = M*C - (2M^2)/3.\)
This formula represents the sensitivity of the body's reaction to the medication, dR/dM. It shows how the reaction changes with respect to the dose of the medication, M. The term M*C represents the contribution of the constant C to the sensitivity, while the term \((2M^2)/3\) represents the contribution of the dose M itself.
Learn more about sensitivity from this link:
https://brainly.com/question/14472410
#SPJ11
the formula for the sensitivity, or the rate of change of the reaction R with respect to the dose M, is
dR/dM = MC - M\(^2^/^3\)
How do we calculate?We calculate the derivative of the reaction function R(M) with respect to M.
the reaction function: R(M) = M²(C/2 - M/3)
We will apply the power rule and the constant multiple rule of differentiation,
dR/dM = d/dM [M²(C/2 - M/3)]
= 2M(C/2 - M/3) + M²(0 - (-1/3))
= 2M(C/2 - M/3) + M\(^2^/^3\)
dR/dM =\(MC - 2M^2^/^3 + M^2^/^3\)
= \(MC - M^2^/^3\)
Learn more about power rule at:
https://brainly.com/question/29288036
#SPJ4
Calculate the mass of solid NaCl that must be added to 1.50 L of a 0.100 M AgNO3 solution to precipitate all the Ag+ ions in the form of AgCl.
Answers
Answer:
0.1169g .
Explanation:
1. Multiply the concentration (0.5 mols/Liters) by the volume of solution you want (0.5 Liters) to find the moles of NaCl you need. 2. Multiply the moles of NaCl by its molar mass (58.44 g/mol) to find the grams of solute needed.
what is the difference in chemical energy between organic and inorganic materials?
Answers
Answer:
the difference is the presence of a carbon atom
Explanation:
based on the law of multiple proportions, how many grams of hydrogen would you expect 2.19 g of nitrogen to combine with to yield ammonia?
Answers
We're going to use 0.34 grams of nitrogen to resolve this. This would have a molar mass of, uh, 28 2 grams if we converted these two moles. Wu will then combine this as well. One-mole play is also a thing. three of H 2's moles. Grams of hydrogen are what we need. We will learn the answer here. Hydrogen weighs two grams per mole. Each gram weighs 0.501 grams. That would thus stand in for this. 2.34 grams of nitrogen would need to react with 0.501 grams of hydrogen to produce ammonia. 241=2 moles of nitrogen and 243=6 moles of hydrogen will be converted into 4 moles of ammonia. Nitrogen has a molar mass of 28 g/mol and hydrogen has a molar mass of 2 g/mol.
To know more about law of multiple proportions refer to http://brainly.com/
which statement(s) is/are true? i. compound x sublimes at 1 atm. ii. at point h, x exists entirely as x(g). iii. x(l) is more dense than x(s). iv. moving from point f to point g, x melts. v. at t
Answers
This statement is incomplete and does not provide enough information to determine its truth or falsehood. The statement should include what happens to compound x at a specific temperature or temperature range.
i. Compound x sublimes at 1 atm - This statement does not provide enough information about compound x to determine if it is true or false. Sublimation occurs when a substance transitions from a solid to a gas without passing through a liquid phase. Whether or not compound x sublimes at 1 atm depends on its properties and the conditions at 1 atm.
ii. At point h, x exists entirely as x(g) - This statement is true. Point h on a phase diagram represents the temperature and pressure conditions where compound x exists entirely as a gas.
iii. X(l) is more dense than x(s) - This statement is generally true for most substances, but it depends on the specific properties of compound x. Generally, the density of a substance increases as it transitions from a gas to a liquid to a solid.
iv. Moving from point f to point g, x melts - This statement is false. Moving from point f to point g on a phase diagram represents a decrease in temperature and pressure, which causes compound x to transition from a gas to a solid (deposition), not from a solid to a liquid (melting).
v. At t - This statement is incomplete and does not provide enough information to determine its truth or falsehood. The statement should include what happens to compound x at a specific temperature or temperature range.
Learn more about temperature range
brainly.com/question/23303647
#SPJ11
What life process breaks down dead organic matter for energy using oxygen and releasing carbon dioxide? a. Respiration b. Combustion c. Decomposition d. Photosynthesis
Answers
Respiration breaks down dead organic matter for energy using oxygen and releasing carbon dioxide.
What is Respiration?
