Answers
Answer:
For photosynthesis to take place, plants need to take in carbon dioxide (from the air), water (from the ground) and light (usually from the sun)
Explanation:
Answer:
water carbon dioxide sunlight and the plant
Explanation:
Related Questions
Which of the following represents a beta decay?
O A. 220/86 Rn → 294/84Po + 4/2 He
O B. 60/28 Ni → 60/28 Ni + y
C. 235/93 Np → 23/91 Pa+ x
O D. 213/83 Bi → 214/84 Po+0/-1e

Answers
Answer:
Option D
Explanation:
Parent isotope has 83 protons, daughter isotope has only one more proton (84). Beta particle is very small, atomic mass of 212 stays the same in beta decay.
\(^2^1^2_8_3Bi\;\rightarrow\;^2^1^2_8_4Po\;+\;^0_-1e\) represents a beta decay. Parent isotope has 83 protons, daughter isotope has only one more proton (84). Beta particle is very small, atomic mass of 212 stays the same in beta decay.
What beta decay?In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which a beta particle (fast energetic electron or positron) is emitted from an atomic nucleus, transforming the original nuclide to an isobar of that nuclide.
\(^2^1^2_8_3Bi\;\rightarrow\;^2^1^2_8_4Po\;+\;^0_-1e\)
In beta decay, one of the neutrons in the nucleus suddenly changes into a proton, causing an increase in the atomic number of an element.
Hence, option D is correct.
Learn more about beta decay here:
https://brainly.com/question/25455333
#SPJ2
2. What is the percent sulfur in iron (III) sulfate?
Answers
Step 1
The chemical formula of iron (III) sulfate is the next one:
Fe2(SO4)3
As we can see, there are 3 x 1 atom of S, 3 atoms of S
----------------
Step 2
Information needed:
The atomic masses of:
Fe) 55.8 g/mol
S) 32.0 g/mol
O) 16.0 g/mol
(Please, the periodic table is useful here)
----------------
Step 3
The % of S in Fe2(SO4)3 is calculated as follows:
