In designing the experiment, the researchers used which type of 32p labeled atp?.
Answers
In designing the experiment, the researchers used Y32P-ATP.
What is ATP?Adenosine triphosphate (ATP) is an organic substance and a hydro-trope that provides energy for a variety of functions in
living cellsincluding muscular contractionnerve impulse propagationcondensate dissolvingand chemical synthesisResearchers used Y32P-ATP Because the Y-phosphate of ATP is used for phosphoryl transfer by kinases. As a result, Y32P-ATP is required for the experiment.
Hence the used ATP is Y32P-ATP.
To know more about ATP, visit the below link:
https://brainly.com/question/893601
#SPJ1
Related Questions
What is the Great Red Spot on Jupiter?
Answers
Answer:
The Great Red Spot is a storm in Jupiter's southern hemisphere with crimson-colored clouds that spin counterclockwise at wind speeds that exceed those in any storm on Earth. The Great Red Spot has slowly changed over the years, and is currently about 1.3 times as wide as our planet.
Convert 645 Newtons per quart to tons per liter. (The picture is a conversion table for help)

Answers
Answer:
0.067 ton/Litre.
Explanation:
645 Newtons per quart to tons per liter.
We'll begin by converting 645 Newton/quart to gram/quart (g/q). This can be obtained as:
1 N/q = 100 g/q
Therefore,
645 N/q = 645 N/q × 100 g/q / 1 N/q
645 N/q = 64500 g/q
Therefore, 645 Newton/quart is equivalent to 64500 grams per quart.
Next, we shall convert 64500 grams per quart to pounds per quart. This can be obtained as follow:
454 g/q = 1 pound/q
Therefore,
64500 g/q = 64500 g/q × 1 p/q / 454 g/p
64500 gram/quart = 142.07 pound/quart
Therefore, 64500 gram/quart is equivalent to 142.07 pound/quart.
Next, we shall convert 142.07 pound/quart to ton/quart. This can be obtained as follow:
2000 p/q = 1 ton/q
Therefore,
142.07 p/q = 142.07 pq × 1 ton/q / 2000 p/q
142.07 pound/quart = 0.071 ton/quart
Therefore, 142.07 pound/quart is equivalent to 0.071 ton/quart.
Finally, we shall convert 0.071 ton/quart to ton/litre. This can be obtained as follow:
1 ton/q = 1/1.06 ton/L
Therefore,
0.071 ton/q = 0.071 ton/q × 1/1.06 ton/L / 1 ton/q
0.071 ton/quart = 0.067 ton/Litre
Therefore, 0.071 ton/quart is 0.067 ton/Litre.
From the above illustration, 645 Newton/quart is equivalent to 0.067 ton/Litre.
One of your classmates tells you, “Since atoms are the basic building blocks of matter, all atoms have to be the same. Do you agree or disagree with this statement? Explain your reasoning.
Answers
41) What is the gist reaction of the CNO cycle?
a. Two 1H atoms e ventually form one 4He
atom.
b. Four 1H atoms eventually form two 4He
atom.
c. Four 1H atoms eventually form one 4He
atom.
d. One 1H atom eventually form one 4He
atom.
Answers
The correct optiob is c - Four 1H atoms eventually form one 4He atom.
The CNO cycle is a set of nuclear reactions that occur in stars, particularly in massive stars, as a means of converting hydrogen into helium.
It involves the interaction of carbon (C), nitrogen (N), and oxygen (O) isotopes as catalysts in the fusion process.
The CNO cycle consists of several steps:
The first step involves the fusion of two protons (1H) to form deuterium (2H) through a process known as proton-proton fusion.
In the second step, the deuterium nucleus (2H) reacts with another proton (1H) to form helium-3 (3He).
Next, two helium-3 nuclei (3He) combine to produce helium-4 (4He) and release two protons.
The overall reaction can be summarized as:
4(1H) → 4He
Therefore, four hydrogen nuclei (1H atoms) eventually combine to form one helium-4 (4He) atom.