Respiration is the biological process by which living organisms exchange gases, usually oxygen and carbon dioxide, with the environment. In animals, respiration involves the absorption of oxygen from the air or water, the transportation of oxygen to the cells of the body, the use of oxygen by the cells to generate energy through cellular respiration, and the elimination of carbon dioxide, which is a waste product of this process.
In the case of dead organic matter, microorganisms such as bacteria and fungi carry out respiration as a means of obtaining energy. These microorganisms use the organic matter as a source of food, breaking it down into simpler compounds through a process called decomposition. During this process, oxygen is consumed and carbon dioxide is released as a byproduct.
Therefore, respiration breaks down dead organic matter for energy using oxygen and releasing carbon dioxide.
To know more about Respiration, go to the given link;
brainly.com/question/22673336
#SPJ1
What is the Molar mass of NH4C2H3O2
Answers
Ammonium acetate, or NH4C2H3O2, has a molar mass and molecular weight of 77.082.
What does the chemical formula NH4C2H3O2 mean?Ammonium acetate is known by this name. There are no synonyms. Acetic acid, ammonium salt is the chemical name.Thus, the equation is NH 4 C 2 H 302. Thus, we have the following four elements, nitrogen, hydrogen, carbon, and oxygen. One nitrogen atom makes up the entire formula. The amount of carbon atoms is excessive, and the number of hard-line tries is four plus three, which equals seven.In ammonium acetate, hydrogen makes up 5.23% of the mass composition.There are two carbon atoms, seven hydrogen atoms, one nitrogen atom, and two oxygen atoms in ammonium acetate.To learn more about Ammonium acetate refer to:
https://brainly.com/question/29570260
#SPJ1
In a particular experiment, a 4. 00 g sample of CaO is reacted with excess water and 2. 14 g of Ca(OH)2 is recovered. What is the percent yield in this experiment
Answers
\textbf{Question:}
In a particular experiment, a 4.00 g sample of CaO is reacted with excess water, and 2.14 g of Ca(OH)2 is recovered. What is the percent yield in this experiment?
\textbf{Answer:}
The Percent yield in this experiment is 40.5%.
What is the percent yield?
In chemistry, the percentage yield is used to compare a reaction's actual outcome to the maximum anticipated outcome.
The actual yield and the theoretical yield must be compared in order to calculate the percent yield.
Given: \ce{CaO}mass equals 4.00 g
\ce{Ca(OH)2}
recovered mass (actual yield): 2.14 g
The reaction's chemical equation is balanced as follows:
\ce{Ca(OH)2(s)} is created when \ce{CaO(s)} and water combine.
The stoichiometric ratio of \ce{CaO}to \ce{Ca(OH)2} is 1:1, as can be shown from the equation. It follows that 1 mole of \ce{CaO} interacts to become 1 mole of \ce{Ca(OH)2}.
We first compute the amount of moles of \ce{CaO} to be used in the theoretical yield calculation:
Moles of \ce{CaO} = \(\frac{{\text{{Mass of \ce{CaO}}}}}{{\text{{Molar mass of \ce{CaO}}}}}\)
\ce{CaO}'s molecular weight is 40.08 \, \text{g/mol}.
Moles of CaO = \(\frac{{4.00 \, \text{{g}}}}{{40.08 \, \text{{g/mol}}}}\)
Next, we determine the theoretical yield of \ce{Ca(OH)2} in grams:
Theoretical yield = Moles of CaO \times \text{{Molar mass of \ce{Ca(OH)2}}}
The molar mass of \ce{Ca(OH)2} is 74.09 \, \text{g/mol}.
Theoretical yield = Moles of CaO \times 74.09 \, \text{{g/mol}}
Finally, we can calculate the percent yield:
Percent yield = \(\frac{{\text{{Actual yield}}}}{{\text{{Theoretical yield}}}} \times 100\%\)
Percent yield = \(\frac{{2.14 \, \text{{g}}}}{{(\text{{Moles of CaO}}) \times 74.09 \, \text{{g/mol}}}}\) \times 100\%
Hence, the percent yield in this experiment is approximately 40.46\%.
---
To know more about percent yield from the given link:
https://brainly.com/question/2451706
#SPJ4
In the set up below, which air should show an increase in temperature?

Answers
Answer:
My guess is A.