\(\frac{3\text{ x 32.0 g/mol of S}}{(2\text{ x 55.8 g/mol Fe + 3 x 32.0 g/mol S + 3 x 4 x 16.0 g/mol O\rparen}}x100\text{ = 24.0 \%}\)Answer: 24 % of S
At what temperature will the following reaction be spontaneous at 1 atm? H2(g) + 1/2O2(g) → H2O(g)
ΔH = +12 kJ/mol and ΔS = +40 J/K-mol
Answers
Answer:
The temperature at which the reaction will be spontaneous at 1 atm is 298 K (25°C).
Explanation:
Solve this organic transformation....use - Br2,CCl4,KOH,CH3OH,Hg+2,diluted H2SO4, PCC,HBr,Mg,Dry ether,Na,H2,Pd,quinoline
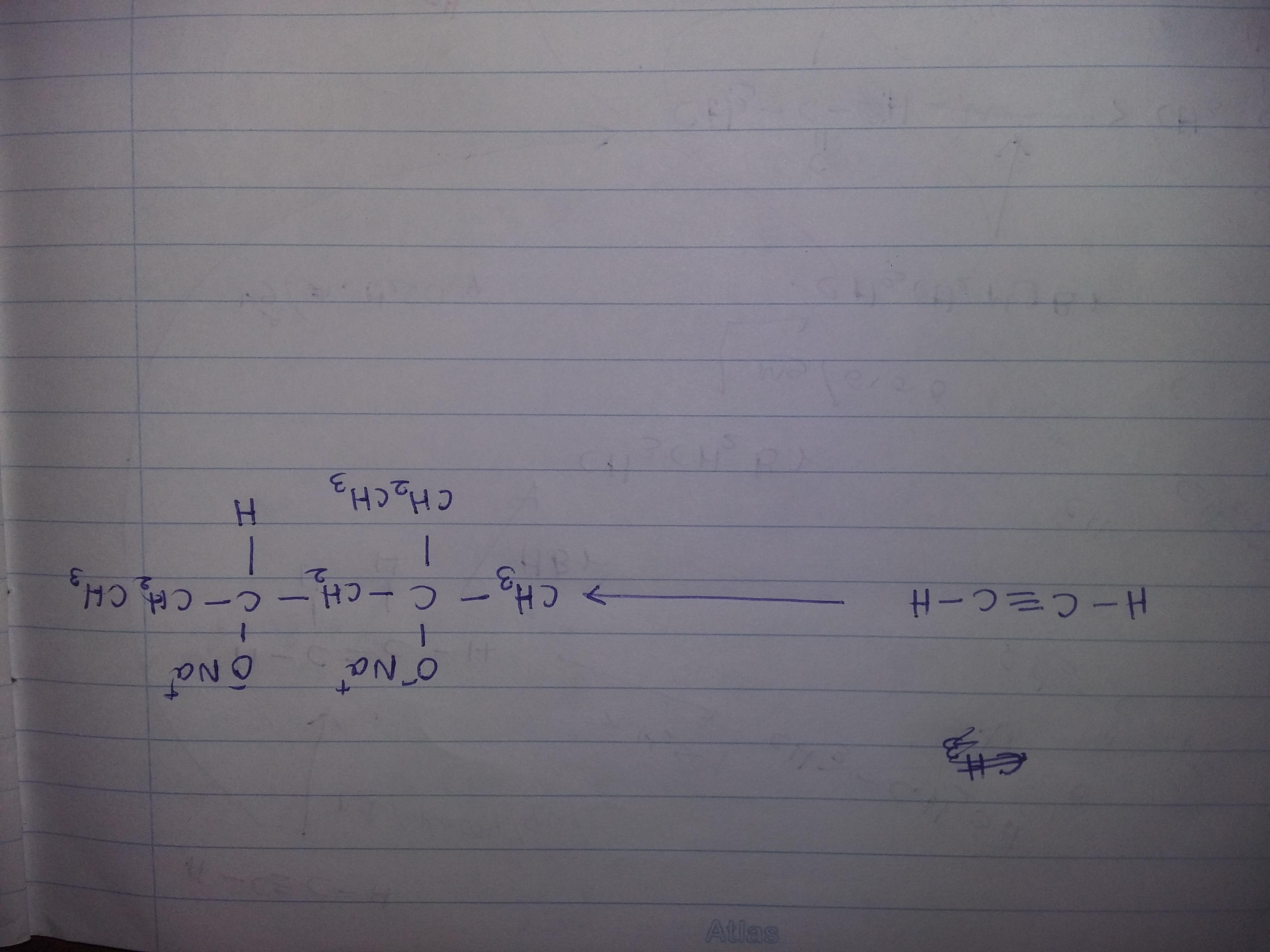
Answers
Organic transformation sequential equation using catalysts will be as follows:
2CH3-CH2-O => (alc. KOH) => CH2=CH2 + KCl + H2O => (Br2/CCl4) => CH2Br-CH2Br + Zn
CH2Br-CH2Br + Zn => (HBr /Pd) => CH2=CH2+ZnBr2
As can be visualized from above organic transformation equation, conversion of dry ether in presence of alkaline potassium hydroxide results in formation of unstable ethene. This dry unsaturated compound of ethene is stabilized by reaction that happens in presence of bromine or calcium tetrachloride as the catalyst which results in formation of ethylene bromide which in presence of highly efficient palladium as catalyst results in formation of stable ethene as byproduct. Thereby with formation of stable compound of ethylene, it releases zinc bromide as byproduct resulting completion of reaction equation. This stable product ethene is a double bonded carbon structure that is chemically extremely flammable and has planar structure.
To know more about organic transformation:
brainly.com/question/14413579
#SPJ1
Vsepr diagram for phcl2
Answers
what is the formula for co3+ and se2-?
Answers
The formula for Co3+ is Co3+ because it represents the ion of cobalt that has lost three electrons, leaving it with a 3+ charge.
What is chemical formula and how they are formed ?