This is the gist reaction of the CNO cycle.
It is important to note that the CNO cycle is one of the primary energy-producing mechanisms in stars, particularly those with high temperatures and densities, such as massive main-sequence stars.
Learn more about atoms from the given link
https://brainly.com/question/17545314
#SPJ11
why is stability of compounds essential
Answers
Answer:
chemical stability is important to consider in the comprehensive assessment of pharmaceutical properties, activity, and selectivity during drug discovery.
Answer:
In general, molecules are stable because all their constituent atoms have sufficient "valence" electrons-that is, electrons that participate in chemical bonding. Such electrons reside in the outer, or valence, "shell," which must fill up with the appropriate number of electrons for the bound atoms to be come stable.
Explanation:
8. Draw the lewis dot diagram for the following electron configuration
1s² 2s² 2p 3s² 3p6 4s²3d¹d 4p5
Answers
Answer:
Bromine has 7 electrons but to complete the octet you add one more electron so it has a net charge of 1-
Explanation:

Which of the following statements about metals and nonmetals is correct? (1 pt)
*
1 point
A.Metals and nonmetals can both be found in any column of the periodic table.
B.Most metals are solid under normal room temperatures while most nonmetals are a liquid.
C.Metals can be hammered into thin sheets while nonmetals are brittle.
D.Metals tend to be poor conductors of electricity, while nonmetals are good conductors of electricity.
Answers
The statement metals can hammered into thin sheets and non metals are brittle is correct. Thus option C is true.
What are metals?Metals is defined as a substance with good conductor of electricity, luster and malleability, which are ready to lose electron to form a positive ion i.e. cation. Metals can also be defined according to their periodic table.
Non metals is defined as a substance which are poor conductor of electricity, luster and malleability which are ready to gain electron to form a negative ion i.e. anion. They can be gas, liquid or solid.
Thus, metals can hammered into thin sheets and non metals are brittle is correct. Thus option C is true.
To learn more about metals, refer to the link below:
https://brainly.com/question/18153051
#SPJ1
The solubility of Ca(OH)2 is measured and found to be 0.953 g/L. Use this information to calculate a Ksp value for calcium hydroxide. Ksp =
Answers
The solubility of Ca(OH)2 is 0.953 g/L. The Ksp value can be determined by raising the concentrations of the calcium and hydroxide ions to the power of their stoichiometric coefficients and multiplying them together.
1. The solubility product constant (Ksp) represents the equilibrium constant for the dissociation of a sparingly soluble salt in water. For calcium hydroxide (Ca(OH)2), it dissociates into one calcium ion (Ca2+) and two hydroxide ions (OH-) in an aqueous solution, according to the balanced equation:
Ca(OH)2(s) ⇌ Ca2+(aq) + 2OH-(aq)
2. To calculate the Ksp value, we need to determine the concentration of the dissolved ions. Since the solubility of Ca(OH)2 is given as 0.953 g/L, we can convert this value to moles per liter using the molar mass of Ca(OH)2, which is approximately 74.093 g/mol.
3. Converting the solubility to moles gives:
0.953 g/L / 74.093 g/mol = 0.01287 mol/L
4. Since Ca(OH)2 dissociates into one Ca2+ ion and two OH- ions, the concentration of Ca2+ is equal to 0.01287 mol/L, and the concentration of OH- is equal to 2 * 0.01287 mol/L = 0.02574 mol/L.
5. To calculate the Ksp value, we multiply the concentrations of the ions raised to the power of their stoichiometric coefficients:
Ksp = [Ca2+][OH-]^2
= (0.01287 mol/L) * (0.02574 mol/L)^2
6. Calculating this expression yields the Ksp value for calcium hydroxide.
Learn more about Ksp value here: brainly.com/question/1304946
#SPJ11
Which substances are acids? Check all that apply.