Explanation:
the normal boiling point of mercury (hg) is 356.7 °c. what is the vapor pressure of mercury at 340.5 °c in atm? (∆hvap = 58.51 kj/mol)
Answers
The vapour pressure of mercury at 340.5°C in atm is 1.06 x 10^(-13) atm
The normal boiling point of mercury is 356.7 °C.
Given that, the ∆Hvap of mercury = 58.51 kJ/mol
We know that at boiling point, the vapour pressure of the substance is 1 atm and with the increase in temperature, the vapour pressure of the substance also increases. We can use Clausius-Clapeyron Equation to calculate the vapour pressure of mercury at 340.5°C.
Clausius-Clapeyron equation is given by :
log(P2/P1) = - ∆Hvap/R [1/T2 - 1/T1]
Where, P1 = vapour pressure at boiling point of mercury = 1 atm
T1 = boiling point of mercury = 356.7 °C = 629.85 K
T2 = 340.5 °C = 613.65 K
∆Hvap = 58.51 kJ/mol
R = gas constant = 0.0821 L atm/mol K
On substituting the values,
log(P2/1) = - 58510/(0.0821 ) x [1/613.65 - 1/629.85]
log(P2) = 29.87
(P2) =1.06 x 10^(-13) atm
Thus, the vapour pressure of mercury at 340.5 °C is 1.06 x 10^(-13) atm(approx).
To learn more about Clausius Clapeyron Equation :
https://brainly.com/question/29414397
#SPJ11
(free question so use your creativity!!)
Physical science is the ____________ class in the world!
Answers
Answer:
Best
Explanation:
physical, best, greatest.
If there are 0.090 moles of sugar (C₁₂H₂₂O₁₁) in the average can of soda, how many sugar molecules would this be
Answers
Given :
There are 0.090 moles of sugar (C₁₂H₂₂O₁₁) in the average can of soda.
To Find :
How many sugar molecules would this be ?
Solution :
We know, in 1 mole of any compound their are \(6.022\times 10^{23}\) molecules of that compound.
So, number of molecules in 0.090 moles of compound is :
\(n = 0.090 \times 6.022 \times 10^{23}\\\\n = 5.42\times 10^{22}\)
Therefore, number of molecules in 0.090 moles of compound is \(5.42 \times 10^{22}\) .
I’ll really appreciate it if you help me out on this one .

Answers
Answer:
i'd say D
Explanation:
the dependent variable is the change that happens
A donut has a density of 0.75 g/cm cubed and a mass of 100.0g. What is the volume of the donut?
Answers
Answer:
133.333333333 cm^3
Explanation:
Volume = Mass/Density
How do you identify conduction?
Answers
Direct contact between objects causes conduction or heat transfer. The heat is transferred inside the fluid during convection. Heat transfer in radiation happens by electromagnetic waves without the use of particles.
How can conduction be distinguished?First, ascertain whether the two things are in contact. If they are, conduction is how heat is transferred between them. Determine whether there is a fluid medium, such as a liquid or gas, connecting the items if they are not in contact.
How do you recognize conduction, a type of heat transfer?Evidence of heat transport is apparent. Convection is the phenomenon that causes the air to shimmer over radiators. Conduction is the phenomenon that causes you to feel warm when you place your hand on a spoon that has been sitting in a hot bowl of soup (radiation).
to know more about conduction here:
brainly.com/question/15085692
#SPJ4
A bus started from kathmandy and reached Khanikhola 26 km far from Kathmandu, in hour, If the bus had uniform acceleration calculate the final velocity of the bus and acc- eleration
Answers
If the bus had uniform acceleration, the final velocity of the bus is 14.4 m/s and acceleration is 0.0040 m/s²
According to question
The distance between Khanikhola and Kathmandu
d = 26 km
= 26000 m
Time,
t = 1 hour
= 3600 seconds
Step-wise explanation:
Consider a is the acceleration of the bus. By using second equation of motion,
d = ut + \(\frac{1}{2} at^{2}\)
Where
u is the initial speed of the bus,
u = 0
a = \(\frac{2d}{t^2}\)
a = \(\frac{2 \times 26000}{3600^2}\)
a = 0.0040 m/s²
By using first equation of motion.
Final velocity, v = u +at
So,
v = 0+0.0040(3600)
v = 14.4 m/s
a = 0.0040 m/s², v = 14.4 m/s.
If the bus had uniform acceleration, the final velocity of the bus is 14.4 m/s and acceleration is 0.0040 m/s².