A chemical formula is a symbolic representation of a chemical compound that shows the types of elements present in the compound and the relative number of atoms of each element. For example, the chemical formula for water is H2O, which indicates that it is made up of two hydrogen atoms and one oxygen atom.
Chemical formulas are formed by identifying the elements that make up a compound and determining the relative number of each element in the compound. The number of each element is represented by a subscript following the chemical symbol of the element. For example, the chemical formula for methane is CH4, which indicates that there is one carbon atom and four hydrogen atoms in each molecule of methane.
The formula for Se2- is Se2- because it represents the ion of selenium that has gained two electrons, giving it a 2- charge.
To know more about reaction visit :-
https://brainly.com/question/11231920
#SPJ1
The question is in an image. You need to use "CER" which is in one of the images and the reference table G which is in another of the images. "CER" means 3 words: Claim (The answer), Evidence (Why the answer is right) and Reasoning (Connect answer to evidence).



Answers
Answer:
C: Claim the answer.
No, 100 g of NH4Cl will not form a saturated solution with 200 g of water.
E: Evidence.
According to table G and by doing a rule of three, we obtained 124 g of NH4Cl and the question is asking if a solution with 100 g of NH4Cl with 200 g of water.
R: Reasoning.
According to the result that we obtained from the rule of three, we need 124 g of NH4Cl to form a saturated solution with 200 g of water, so 100 g of NH4Cl with 200 g of water will form an unsaturated solution.
Explanation:
First, let's review the concept of a saturated solution: A saturated solution is a solution that contains the maximum amount of solute that is capable of dissolving.
You can see in the graph that at 70 °C, we can dissolve approximately 62 g of NH4Cl in 100 g of water to form a saturated solution. But, we want to confirm if 100 g of NH4Cl can be dissolved in 200 g of water to form a saturated solution, so let's state a rule of three:
\(\begin{gathered} 62\text{ g NH}_4Cl\rightarrow100\text{ g water} \\ x\text{ g NH}_4Cl\rightarrow200\text{ g water} \end{gathered}\)The calculation of this rule of three would be:
\(62\text{ g NH}_4Cl\cdot\frac{200\text{ g water}}{100\text{ g water}}=124\text{ g NH}_4Cl.\)To form a saturated solution of NH4Cl with 200 g of water, we will need 124 g of NH4Cl. As we obtained 124 g of NH4Cl and the question is asking if a solution with 100 g of NH4Cl with 200 g of water will form a saturated solution would be wrong because this amount doesn't reach the maximum of solute. This would be an unsaturated solution (an unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved).
C: Claim the answer.
No, 100 g of NH4Cl will not form a saturated solution with 200 g of water.
E: Evidence.
According to table G and by doing a rule of three, we obtained 124 g of NH4Cl and the question is asking if a solution with 100 g of NH4Cl with 200 g of water.
R: Reasoning.
According to the result that we obtained from the rule of three, we need 124 g of NH4Cl to form a saturated solution with 200 g of water, so 100 g of NH4Cl with 200 g of water will form an unsaturated solution.
Besides solubility, state two other physical properties that are different for salt and sand.
Answers
Answer:Electrical Conductivity,soluble
Explanation:
Salt is a non-magnetic solid and is soluble in water. Sand is a non-magnetic solid and is insoluble in water.
Electrical Conductivity: Salt is an electrolyte and conducts electricity when dissolved in water or in a molten state. This is because salt dissociates into ions (Na+ and Cl-) that can carry electric current. In contrast, sand is a covalent compound and does not conduct electricity, as it does not dissociate into ions in the same way as salt. Sand is considered an insulator in terms of electrical conductivity.