AgOH
HF
NiOOH
NH4OH
HMnO4
Zn(OH)2
Answers
Answer:
Explanation:
Hope this helps:
AgOH is silver hydroxide
HF is hydrofluoric acid
NiOOH is nickel oxide hydroxide
NH4OH is ammonium hydroxide
HMnO4 is Permanganic acid
Zn(OH)2 is zinc hydroxide
Answer:
HF
HMnO4
Explanation:
bc i know everything
If the decomposition of a sample of k c l o 3 kclox3 produces 3. 29 g of o 2 ox2 , what was the mass (g) of the original sample?
Answers
The mass (g) of the original sample after decomposition is 8.3983 g.
A decomposition reaction can be described as a chemical reaction wherein one reactant breaks down into or extra merchandise.
explanation:
Reaction 2KClO₃ ⇒ 2KCl + 3O₂
moles 2 2 3
molar mass 122.55 74.55 32
Given, Mass of O₂ = 3.29g ⇒ moles of O₂
= (3.29/32) = 0.1028
3 moles of O₂ produced by 2 moles of KClO₃
Therefore, 0.1028 moles of O₂ produced by (2*0.1028/3) = 0.06853 moles of Kclo₃
Mass of KClo₃ in original sample is = moles * molar mass
= 0.06853 * 122.55
= 8.3983 g
A decomposition response occurs whilst one reactant breaks down into or extra merchandise. this may be represented through the general equation: XY → X+ Y. Examples of decomposition reactions consist of the breakdown of hydrogen peroxide to water and oxygen, and the breakdown of water to hydrogen and oxygen.
Learn more about decomposition here:-https://brainly.com/question/27300160
#SPJ4
When performing calculations relating to electrolysis, the number of moles of electrons can be derived from: Select the correct answer below: O the atomic mass of an electron O the stoichiometry of the reaction O the standard cell potential O none of the above
Answers
When performing calculation relating to electrolysis, no of moles of electron can be derived from The stoichiometry of the reaction.
Option B is correct.
Because that is the only way, no moles of electron can be derived from the reaction stoichiometry when calculating electrolysis. How can we tell? number of electrons released or captured in the electrolysis reaction is correct .
Electrolysis reaction :The process of separating hydrogen and oxygen from water through the use of electricity is called electrolysis. This response happens in a unit called an electrolyzer. Electrolysis is a chemical change that happens naturally. The process of breaking down a compound using electrical energy is what the term "electrolysis" means, which is also apt. Chemical reactions are altered by electrical energy.
Incomplete question :
When performing calculations relating to electrolysis, the number of moles of electrons can be derived from: Select the correct answer below: A. the atomic mass of an electron
B. the stoichiometry of the reaction
C. the standard cell potential
D. none of the above
Learn more about electrolysis reaction :
brainly.com/question/24063038
#SPJ4
PLEASE ANSWER ASAP I WILL GIVE BRAINLIEST!!

Answers
Answer: It’s Lower
What can you say about the pH of a carbonic acid solution compared to that of a sulfuric acid soloution with the same contention: Strong acids dissociate fully in water to produce the maximum number of \(H^{+}\)ions. The pH levels of strong acids are smaller than those of low acids, which is why the reactions rates for strong acids are greater than the reactions of weak acids with products like metals, metal carbonates, etc. Meaning The pH Level is lower.
take into account the speed of the top surface of the tank and find the speed of fluid leaving the opening at the bottom, if h=y2?y1, and A1 and A2 are the areas of the opening and of the top surface, respectively. Assume A1?A2 so that the flow remains nearly steady and laminar.
Express your answer in terms of the variables h, A1, A2, and appropriate constants.
Answers
The speed of the fluid leaving the opening at the bottom is approximately equal to v₂ ≈ (A₁v₁) / A₂
The speed of the fluid leaving the opening at the bottom can be determined using the principle of continuity, which states that the mass flow rate of a fluid remains constant in a steady and laminar flow.