Learn more about velocity, here:
https://brainly.com/question/19561022
#SPJ1
What is the pH of a substance that has a hydrogen ion concentration of 1.2 x 10^-2 M
Answers
Answer:
1.92
Explanation:
A 0.205 g sample of caco3 (mr = 100.1 g/mol) is added to a flask along with 7.50 ml of 2.00 m hcl. caco3(aq) + 2hcl(aq) → cacl2(aq) + h2o(l) + co2(g) enough water is then added to make a 125.0 ml solution. a 10.00 ml aliquot of this solution is taken and titrated with 0.058 m naoh. naoh(aq) + hcl(aq) → h2o(l) + nacl(aq) how many ml of naoh are used?
Answers
Answer:
118MOLS
Explanation:
M1VI=M2V2
THE REST WORK OUT ( BA UNZA STUDENTS HE HE HE )
PLease help my imma mark brainlist plsss
Did the chemical reaction absorb or release energy? How do you know?
Answers
Answer:
Chemical reactions that absorb (or use) energy overall are called endothermic. In endothermic reactions, more energy is absorbed when the bonds in the reactants are broken than is released when new bonds are formed in the products.
which element has the highest ionization energy in period 3
Answers
After considering the given the data we conclude that the ionization energy generally increases from left to right across a period. Therefore, the element with the highest ionization energy in period 3 would be located on the right side of the periodic table.
We can also see from the search results that helium has the highest ionization energy of all the elements, while sodium has the lowest ionization energy in period 3. Therefore, we can conclude that the element with the highest ionization energy in period 3 is located to the right of sodium.
Based on the periodic table, we can see that the elements in period 3 are:
Sodium (Na)
Magnesium (Mg)
Aluminum (Al)
Silicon (Si)
Phosphorus (P)
Sulfur (S)
Chlorine (Cl)
Argon (Ar)
Therefore, the element with the highest ionization energy in period 3 is most likely Argon (Ar), which is located on the far right side of the period.
In summary, the element with the highest ionization energy in period 3 is most likely Argon (Ar).
To learn more about periodic table
https://brainly.com/question/25916838
#SPJ4
Is the following compound chiral? he following compound chiral? OH Does this compound have a plane of symmetry? How many stereocenters do you count? Submit Answer Try Another Version 1 item attempt remaining
Answers
The terms you've mentioned are essential for understanding chirality. A compound is considered chiral if it cannot be superimposed onto its mirror image. Chiral compounds often have stereocenters, which are atoms bearing groups in a spatial arrangement that creates non-superimposable mirror images.
In your question, it seems that the specific compound is not provided. However, I can still explain the terms:
1. Compound Chiral: A molecule that is not superimposable on its mirror image, resulting in two enantiomers.
2. OH Plane of Symmetry: A plane that divides a molecule into two mirror-image halves. If a compound has a plane of symmetry, it is achiral.
3. Stereocenters: Atoms within a molecule where the exchange of two groups would generate a different stereoisomer (e.g., a chiral carbon with four different groups attached).
To determine if a compound is chiral, identify stereocenters and check for a plane of symmetry. If there are no stereocenters or a plane of symmetry exists, the compound is achiral.
Identify stereocenters in compound, look for any atom with four different groups attached to it, determine plane of symmetry. If one or more stereocenters and no plane of symmetry, the compound is chiral. Otherwise, it is not chiral.
It seems like some parts of the compound description are missing.
To determine if a compound is chiral, you need to look for the presence of stereocenters (also known as chiral centers). A stereocenter is an atom, usually a carbon, that has four different groups attached to it. If a molecule has one or more stereocenters and no internal plane of symmetry, it is considered chiral.
A plane of symmetry is an imaginary plane that divides a molecule into two equal halves that are mirror images of each other. If a compound has a plane of symmetry, it is achiral, which means it is not chiral.
To answer your question, follow these steps:
1. Identify the stereocenters in the compound. Look for any atom with four different groups attached to it.
2. Determine if there is a plane of symmetry in the molecule.
3. If there are one or more stereocenters and no plane of symmetry, the compound is chiral. Otherwise, it is not chiral.
Without the complete structure of the compound you're asking about, I cannot provide a specific answer. If you can provide the full structure, I'd be happy to help you determine its chirality and other properties.
Learn more about chiral here:
https://brainly.com/question/31755655
#SPJ11
Julio has started his very own floral business. He sells centerpieces for $19. 50 each. His fixed costs are $500 per month, and each centerpiece cost $8 to produce.