What is STUTE IN
h आधुनिक पेरियोडिक तालिकामा मन कन समूह र पिरियडमा पर्छ ।
In what group and period does zold le in the modern periodic table"
1 बैकलाइट बनाउन कन कन कच्चा पदार्थहरु प्रयोग गरिन्छ ।
Answers
State yes no whether each of the following orbital diagrams conforms to the rules governing electron configuration if not explain what is wrong with the diagram

Answers
Answer:yes,no,no,yes
Using the guideline for oxidation numbers, write the reduction half-reactions for the following:
• O
• P
• Cu
Answers
The reduction half-reactions for O, P, and Cu:
• O: O2 + 4 e- → 2 O2-
• P: HPO42- + 2 H+ + 2 e- → H3PO4
• Cu: Cu2+ + 2 e- → Cu+
To write the reduction half-reactions for O, P, and Cu, we need to determine the oxidation numbers for each element. The guidelines for assigning oxidation numbers are:
The oxidation number of an atom in its elemental form is 0.The oxidation number of a monatomic ion is equal to its charge.The sum of the oxidation numbers of all atoms in a neutral molecule must be 0.The sum of the oxidation numbers of all atoms in a polyatomic ion must be equal to the charge of the ion.Using these guidelines, we can determine the oxidation numbers for O, P, and Cu:
O: Oxygen is a diatomic molecule, so its oxidation number is 0 in O2.P: The most common oxidation state for phosphorus is +5 in its compounds, but it can also have oxidation states ranging from -3 to +5.Cu: The most common oxidation state for copper is +2, but it can also have oxidation states ranging from +1 to +4.To know more about the Reduction half-reaction, here
https://brainly.com/question/18403544
#SPJ4
. Which of the following statements is false?
a. The actual yield is the amount of product
actually produced by a chemical reaction.
B. The percent yield= actual yield/ theoretical yield (X) 100%
C. The theoretical yield is the amount of product
that can be made based on the amount of
limiting reagent.
d. The limiting reactant is completely consumed in
a chemical reaction.
e. All of the above are true statements.
Answers
Answer: A. The actual yield is the amount of product actually produced by a chemical reaction.
The actual yield is the amount of product actually produced by a chemical reaction. This statement is false. Therefore, the correct option is option A.
What is percent yield?Chemists use the calculation percent yield to assess the effectiveness of chemical reactions. Every chemical reaction that they deal with has a predicted outcome. The great majority of the time, even with a desired outcome in mind that should occur chemically, this doesn't actually happen. The percent yield equation gives chemists an indication of how successfully the reaction actually went, as opposed to the best possible outcome they had anticipated.
The percent yield equation is useful for various purposes, such as finances, in addition to helping us understand chemical reactions better. The percent yield metric is used by businesses that produce chemical-based goods to assess their financial health and productivity. The actual yield is the amount of product actually produced by a chemical reaction. This statement is false.
Therefore, the correct option is option A.
To know more about percent yield, here:
https://brainly.com/question/30700754
#SPJ2
Mass box A = 10 grams; Mass box B = 5 grams; Mass box C-made of one A and one B
Predicted mass C =
grams
Answers
C = 15 grams
Take a balloon that we filled up with our breath. Tell me what would happen if we put it outside and let it sit in the sun for a bit. Support your answer using the KMT.Keep in mind we are going to be under the impression that no air can escape from the balloon.
Answers
The balloon will be filled by a gas, and as the gas is not escaping we can assume that it is at a constant pressure.
The KMT (Kinetic Molecular Theory) explains the macroscopic properties of gases. This theory states that gases are formed by particles in constant motion, and that their kinetic energy is directly proportional to the temperature of the gas.
If we leave the balloon filled with gas in the sun we assume that the temperature of the gas inside will start to rise. When the temperature starst to rise, and the pressure is constant, particles will gain kinetic energy. This will result in particles moving faster and coliding with each other and with the balloon's walls more often. As a consecuence the particles will tend to stay farther from one another and the volume of the balloon will increase.
This is an example of Charles's Law that states that at constant pressure the volume of a gas is directly proportional to its temperature. In other words, when the temperature of the gas increases the volume increases as well.
To summarize, if we fill a balloon and we leave it in the sun its volume will increase.
c) Discuss precision and Accuracy as they relate to types of errors.
what is the answer
Answers
Precision relates to the consistency and reproducibility of measurements, while accuracy reflects how close measurements are to the true value.
Precision and accuracy are two important concepts in the context of errors in measurements. While they both pertain to the quality of data, they refer to different aspects.