According to the principle of continuity, the equation can be expressed as:
A₁v₁ = A₂v₂
Where A1 and A2 are the areas of the opening and the top surface respectively, v1 is the speed of the fluid at the top surface, and v2 is the speed of the fluid leaving the opening at the bottom.
Given that h = y₂ - y₁, where h is the height difference between the top surface and the opening, and assuming A₁ ≈ A₂, we can rewrite the equation as:
A₁v₁ ≈ A₂v₂
Now we can solve for v₂:
v₂ ≈ (A₁v₁) / A₂
Expressing the answer in terms of the given variables and appropriate constants, the speed of the fluid leaving the opening at the bottom is approximately equal to (A₁v₁) / A₂
To know more about fluid refer here:
https://brainly.com/question/6329574
#SPJ11
Select the correct answer from each drop-down menu. a molecule of chlorine has atoms of the same element, and a molecule of carbon dioxide has different kinds of atoms. based on this information, chlorine is and carbon dioxide is______ .
Answers
Chlorine is an element and carbon dioxide is a compound, this is the answer to the above question.
The matter is divided into categories using elements, compounds, and mixtures. Because all of the atoms in the simplest chemical compounds are the same size, they are referred to as elements. Compounds are made up of two or more elements that are chemically linked together in a certain sequence.
Elements, which are subcategories of atoms, may be defined by the same amount of protons. An element's atoms have the same number of protons, but its mass and number of neutrons may differ.
A compound is a substance that combines two or more distinct elemental kinds in a certain ratio of its atoms. During the process of fusion, some of the distinctive attributes of the constituent pieces are lost while the newly formed molecule picks up new traits. Compounds are represented using chemical formulae.
To know more about Chlorine visit:
https://brainly.com/question/14962130
#SPJ4
Answer:
Explanation:
chlorine is an element while carbon dyxoide is a compound
describe what gas pressure is. explain how concentration and temperature both effect gas pressure in a sealed container.
Answers
Gas pressure can be described as the force exerted per unit area by gas molecules as they collide with the surface of a container. It is a measure of the average kinetic energy of the gas molecules.
The pressure of a gas depends on several factors including the temperature, the volume, and the number of gas molecules present in a container. Changes in temperature and concentration can have an effect on gas pressure within a sealed container.Concentration can affect gas pressure because increasing the number of gas molecules in a container will result in more collisions and a greater force being exerted on the container walls. The pressure of the gas will increase. If the concentration of gas molecules decreases, then there will be fewer collisions and the pressure of the gas will decrease.Temperature is another important factor that can affect gas pressure. According to Charles' Law, if the temperature of a gas increases, then the volume of the gas will also increase. This is because the gas molecules will be moving faster and will require more space to move around in. The pressure of the gas will increase. An increase in concentration or temperature will lead to an increase in gas pressure, while a decrease in concentration or temperature will lead to a decrease in gas pressure.For such more questions on Gas pressure
https://brainly.com/question/30235826
#SPJ8
Based on the law of conservation of mass, what mass of reactants are used during the reaction

Answers
The mass of the reactant during the reaction base on the law of conservation of mass is 27.50 grams
How do i determine the mass of the reactants?The law of conservation of matter states that matter can neither be created nor destroyed during a chemical reaction but can be transferred from one form to another. Thus, the total mass of reactants must equal to the total mass of the product obtained in a chemical reaction.
Now, we shall obtain the mass of the reactants during the reaction. Details below:
Equation: Iron + sulfur -> Iron sulfideMass of iron sulfide = 27.50 gMass iron + sulfur = mass of reactants =?Iron + sulfur -> Iron sulfide
Mass of iron + mass of sulfur = Mass of iron sulfide
Mass of iron + mass of sulfur = 27.50
Thus, we can conclude from the above calculation that the mass of reactants is 27.50 grams
Learn more about law of conservation of matter:
https://brainly.com/question/9434062
#SPJ1
Calculate the mass of hydrogen produced when 72 g of magnesium
reacts with sulfuric acid.