Answers
Julio established his very own floral shop. He charges $19.50 for each centerpiece. Each centerpiece he produces costs $8, and his fixed monthly expenses total $500.
The total cost, C, is the sum of the cost to produce the centerpieces and the fixed cost. The equation for C is C = 8n + 500.
The total revenue, R, of Julio's floral business is the product of the cost per centerpiece and the number of centerpieces sold. The equation for R is R = 19.50n.
$19.50x - $8x-500 = 0 (Break even) x+=+500%2F11.50
25P - 25*8 - 500 = 0 (Break even with 25 sold P+=+700%2F25 = $28
learn more about fixed cost here:
https://brainly.com/question/17100497
#SPJ4
Help a person out please
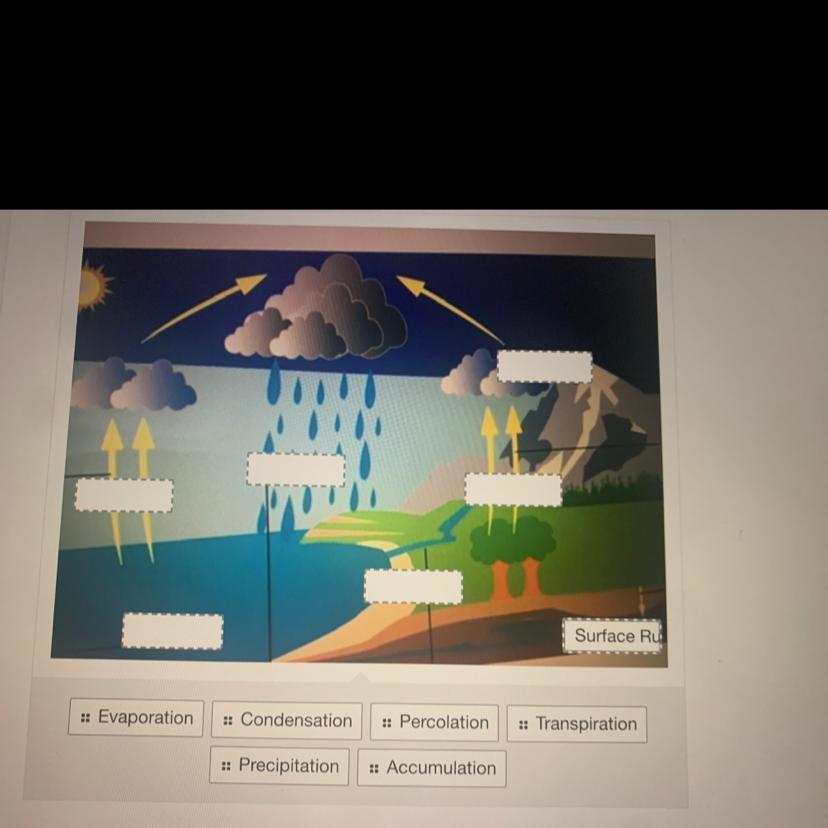
Answers
Explanation:
here I haven't drawn the diagram but I have marked them where they will be .
So if it helps don't forget to like and Mark me

A sample of gas occupies 2.30 L at 825 mmHg and 70.0°C. What is its volume at STP
Answers
Answer:
V₂ = 2.0 Liters at STP conditions
Explanation:
Solving problem using the combined gas law
Given:
Case I Conditions Cast II Conditions
P₁ = 825mmHg P₂ = 760mm
V₁ = 2.30 Liters V₂ = ?
T₁ = 70°C + 273 = 343K T₂ = 0°C = 273K
Substitute into combined gas law assuming moles of gas remains constant; solve for unknown volume under case 2 conditions
P₁·V₁/n₁·T₁ = P₂·V₂/n₂T₂ => V₂ = P₁·V₁·T₂ / P₂·T₁ => note: in this solution, moles of gas remains constant as is disregarded in final calculation.
V₂ = (825mm)(2.3L)(273K) / (760mm)(343K) = 1.987 Liters (calculator answer) ≅ 1.9 Liters (2 sig figs based on given volume (V₁)
V₂ = 1.987 Liters ≅ 2.0 Liters (2 sig figs based on given volume (V₁)
Elements and compounds are ___
Answers
Answer:
an element os3a material that consists of a single type of atom each type contains the same number of protons.