Precision refers to the degree of consistency or reproducibility in a series of measurements. It reflects the scatter or spread of data points around the average value. If the measurements have low scatter and are tightly clustered, they are considered precise. On the other hand, if the measurements have a high scatter and are widely dispersed, they are considered imprecise.
Accuracy, on the other hand, refers to the closeness of measurements to the true or target value. It represents how well the measured values align with the actual value. Accuracy is achieved when measurements have a small systematic or constant error, which is the difference between the average measured value and the true value.
Errors in measurements can be classified into two types: random errors and systematic errors.
Random errors are associated with the inherent limitations of measurement instruments or fluctuations in the measurement process. They lead to imprecise data and affect the precision of measurements. Random errors can be reduced by repeating measurements and calculating the average to minimize the effect of individual errors.
Systematic errors, on the other hand, are caused by consistent biases or inaccuracies in the measurement process. They affect the accuracy of measurements and lead to a deviation from the true value. Systematic errors can arise from factors such as instrumental calibration issues, environmental conditions, or experimental techniques. These errors need to be identified and minimized to improve the accuracy of measurements.
In summary, precision refers to the degree of consistency or reproducibility of measurements, while accuracy refers to the closeness of measurements to the true value. Random errors affect precision, while systematic errors affect accuracy. To ensure high-quality measurements, both precision and accuracy need to be considered and appropriate techniques should be employed to minimize errors.
Know more about Precision here:
https://brainly.com/question/30461151
#SPJ8
what would lead to a higher final temperature of water, a metal with a low heat capacity of a high heat capacity
Answers
A hot metal with a low heat capacity will raise the final temperature of water more than a hot metal with high heat capacity.
Heat capacityIt is the amount of heat required to change the temperature of a substance by just a unit.
Substances that heat up quickly are said to have low heat capacities while those that take time to heat up are said to have high heat capacities.
Substances with low heat capacity also release their thermal energy quicker than those with high heat capacity.
Thus, a hot metal with low heat capacity will readily transfer its thermal energy to water than a hot metal with high heat capacity.
More on heat capacities can be found here: https://brainly.com/question/11194034
What type of chemical reaction requires oxygen gas as reactant and releases heat?
Answers
Answer:
A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat.
Which of the following molecules will exhibit hydrogen bonding?
A. CH3OCH3
B. HF
C. CH4
D. LiH
Answers
Answer:
B. HF
Explanation:
Hydrogen bonding is basically a bond between hydrogen and an electronegative element particularly the second-row elements nitrogen (N), oxygen (O), or fluorine (F).
option A.
Although oxygen is present, there are no O - H bond present. The oxygen is bonded to two carbon atoms hence hydrogen bonding is absent.
option B.
Hydrogen is bonded directly to Flourine. Hydrogen bond is present.
option C.
This is an organic compound, only covalent bonds are present in this molecule. No hydrogen bonds,
option D.
Lithium does snot have high electronegativity hence hydrogen bonding is absent.
d = 7 V = 950 cm M = 95 g
0.1 g/cm3
0.1 cm3
90,250 g
90,250 g/cm3
Answers
Answer:
daddy chill
Explanation:
net ionic equation for SrBr2 + K2SO4 --->
Answers
Answer:
\(2K^+\text{ + 2Br}^-\text{ }\rightarrow\text{ 2KBr}_{(s)}\)Explanation:
Here, we want to write the net ionic equation for the given equation;
\(SrBr_2\text{ + K}_2SO_4\text{ }\rightarrow\text{ SrSO}_4\text{ + 2KBr}\)Now, what is left is to write the participating ions:
\(Sr^{2+}\text{ + 2Br}^-\text{ + 2K}^+\text{ + SO}_4^{2-}\text{ }\rightarrow\text{ Sr}^{2+}\text{ + SO}_4^{2-}\text{ + 2KBr}\)We must identify that the Potassium Bromide is the precipitate and thus would not be dissociated into ions
Thus, we have it that:
The Strontium ion cancels out, including the sulphate
We have left:
\(2K^+\text{ + 2Br}^-\text{ }\rightarrow\text{ 2KBr}\)a 15.7 g aluminum block at 53.2 degrees c is plunged into an insulated beaker containing 32.5g of water, initially at 24.5 degrees c
Answers
The final temperature of water and aluminum will be 27.2 °C., after they are allowed to come to thermal equilibrium.
