imagine a radioactive isotope called z has a half-life of 12 hours. after 2 days, the fraction of the original amount remaining is
Answers
The remaining fraction of the original amount is 0.063 that can be calculated using the half life formula.
The half-life of a reaction is the time required for the reactant concentration to decrease to one-half its initial value. The half-life of a first-order reaction is a constant that is related to the rate constant for the reaction:
t1/2 = 0.693/k.
k = 0.693/t1/2
= 0.693/12
=0.05775 hour-1
By using the rate constant equation,
[A] = [Ao]*e^(-k*t)
[A] / [Ao] = e^(-k*48)
= e^ (-0.05775*12)
=0.063
Answer: 0.063
To learn more about half-life check the link below:
https://brainly.com/question/1160651
#SPJ4
Related Questions
what is an example of an atomic number
Answers
The circled number in red is the atomic number.
(The site I used for this example is ptable.com. Very useful periodic table!)

Answer: in a sodium atom, there are 11 electrons and 11 protons. Thus the atomic number of Na atom = number of electrons = number of protons = 11.
Explanation:
100 POINTS PLS HELP!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!

Answers
Answer:
i need this im struggling with my work
Explanation:
Last one and the first
compare the energies of excitation and emission for fluorescence. which is greater? choose... why is this the case? choose...
Answers
In fluorescence, the energy of excitation is typically greater than the energy of emission.
This is because fluorescence involves the absorption of a photon of light by a molecule, which causes an electron to move from its ground state to an excited state of higher energy.
The excited electron then returns to its ground state by emitting a photon of light, but the energy of this emitted photon is typically lower than the energy of the absorbed photon. This energy difference is dissipated as heat, and is known as the Stokes shift.
The energy difference between the absorbed and emitted photons is due to a combination of factors, including the nature of the molecule, the electronic structure of the excited state, and the environment surrounding the molecule.
In general, the energy of the emitted photon is lower than the energy of the absorbed photon because the excited electron loses some energy as it interacts with its surroundings before emitting a photon.
To know more about fluorescence here
https://brainly.com/question/14742655
#SPJ4
What is cells function of animal cell??
PLZ HELP ME
Answers
Answer:
Controls the movement of substances into and out of the cell.
he model below shows a calcium atom.
An image has a mix of red and blue balls in its center and 4 concentric black rings around the center. The first ring from the center has 2 small green balls on it. The second ring has 8 small green balls on it. The third ring has 8 small green balls on it. The outermost ring has 2 small green balls on it.
How many electrons are in the third energy level?
2
8
18
20
Answers
Answer:
8
Explanation:
At most, how many covalent bonds can a phosphorous atom form?
(A) 1
(B2
(C)3
(D)4
Answers
Answer:
(c)
Explanation:
in both black and red
Answer:
I think 3 im am not sure
Explanation:
A decomposition reaction starts with one reactant and ends up with two or more
products. Which of the following reactions are decomposition reactions?
NaCl --> Na+ Cl2
H2+02 --> H20
NaOH + HCI--> HOH + NaCl
O H2O --> H2 + O2
O Na + Cl2--> NaCl
Answers
Answer:
2H₂O → 2H₂ + O₂
2NaCl → 2Na + Cl₂
Explanation:
2H₂O → 2H₂ + O₂
2NaCl → 2Na + Cl₂
The reactions shown above is a decomposition.
In a decomposition reaction or cracking, two or more products are formed from a single product.
The breakdown of a compound into its components as individual elements or other compounds falls into this category of reactions.
Cracking is a sort of decomposition reactions that is used in petroleum industries to sort the fractions of a compound.
Need help in this I don’t understand what the mass would be so confusing there are no labels to this exercise

Answers
Answer:
\(10\text{ a.m.u}\)Explanation:
Here, we want to get the mass of the given atom
The atomic mass is simply the sum of the mass of the protons and neutrons
From the nucleus, we can see that we have 5 protons and 5 neutrons
Although they have different charges, they have roughly the same mass
The mass of each is approximately equal to 1
Thus, we have the mass of the atom as;
\(5(1)\text{ + 5(1) = 10 a.m.u}\)7. A strong acid has a pH of
A) 0
B) 6
C) 7
D) 14
Answers
because acids are from PH0-PH6 and basics are from PH7-PH14
Hope this helps!