Answers
Since this is a single replacement reaction, the equation for the reaction is:
\(\text{Mg}+\text{H}_{2}\text{SO}_{4} \longrightarrow \text{MgSO}_{4}+\text{H}_{2}\)
From this, we know that for every mole of magnesium consumed, 1 mole of hydrogen is produced.
The atomic mass of magnesium is 24.305 g/mol, so 72 grams of magnesium is 72/24.305 = 2.9623534252211 moles.
This means we need to find the mass of 2.9623534252211 moles of hydrogen.
Hydrogen has an atomic mass of 1.00794 g/mol, so doubling this to get the formula mass of of \(\text{H}_{2}\), we get 2.01588 g/mol, which his a mass of:
(2.01588)(2.9623534252211). which is about 5.97 g
please help me quick

Answers
Different rock and mineral ,soil profile
Which of the following 0.150 m solutions has the
greatest boiling-point elevation?
Mg(NO3)2
NaNO3
C2H4(OH)2
Answers
The solution with the greatest boiling-point elevation among the given options is Mg(NO₃)₂.
The boiling-point elevation of a solution depends on the concentration of solute particles. In this case, we have three solutions: Mg(NO₃)₂, NaNO₃, and C₂H₄(OH)₂.
Mg(NO₃)₂ dissociates into three ions: Mg²⁺ and two NO₃⁻ ions. NaNO₃ dissociates into two ions: Na⁺ and NO₃⁻. C₂H₄(OH)₂ does not dissociate, so it remains as one molecule.
Since the boiling-point elevation is directly proportional to the number of solute particles, Mg(NO₃)₂, with three ions per formula unit, will have the greatest boiling-point elevation. NaNO₃ has two ions per formula unit, and C₂H₄(OH)₂ has no ionization, resulting in fewer solute particles and lower boiling-point elevation compared to Mg(NO₃)₂.
Learn more about molecule here:
https://brainly.com/question/19922822
#SPJ11
The pH of a solution of Ca(OH)2 is 8.57. Find the [Ca(OH)2]. Be careful, the fact that this base produces 2 OH- is important!
Answers
The concentration of Ca(OH)2 in the solution is approximately 1.33 x 10^(-6) M.
To find the concentration of Ca(OH)2 in a solution with a pH of 8.57, we need to use the concept of pOH, which is the negative logarithm of the hydroxide ion concentration ([OH-]). The pOH can be calculated by subtracting the pH from 14, which gives us 14 - 8.57 = 5.43.
Since Ca(OH)2 produces two OH- ions for every molecule of Ca(OH)2 that dissolves, the concentration of OH- ions will be twice the concentration of Ca(OH)2. Thus, we have [OH-] = 2x, where x represents the concentration of Ca(OH)2.
Taking the antilogarithm of the pOH, we find that [OH-] = 10^(-pOH) = 10^(-5.43).
Since [OH-] = 2x, we can write 2x = 10^(-5.43) and solve for x.
x = (10^(-5.43))/2 ≈ 1.33 x 10^(-6) M
For more such questions on Ca(OH)2
https://brainly.com/question/31035177
#SPJ8
which is not a redox reaction?
a. Formation of ammonium sulphate from ammonia and sulphuric acid
b. formation of nitrogen monoxide from ammonia
c. formation of sulphuric acid from sulphur
d. formation of zinc from zinc sulphide
help me please
Answers
Answer:
D. formation of zinc from zinc sulphide
Explanation:
A redox reaction could be explained as an artificial reaction in which electrons are moved between two reactants partaking in it. This substitution of electrons can be recognized by examining the variations in the oxidation states of the reacting classes. The generation of hydrogen fluoride is an illustration of a redox reaction. We can crack the reaction down to investigate the oxidation and loss of reactants.