Here,
Physical principle:a) According to first law of thermodynamic the energy is conserved.
b) In an insulated system there is no loss of heat
So, Heat lost by aluminum will be equal to heat gained by water
c) At thermal equilibrium the, final temperature of water will be equal to final temperature of aluminium
Formulae:
Heat gain or loss = m×Cs×ΔT,
where m = mass of the substance,
Cs is the specific heat of the substance,
ΔT is the change in temperature
Datamass of aluminum, Ma = 15.7g
Temprature of aluminium = 53.2 °C
mass of water, Mw = 32.5 g
Temrature of water= 24.5°C
Known values:
Specific heat liquid water: Cs = 1 cal/g°C
Specific heat aluminum: Cs = 0.215 cal/g°
So Calculation will be done,
Heat of aluminium = Heat of water
Heat of water = m Cs ΔT = 32.5g × 1× (T - 24.5)
Heat of aluminium = m CsΔT = 15.7× (0.215) (53.2 - T)
32.5 (1 ) (T - 24.5) = 15.7 (0.215) (53.2 - T)
32.5T - 796.25 = 179.576 - 3.375T
35.8755Tf = 975.8266
T = 27.2°C
To learn more on calculating temperature on thermal equilibrium please refer:
https://brainly.com/question/14036541
#SPJ1
Your question is incomplete, for full question refer below:
A 15.7 g aluminum block is warmed to 53.6 ∘C is plunged into an insulated beaker containing 32.5 g of water initially at 24.5 ∘C. The aluminum and the water are then allowed to come at thermal equilibrium.
Assuming that no heat is lost in the process, Determine is the final temperature of the water and aluminum?
which type of severe weather is related to high pressure systems?
A.Hurricane
B.Heat Wave
C.Tornado
D.Thunderstorm
Answers
Answer:
Heat waves
Explanation:
The rock sample on the left is basalt, a type of igneous rock. Heat and intense
pressure changed the basalt into blueschist, a type of metamorphic rock.
Which characteristics of the rock sample changed?

Answers
The rock sample on the left is basalt, a type of igneous rock. Heat and intense pressure changed the basalt into blueschist, a type of metamorphic rock glaucophane schist
Blueschist facies is determined by the particular temprature and pressure condition required to metamorphose basalt to form blueschist and felsic rock and pelitic sediment which are subjected to blueschist facies condition will form different mineral assemblages then metamorphosed and blueschist facies rock are generally formed in subduction zones where oceanic crust is being stuffed into a trench that will become true blueschist once they were pressure cooked and also called glaucophane schist and is a metavolcanic rock that with similar composition at high pressure and low temprature
Know more about rock
https://brainly.com/question/12586610
#SPJ1
The scientific method is great, but how do you think we answer the questions which cannot be tested with an experiment?
Answers
Answer:
We do something called hypothesis
Answer:
1. Make an observation.
2. Ask a question.
3. Propose a hypothesis.
4. Make predictions.
5. Test the predictions.
6. Iterate.
and if it can't be trsted the you did something wrong
Explanation:
The scientific method
At the core of biology and other sciences lies a problem-solving approach called the scientific method. The scientific method has five basic steps, plus one feedback step:
Make an observation.
Ask a question.
Form a hypothesis, or testable explanation.
Make a prediction based on the hypothesis.
Test the prediction.
Iterate: use the results to make new hypotheses or predictions.
The scientific method is used in all sciences—including chemistry, physics, geology, and psychology. The scientists in these fields ask different questions and perform different tests. However, they use the same core approach to find answers that are logical and supported by evidence.
Scientific method example: Failure to toast
Let's build some intuition for the scientific method by applying its steps to a practical problem from everyday life.