Good luck!
A) Calculate the number of molecules in a deep breath of air whose volume is 2.25 L at body temperature, 37 ∘C, and a pressure of 740 torr .
B) The adult blue whale has a lung capacity of 5.0×103L. Calculate the mass of air (assume an average molar mass 28.98 g/mol) contained in an adult blue whale’s lungs at 0.5 ∘C and 1.03 atm , assuming the air behaves ideally.
Answers
The mass of air contained in an adult blue whale’s lungs at 0.5∘C and 1.03 atm, assuming the air behaves ideally is 6.03 kg.
a) Calculation for the number of molecules in a deep breath of air whose volume is 2.25 L at body temperature, 37∘C and a pressure of 740 torr, can be given by the following steps:
Volume of air in a deep breath,
V = 2.25 L
Temperature of air in a deep breath, T = 37+273 = 310 K
Pressure of air in a deep breath, P = 740 torr
Convert torr to atm. 1 atm = 760 torr
Pressure of air in a deep breath, P = 740/760 = 0.973 atm
The number of molecules in the deep breath, n = PV/RT
Where R is the gas constant. R = 0.08206 L atm K⁻¹ mol⁻¹
n = (0.973 atm × 2.25 L)/(0.08206 L atm K⁻¹ mol⁻¹ × 310 K)
n = 0.0925 mol
Number of molecules = n × Na
Where Na is Avogadro's number which is equal to 6.022 × 10²³ mol⁻¹
Number of molecules = 0.0925 mol × 6.022 × 1023 mol⁻¹
Number of molecules = 5.563 × 1022 molecules.
Therefore, the number of molecules in a deep breath of air whose volume is 2.25 L at body temperature, 37∘C, and a pressure of 740 torr is 5.563 × 10²² molecules.
b) Calculation for the mass of air contained in an adult blue whale’s lungs at 0.5∘C and 1.03 atm, assuming the air behaves ideally, can be given by the following steps:
Lung capacity of an adult blue whale = 5.0 × 103L
Volume of air in blue whale's lungs, V = 5.0 × 103L
Temperature of air in blue whale's lungs, T = 0.5+273 = 273.5 K
Pressure of air in blue whale's lungs, P = 1.03 atm