What element has 2 valence electrons and 2 energy levels
Answers
Answer:
helium
Explanation:
helium has 2 protons and 2 electrons. The 2 electrons are on the first energy level.
Consider the chemical equation.
CuCl2 + 2NaNO3 Right arrow. Cu(NO3)2 + 2NaCl
What is the percent yield of NaCl if 31.0 g of CuCl2 reacts with excess NaNO3 to produce 21.2 g of NaCl?
Use Percent yield equals StartFraction actual yield over theoretical yield EndFraction times 100..
49.7%
58.4%
63.6%
78.7%
Answers
Percent yield = 78.7% , the correct answer is D) 78.7%, which represents the percent yield of NaCl in the reaction.
To calculate the percent yield of NaCl in the given chemical equation, we need to compare the actual yield of NaCl with the theoretical yield. The theoretical yield is the amount of NaCl that would be produced if the reaction went to completion based on stoichiometry.
First, we need to determine the theoretical yield of NaCl. By examining the balanced equation, we can see that the stoichiometric ratio between CuCl2 and NaCl is 1:2. This means that for every 1 mole of CuCl2, 2 moles of NaCl are produced.
Step 1: Convert the mass of CuCl2 to moles using its molar mass.
Molar mass of CuCl2 = 63.55 g/mol (atomic mass of Cu) + 2 × 35.45 g/mol (atomic mass of Cl)
Molar mass of CuCl2 = 134.45 g/mol
Moles of CuCl2 = 31.0 g / 134.45 g/mol ≈ 0.231 mol
Step 2: Use the stoichiometry to calculate the theoretical yield of NaCl.
Since the stoichiometric ratio between CuCl2 and NaCl is 1:2, the moles of NaCl produced will be twice the moles of CuCl2.
Moles of NaCl (theoretical) = 2 × 0.231 mol = 0.462 mol
Step 3: Convert the moles of NaCl to grams using its molar mass.
Molar mass of NaCl = 22.99 g/mol (atomic mass of Na) + 35.45 g/mol (atomic mass of Cl)
Molar mass of NaCl = 58.44 g/mol