30 example of redox reaction
Answers
1. Combustion of gasoline in a car engine
2. Rusting of iron
3. Photosynthesis in plants
4. Respiration in animals
5. Corrosion of metals
6. Bleaching of hair with hydrogen peroxide
7. Formation of ozone in the atmosphere
8. Electroplating of metals
9. Burning of wood
10. Reaction between bleach and ammonia
11. Reaction between copper and nitric acid
12. Reaction between iron and hydrochloric acid
13. Reaction between zinc and sulfuric acid
14. Reaction between magnesium and hydrochloric acid
15. Reaction between aluminum and hydrochloric acid
16. Reaction between sodium and water
17. Reaction between potassium and water
18. Reaction between lithium and water
19. Reaction between calcium and water
20. Reaction between barium and water
21. Reaction between copper and silver nitrate
22. Reaction between lead and silver nitrate
23. Reaction between zinc and copper sulfate
24. Reaction between iron and copper sulfate
25. Reaction between magnesium and copper sulfate
26. Reaction between aluminum and copper sulfate
27. Reaction between sodium and chlorine
28. Reaction between magnesium and chlorine
29. Reaction between aluminum and chlorine
30. Reaction between zinc and hydrochloric acid.
How does the burning of fossil fuels contribute to global warming?
Answers
Answer: The burning of fossil fuels releases carbon dioxide, methane and other greenhouse gases into the atmosphere. These gases trap heat in the atmosphere, leading to a gradual increase in global average temperatures, known as global warming. This phenomenon has serious impacts on our environment and ecosystems, including extreme weather events and rising sea levels.
oxidation number of Ag in Ag2O
Answers
The oxidation number of Ag in Ag2O is +1.
In Ag2O, there are two silver atoms (Ag) and one oxygen atom (O). Oxygen is known to have an oxidation number of -2 in most compounds. Since the compound is neutral, the sum of the oxidation numbers of all the atoms must equal zero.
Therefore, the oxidation numbers of the two silver atoms must add up to +2 to balance out the -2 oxidation number of the oxygen atom. Since there are two silver atoms, each silver atom must have an oxidation number of +1 to yield a total oxidation number of +2 for the compound.
In Ag2O, the silver atoms lose one electron each to form Ag+ ions. This results in an oxidation number of +1 for each silver atom. The oxygen atom gains two electrons from the silver atoms to achieve a stable octet configuration, resulting in an oxidation number of -2 for the oxygen atom. The compound Ag2O is formed through the transfer of electrons, with each silver atom exhibiting an oxidation number of +1.
for such more questions on oxidation
https://brainly.com/question/13182308
#SPJ8
The rotational spectrum of 79BrºF shows a series of equidistant lines spaced 0-714 33 cm - apart. Calculate the rotational constant B, and hence the moment of inertia and bond length of the molecule. Determine the wavenumber of the J = 9+= 10 transition, and find which transition gives rise to the most intense spectral line at room temperature (say 300 K).
and calculate the number of revolutions per second which the Brf molecule undergoes when in (a) the J = 0 state, (b) the J = 1 state, and (c) the J = 10 state. Hint: Use E = {lwin conjunction with Eqs (2.10) and (2.13), but remember that here w is in radians per second.[its Q season 2 from fundamentals of molcular spectruscopy . banwell.c.n]
Answers
In the J = 0 state, the BrF molecule does not undergo any revolutions per second. In the J = 1 state, it undergoes approximately 0.498 revolutions per second, and in the J = 10 state, it undergoes approximately 15.71 revolutions per second.