The number of moles of air, n = PV/RT
n = (1.03 atm × 5.0 × 103L)/(0.08206 L atm K⁻¹ the mass of air contained in an adult blue whale’s lungs at 0.5∘C and 1.03 atm, assuming the air behaves ideally is 6.03 kg.mol⁻¹ × 273.5 K)
n = 208.37 mol
The mass of air contained in an adult blue whale’s lungs, m = n × MM
Where MM is the average molar mass of air which is equal to 28.98 g mol⁻¹m = 208.37 mol × 28.98 g mol⁻¹ m
= 6034.58 g or 6.03 kg.
Therefore, the mass of air contained in an adult blue whale’s lungs at 0.5∘C and 1.03 atm, assuming the air behaves ideally is 6.03 kg.
Learn more about molar mass :
brainly.com/question/21334167
#SPJ11
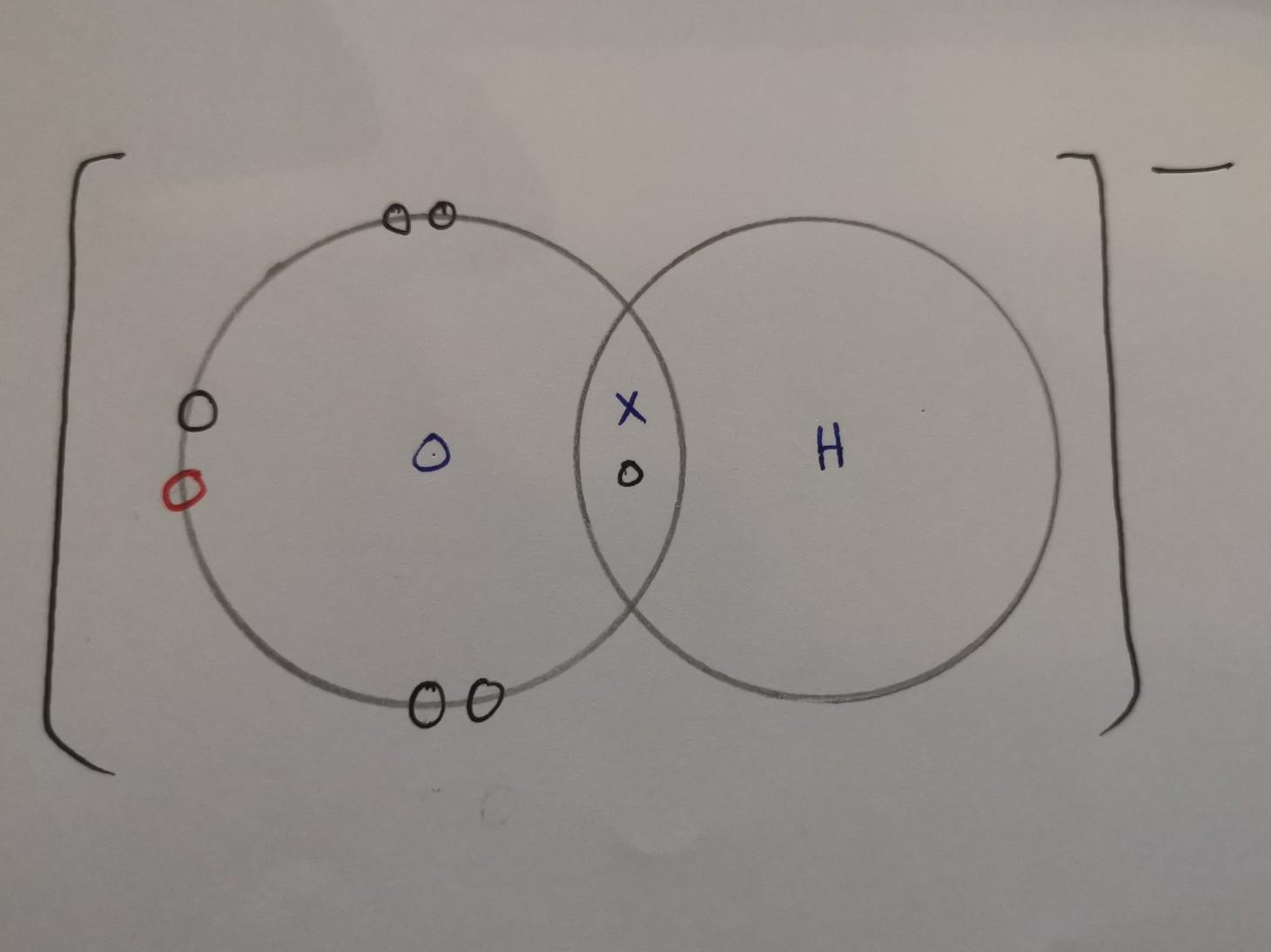
Draw the electron dot structure of the hydroxide ion (OH-).
Answers
Answer:
X = electrons from hydrogen
O (black) = electrons from oxygen
O (red) = electrons from when it was connected to a metal atom, hence why it has a negative charge
1) In this experiment, you will be mixing aqueous solutions of sodium carbonate and calcium chloride to produce solid calcium carbonate.
Na CO2 (aq) +CaCl(aq) — 2 NaCl(aq) +CaCO3
Order the steps required to predict the volume (in mL) of 0. 200 M sodium carbonate needed to produce 2. 00 g of calcium carbonate. There is an excess of calcium chloride.
Comp
Identi
volum
2
>
Calcu
Check
>
Step 1
Convert mass of calcium carbonate 10 moles of calcium carbonate
Step 2
Compare moles of calcium carbonate to moles of sodium carbonate based on balanced equation to calculate moles of sodium carbonate required
Step 3
Compute the volume of sodium carbonate solution required
Step 4
Convert the volume of sodium carbonate solution required from liters to milliers
2) Na2CO3(aq) + CaCl2(ag) + 2NaCl(aq) + CaCO3(-)
Calculate the volume (in mL) of 0. 200 M Na2CO, needed to produce 2. 00 g of CaCO3(s). There is an excess of CaCl.