Theoretical yield of NaCl = 0.462 mol × 58.44 g/mol ≈ 26.96 g
Now, we can calculate the percent yield using the formula:
Percent yield = (Actual yield / Theoretical yield) × 100
Percent yield = (21.2 g / 26.96 g) × 100 ≈ 78.7%
Option D
For more such questions on Percent yield visit:
https://brainly.com/question/14714924
#SPJ8
How many grams are equal to 413 kg in scientific notation
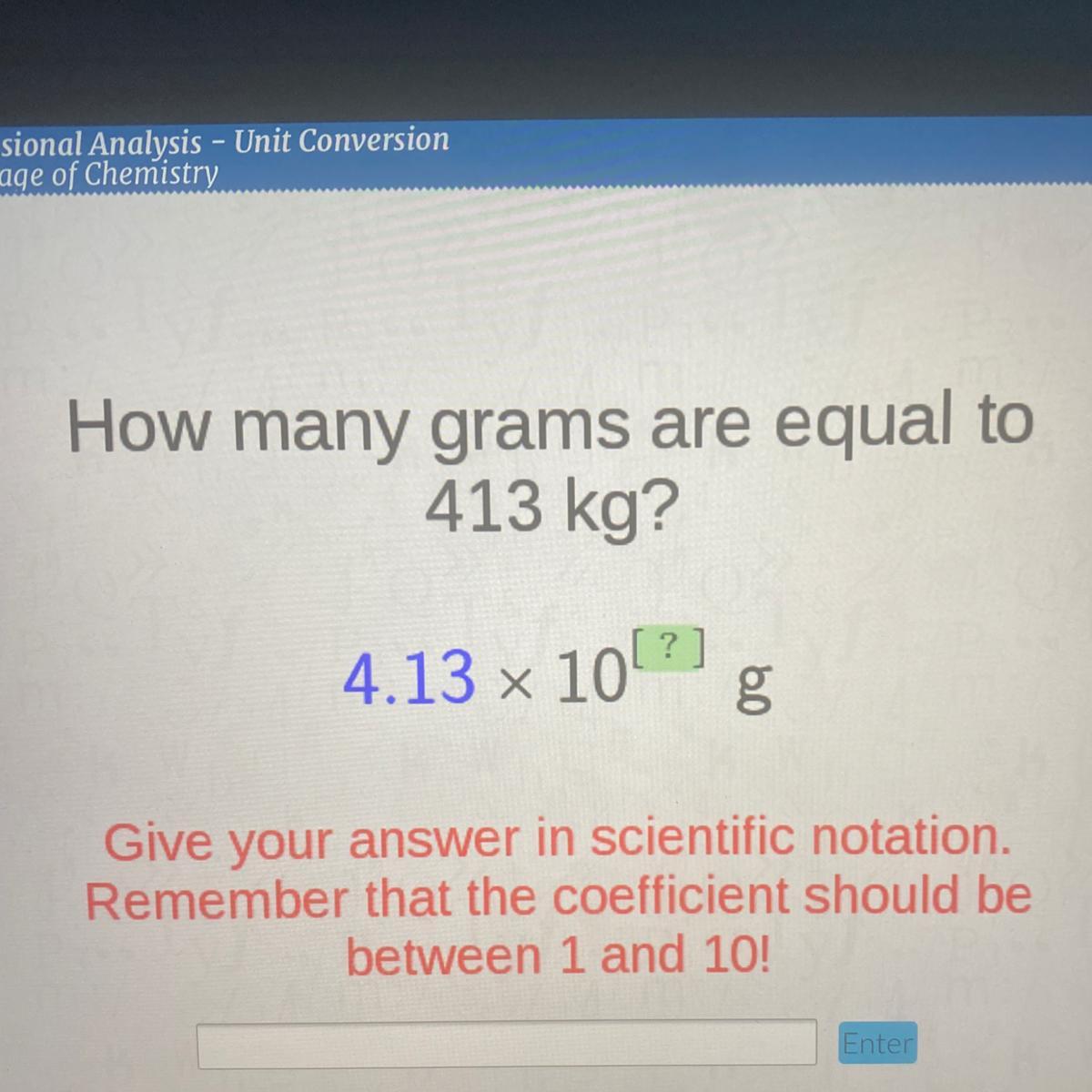
Answers
Answer:
\(4.13\) x \(10^{2}\) = 413 kg
Explanation:
4.13 x 10 = 41.3 then we multiply 41.3 by 10 agian = 41.3 x 10 = 413
so, we multiplied ten two times that = \(10^{2}\)
= 4.13 x \(10^{2}\)= 413 kg
Given that 1 kilogram = 1000grams, the value of 413 kilograms in grams, using scientific notation is 4.13 × 10⁵g.
How many grams are equal to 413 kg in scientific notation?Note that;
1 kilogram = 1000grams
Given that;
Mass in kilograms = 413kgMass in grams = ?Since, 1 kilogram = 1000grams
413 kilograms = ( 413 × 1000 )grams
413 kilograms = ( 413000 )grams
413 kilograms = 4.13 × 10⁵ g
Given that 1 kilogram = 1000grams, the value of 413 kilograms in grams, using scientific notation is 4.13 × 10⁵g.
Learn more about conversion of mass here: https://brainly.com/question/14147088
#SPJ9
Carbon cycle – What are the main reservoirs
of the carbon cycle? Where do the inorganic and organic carbon
cycles interact? What are the major differences and similarities
between the inorganic and organic carbon?
Answers
The main reservoirs of the carbon cycle are the atmosphere, oceans, land (including vegetation and soils), and fossil fuels. In these reservoirs, carbon exists in both inorganic and organic forms.
The inorganic carbon cycle involves the exchange of carbon dioxide (CO2) between the atmosphere and oceans through processes like photosynthesis and respiration.