To calculate the rotational constant B, we can use the formula:
B = 1 / (2 * π * Δν)
Where:
B = rotational constant
Δν = spacing between consecutive lines in the rotational spectrum
Given that the spacing between consecutive lines is 0.71433 cm^(-1), we can substitute this value into the formula:
B = 1 / (2 * π * 0.71433 cm^(-1))
B ≈ 0.079 cm^(-1)
The moment of inertia (I) of the molecule can be calculated using the formula:
I = h / (8 * π^2 * B)
Where:
h = Planck's constant
Given that the value of Planck's constant (h) is approximately 6.626 x 10^(-34) J·s, we can substitute the values into the formula:
I = (6.626 x 10^(-34) J·s) / (8 * π^2 * 0.079 cm^(-1))
I ≈ 2.11 x 10^(-46) kg·m^2
The bond length (r) of the molecule can be determined using the formula:
r = sqrt((h / (4 * π^2 * μ * B)) - r_e^2)
Where:
μ = reduced mass of the molecule
r_e = equilibrium bond length
To calculate the wavenumber (ν) of the J = 9+ to J = 10 transition, we can use the formula:
ν = 2 * B * (J + 1)
Substituting J = 9 into the formula, we get:
ν = 2 * 0.079 cm^(-1) * (9 + 1)
ν ≈ 1.58 cm^(-1)
To determine the most intense spectral line at room temperature (300 K), we can use the Boltzmann distribution law. The intensity (I) of a spectral line is proportional to the population of the corresponding rotational level:
I ∝ exp(-E / (k * T))
Where:
E = energy difference between the levels
k = Boltzmann constant
T = temperature in Kelvin
At room temperature (300 K), the population distribution decreases rapidly with increasing energy difference. Therefore, the transition with the lowest energy difference will have the most intense spectral line. In this case, the transition from J = 0 to J = 1 will have the most intense spectral line.
To calculate the number of revolutions per second, we can use the formula:
ω = 2 * π * B * J
Where:
ω = angular frequency (in radians per second)
J = rotational quantum number
For J = 0:
ω = 2 * π * 0.079 cm^(-1) * 0 = 0 rad/s
For J = 1:
ω = 2 * π * 0.079 cm^(-1) * 1 ≈ 0.498 rad/s
For J = 10:
ω = 2 * π * 0.079 cm^(-1) * 10 ≈ 15.71 rad/s
For more such questiosn on BrF molecule visit;
https://brainly.com/question/30624940
#SPJ8
Which of the following best explains what happens when the kinetic energy of particles in a liquid state incereases
Answers
Answer:
YES
Explanation:
A rigid, 28-L steam cooker is arranged with a pressure relief valve set to release vapor and maintain the pressure once the pressure inside the cooker reaches 150 kPa. Initially, this cooker is filled with water at 175 kPa with a quality of 10 percent. Heat is now added until the quality inside the cooker is 40 percent. The water is stirred at the same time that it is being heated. Determine the minimum entropy change of the heat-supplying source if 100 kJ of work is done on the water as it is being heated. Use steam tables.
Answers
Answer:
\(\Delta S_{source}>-1.204\frac{kJ}{K}\)
Explanation:
Hello!
In this case, given the initial conditions, we first use the 10-% quality to compute the initial entropy:
\(s_1=s_{f,175kPa}+q*s_{fg,175kPa}\\\\s_1=1.4850\frac{kJ}{kg*K} +0.1*5.6865\frac{kJ}{kg*K}=2.0537\frac{kJ}{kg*K}\)
Now the entropy at the final state given the new 40-% quality:
\(s_2=s_{f,150kPa}+q*s_{fg,150kPa}\\\\s_2=1.4337\frac{kJ}{kg*K} +0.4*5.7894\frac{kJ}{kg*K}=3.7495\frac{kJ}{kg*K}\)
Next step is to compute the mass of steam given the specific volume of steam at 175 kPa and the 10% quality:
\(m_1=\frac{0.028m^3}{(0.001057+0.1*1.002643)\frac{m^3}{kg} } =0.274kg\\\\m_2=\frac{0.028m^3}{(0.001053+0.4*1.158347)\frac{m^3}{kg} } =0.0603kg\)
Then, we can write the entropy balance:
\(\Delta S_{source}+\frac{Q}{T_1} -\frac{Q}{T_2} +s_2m_2-s_1m_1-s_{fg}(m_2-m_1)>0\)
Whereas sfg stands for the entropy of the leaving steam to hold the pressure at 150 kPa and must be greater than 0; thus we plug in:
Which is such minimum entropy change of the heat-supplying source.
Best regards!