Molar mass of calcium carbonate = 100. 09 g/mol
Volume of sodium carbonate - 100 mL
METHODS
RESET
MY NOTES
A LAB DATA
Answers
Based on the information provided, the correct order of steps required to predict the volume of 0.200 M sodium carbonate needed to produce 2.00 g of calcium carbonate is:
Step 1: Identify the molar mass of calcium carbonate (CaCO3)
Step 2: Convert the given mass of calcium carbonate to moles using its molar mass
Step 3: Use the balanced chemical equation to determine the mole ratio between calcium carbonate and sodium carbonate
Step 4: Calculate the amount of moles of sodium carbonate required
Step 5: Convert the moles of sodium carbonate to volume in liters using its molarity
Step 6: Convert the volume in liters to milliliters
Therefore, the correct order of steps is:
Step 1: Identify
Step 2: Convert
Step 3: Compute
Step 4: Convert
Step 5: Convert
Step 6: Check
Using the given information, the calculation can be done as follows:
Step 1: The molar mass of calcium carbonate (CaCO3) is given as 100.09 g/mol.
Step 2: The given mass of calcium carbonate is 2.00 g. Therefore, the number of moles of calcium carbonate can be calculated as follows:
2.00 g / 100.09 g/mol = 0.01998 mol ≈ 0.020 mol
Step 3: According to the balanced chemical equation, the mole ratio between calcium carbonate and sodium carbonate is 1:1. Therefore, the amount of moles of sodium carbonate required is also 0.020 mol.
Step 4: The molarity of the sodium carbonate solution is given as 0.200 M. Therefore, the volume of sodium carbonate solution required in liters can be calculated as follows:
0.020 mol / 0.200 mol/L = 0.100 L = 100 mL
Step 5: The volume in liters needs to be converted to milliliters:
0.100 L x 1000 mL/L = 100 mL
Step 6: Check the answer to make sure it is reasonable and makes sense.
To know more about sodium carbonate, visit: brainly.com/question/13565765
#SPJ4
Which of the following represents the lattice energy for AlCl3?a)Al(s) + 3/2 Cl2(g) --> AlCl3(s)b)Al(s) --> Al(g)c)3/2 Cl2(g) --> 3 Cl(g)d)Al3+(g) + 3 Cl-(g) --> AlCl3(s)e)Cl(g) + e- --> Cl-(g)
Answers
The correct representation for the lattice energy of AlCl3 is option (d): Al3+(g) + 3 Cl-(g) → AlCl3(s).
Lattice energy is the energy released when gaseous ions combine to form a solid ionic lattice. In the case of AlCl3, the reaction that represents the formation of the ionic lattice is the combination of Al3+ ions and Cl- ions to form solid AlCl3.
Option (a), Al(s) + 3/2 Cl2(g) → AlCl3(s), represents the formation of AlCl3 from its elements, but it does not represent the lattice energy specifically. It shows the formation of the compound, but not the process of ionic lattice formation.
Option (b), Al(s) → Al(g), represents the sublimation of aluminum, which is not directly related to the formation of AlCl3 or the lattice energy.
Option (c), 3/2 Cl2(g) → 3 Cl(g), represents the dissociation of chlorine molecules into chlorine atoms, which is not directly related to the formation of AlCl3 or the lattice energy.
Option (e), Cl(g) + e- → Cl-(g), represents the process of electron capture by chlorine, which is not directly related to the formation of AlCl3 or the lattice energy.
Therefore, option (d) correctly represents the formation of AlCl3 through the combination of gaseous Al3+ ions and Cl- ions, which corresponds to the lattice energy of AlCl3.
Know more about Lattice Energy here:
https://brainly.com/question/29735933
#SPJ11
Doctors are seeing an alarming increase in the number of cases of tuberculosis (TB) that is resistant to drugs commonly used to treat the disease. TB is difficult to pass to other people without prolonged contact, but it is often fatal if it is not treated.
How will doctors most likely respond to someone who has an active case of drug-resistant tuberculosis?
Answers
Answer:
Doctors will use other drugs that are known to be able to treat TB, even if they are less effective than the common drugs.
Explanation:
When a case of drug resistance is established, the doctor has to change the prescription and give other drugs that are also known to be effective against the organism.
Bacterial drug resistance is a common clinical experience. Many established drugs have been found to be ineffective in treating common infections leading to the continuous quest for improved antibiotics that are active against most organisms.
When doctors discover that someone who has an active case of drug-resistant tuberculosis, the doctor may need to change the prescription to other drugs that are also known to be effective against Mycobacterium tuberculosis, the causal agent of tuberculosis.