Organic carbon, on the other hand, is found in living organisms, dead organic matter, and soil organic matter. It is cycled through processes such as decomposition and consumption by organisms. The interactions between the inorganic and organic carbon cycles occur primarily in the biosphere, where photosynthesis converts inorganic carbon into organic carbon compounds. While inorganic carbon is primarily in the form of CO2, organic carbon is present in complex organic molecules. Both forms of carbon play crucial roles in energy transfer, nutrient cycling, and climate regulation.
Learn more about Carbon Cycle
brainly.com/question/13729951
#SPJ11
Select an acceptable name for each compound. a) CH3(CH2)4CO2CH2CH3
a. ethyl hexanoate b. propyl pentanoate c. methyl pentanoate d. ethyl pentanoate
Answers
The acceptable name for the compound CH₃(CH₂)₄CO₂CH₂CH₃ is ethyl hexanoate,
So, the correct answer is A.
To select an acceptable name for the compound CH₃(CH₂)₄CO₂CH₂CH₃, we need to first identify the functional groups present in the molecule. In this case, we have a carboxylic acid (COOH) and an alcohol (CH₃CH₂) functional group.
To name the compound, we follow the standard naming conventions for esters. The first part of the name comes from the alkyl group attached to the carboxylic acid (COOH) functional group, which is hexanoate in this case. The second part of the name comes from the alcohol (CH₃CH₂) group, which is ethyl in this case.
Therefore, the acceptable name for this compound is ethyl hexanoate, as it follows the standard naming conventions for esters and correctly identifies the alkyl and alcohol groups present in the molecule.
Learn more about carboxylic acid at https://brainly.com/question/26855500
#SPJ11
What must occur before a newly made polypeptide is secreted from a cell?.
Answers
Answer: Your answer is Its signal sequence must target it to the ER, after which it goes to the Golgi.
Hope this helps!
hg
Identify what type of reaction is taking place, Explain:
2BaCl2+2KI 2KCl + BaI2
2 NH3+ 1 H2SO4 (NH4)2SO4
CaCO3 CaO + CO2
C11H24+17O2 11CO2 + 12H2O
Answers
2BaCl2+2KI ----> 2KCl + BaI2 Double replacement reaction
2 NH3 + H2SO4 ----> (NH4)2SO4 Synthesis reaction
CaCO3 ----> CaO + CO2 Decomposition reaction
C11H24+17O2 -----> 11CO2 + 12H2O Combustion reaction.
How do you know the type of reaction that is taking place?In a synthesis reaction, two or more reactants combine to form a single product. This type of reaction is also known as a combination reaction. The general equation for a synthesis reaction is A + B → AB.
In a double replacement reaction, two compounds exchange ions to form two new compounds. The general equation for a double replacement reaction is AB + CD → AD + CB.
Learn more about reaction:https://brainly.com/question/12080852
#SPJ1
What is the mass of 1.55 x 10^24 molecules of chlorine?
Answers
Explanation:
Use the molecular formula to find the molar mass; to obtain the number of moles, divide the mass of compound by the molar mass of the compound expressed in grams.
Now write the formulas of TWO compounds we can investigate--one that has an
element substituted for H, and one that has an element substitute for O. Write the
formulas separate by a comma, like this: X20, H2X
Answers
The term chemical formula is also called the molecular formula of a compound which represents the total number of atoms of each constituent elements present in one molecule of the compound.
What is chemical formula?The chemical formula is defined as the symbolic representation which gives the chemical composition of a compound. They also denotes the ratios in which the constituent elements combine to form the compound.
The chemical formula of a compounds with an element hydrogen are HCl, NH₃, HNO₃, etc. whereas the chemical formula of the compounds with an element 'O' are H₂O, H₂O₂, OH⁻, etc.
To know more about chemical formula, visit;
https://brainly.com/question/29031056
#SPJ9