Learn more: https://brainly.com/question/2124849
Suspensions are a homogeneous mix.
Question 1 options:
True
False
Answers
Answer:
Suspensions are heterogeneous mixtures. Like heterogeneous goes way better with it.
Explanation:
Like suspensions just go with heterogeneous mixtures. It sounds way cutter when paired up together! For example, some of the material in suspensions will settle out standing? If that makes sense or whatever. Here is the definition of Suspensions, (suspension: A heterogeneous mixture in which some of the particles settle out of the mixture upon standing.) but yeah, suspensions so in with a mixture of those heterogeneous lol heehee!
Answer:
Suspensions is a homogeneous mix. true or false
Explanation: I think it's True
19. I accidentally mixed bleach and ammonia when cleaning one day. It released 55 liters of deadly chlorine (CI) gas. How many grams of
chlorine gas is this?

Answers
Hope this helps you out :)
If the liquid remains water and the copper is rep laced with a brick, in which direction will the equilibrium temperature move? Why? Answer in terms of the relative specific heats of the two materials.
Answers
If the liquid remains water and the copper is replaced with a brick, the equilibrium temperature will likely decrease. This is because bricks typically have a lower specific heat capacity compared to copper.
The specific heat capacity is the amount of heat energy required to raise the temperature of a given mass of a substance by a certain amount.
Since bricks have a lower specific heat capacity than copper, they tend to heat up and cool down faster when exposed to the same amount of heat.
When the copper was present, it would absorb more heat energy from the water due to its higher specific heat capacity, resulting in a higher equilibrium temperature.
However, when the copper is replaced with a brick, the brick will absorb less heat energy from the water compared to copper. As a result, the equilibrium temperature will likely decrease as the water transfers less heat energy to the brick.
The specific heat capacities of the materials play a crucial role in determining the direction of the equilibrium temperature change.
To know more about "Specific heat capacity" refer here:
https://brainly.com/question/14646878#
#SPJ11
MOLES AND MOLAR MASS

Answers
2030g C6H8O7 (1 mol/ 192.124g) = 10.6 mol C6H8O7
11 B
5
Mass =
Protons =
Neutrons =
Element =

Answers
protons:5
neutrons:6
element:boron
List the elements and the amounts of each element below for 5 Li2S04.
Answers
Answer:
Lithium has 5 elements, sulphur has 2 elements and oxygen has 4 elements
Which of the following describes what a scientist does?
Answers
Answer:
people who discover new things fyi you dont have any answer choices on there
Explanation:
there are different type of scientist
Answer:
A scientist asks questions about the world.
Explanation:
Which of the following is not true of deciduous forests?
a.
characterized by four seasons
b.
trees lose their leaves in the fall.
c.
precipitation is concentrated in one season
d.
averages 30-60 inches of precipitation annually
Answers
The Option C is correct precipitation is concentrated in the season
Hope it help
Answer:
C) precipitation is concentrated in one season
Explanation:
edge 2020
5. How many grams of Al2O3 are dissolved in 852 g of water in a 0.329 m solution of Al2O3?
Answers
Answer:
Al2o3 grams to moles
Explanation:
You can view more details on each measurement unit: molecular weight of Al2O3 or grams This compound is also known as Aluminium Oxide. The SI base unit for amount of substance is the mole. 1 mole is equal to 1 moles Al2O3, or 101.961276 grams.
In which state of matter has the LEAST kinetic energy?
A gasgas
B liquidliquid
C solidsolid
D plasma
Answers
I think it's c I'm not sure
Why does the radius of the elements decrease moving across a row when the atomic masses increase going in the same direction?
Answers
Answer:
"The effect of increasing proton number is greater than that of the increasing electron number therefore, there is a greater nuclear attraction."
Explanation:
I got this off of Gogle, I would suggest using it for questions like this. I hope you have good luck on other questions! :)
Explain why I2 is a solid, Br2 is a liquid but Cl2and F2 are gases even though they are all Halogens
Answers
I₂ is a solid, Br₂ is a liquid, while Cl₂ and F₂ are gases because of their increasing molecular size and decreasing strength of their intermolecular forces.
The main factor influencing the physical states of halogens is the strength of the intermolecular forces (Van der Waals forces) between their molecules.
As you move down Group 17 in the periodic table (from F₂ to I₂), the size and mass of the halogen molecules increase. Larger molecules have a greater number of electrons, leading to stronger dispersion forces (a type of Van der Waals forces) between molecules.
For I₂, these forces are strong enough to hold the molecules together in a solid form. For Br₂, the forces are slightly weaker but still strong enough to form a liquid. However, in Cl₂ and F₂, the forces are weaker, allowing the molecules to be in a gaseous state at room temperature.
In summary, the physical states of the halogens depend on the strength of their intermolecular forces, which is influenced by the size and mass of the molecules.
To know more about intermolecular forces click on below link:
https://brainly.com/question/9007693#
#SPJ11
If lead (II) nitrate is decomposed and produces .0788 grams of oxygen gas how much nitrogen dioxide is also produced
Please help me I’m in the middle of a final
Answers
To determine the amount of nitrogen dioxide (NO2) produced when lead (II) nitrate (Pb(NO3)2) decomposes and produces 0.0788 grams of oxygen gas (O2), we need to consider the balanced chemical equation for the decomposition reaction.
The balanced chemical equation for the decomposition of lead (II) nitrate is:
2Pb(NO3)2(s) → 2PbO(s) + 4NO2(g) + O2(g)
From the balanced equation, we can see that for every 2 moles of lead (II) nitrate decomposed, we get 4 moles of nitrogen dioxide (NO2) gas produced.
To calculate the amount of nitrogen dioxide (NO2) produced, we need to determine the number of moles of oxygen gas (O2) produced.
First, we need to calculate the molar mass of oxygen gas (O2), which is 32.00 grams/mol (16.00 g/mol for each oxygen atom).
Now, we can calculate the number of moles of oxygen gas (O2) produced:
Moles of O2 = Mass of O2 / Molar mass of O2
Moles of O2 = 0.0788 g / 32.00 g/mol ≈ 0.0024625 mol
Since the balanced equation shows that for every 2 moles of lead (II) nitrate decomposed, we get 4 moles of nitrogen dioxide (NO2) gas, we can use the mole ratio to determine the number of moles of nitrogen dioxide (NO2) produced:
Moles of NO2 = Moles of O2 × (4 moles NO2 / 2 moles O2)
Moles of NO2 = 0.0024625 mol × (4/2) ≈ 0.004925 mol
Therefore, approximately 0.004925 moles of nitrogen dioxide (NO2) are produced when 0.0788 grams of oxygen gas (O2) is generated through the decomposition of lead (II) nitrate.\(\)
Please help asap thank u

Answers
Answer: Xenon- 144
Explanation: It's D
Put the solutes in an aqueous solution of KF in order of increasing concentration.
Highest concentration --> Lowest concentration
OH- (aq)
HF (aq)
H+ (aq)
F- (aq)
K+ (aq)
Answers
The solutes in an aqueous solution of KF in order of increasing concentration are : Lowest concentration: K+ (aq),H+ (aq),OH- (aq),F- (aq),Highest concentration: HF (aq)
In an aqueous solution of KF, K+ ions come from the dissociation of KF, but KF is a strong electrolyte and dissociates almost completely, so the concentration of K+ ions is relatively high. H+ ions are present in water due to the self-ionization of water, but their concentration is relatively low. OH- ions are also present due to the self-ionization of water, but their concentration is lower than that of H+ ions. F- ions come from the dissociation of KF, so their concentration is higher than that of OH- ions. HF is a weak acid that partially dissociates in water, resulting in a higher concentration of HF compared to the other ions in the solution.
Learn more about aqueous solution here: https://brainly.com/question/1382478
#SPJ11
2.Which of the following compounds when dissolved in water will turn blue litmus paper red? copper oxide, calcium oxide, sulphur oxide, sodium oxide, iron oxide
Answers
Answer:
Sulphur oxide
Explanation:
All of the above oxides with the exception of sulphur oxide are basic oxides and so will not turn blue litmus paper red when dissolved in water. Sulphur oxide, SO2 on the other hand, is an acid anhydride (a non-metallic oxide which dissolves in water to produce acid) rendering it the ability of turning blue litmus paper red when dissolved in water.
Answer:sulpher oxide
Explanation:
All of the compounds except sulpher dioxide are metallic oxide which means basic oxide and base change red litmus paper to blue while acid change blue to red so the answer is the acidic oxide or sulpher dioxide