in part one the purpose of the experiment was to setermine the molairty of your naoh solution. during th eittrstion, water was aded to khp to wash down the soldes of the flask. it dilute he concentration o khp. what is it not a concern?
Answers
In the experiment described in part one, the purpose was to determine the molarity of the NaOH solution. During the titration, water was added to the KHP to wash down the solids in the flask, which diluted the concentration of KHP. However, the dilution of KHP concentration is not a concern for the experiment.
The reason it is not a concern is because the concentration of KHP does not affect the accuracy of the titration. In this experiment, the goal is to determine the concentration of NaOH, not KHP. By adding water to the KHP, any impurities or residual substances in the flask are washed down, ensuring a clean environment for the titration. The concentration of KHP is not directly related to the accuracy of the NaOH concentration determination. Instead, the focus is on the volume of NaOH solution required to reach the endpoint of the titration, which indicates the amount of NaOH present in the solution.
To summarize, while the dilution of KHP concentration occurs during the experiment, it is not a concern because the accuracy of the NaOH concentration determination is not affected by the concentration of KHP.
To know more about that experiment visit:
https://brainly.com/question/30055326
#SPJ11
Related Questions
Rebecca placed an uncharged object next to an electrically charged object, and they were attracted to each other. She moved the charged object and placed it next to another uncharged object, and they were attracted to each other. What is the best explanation as to why this happened? The charged object kept its charge.
The charged object lost its charge.
The second object had a stronger charge.
The second object had a weaker charge.(PLEASE HELP)
Answers
Answer:
the second object had a weaker charge
Explanation:
the charged object had a stronger charge and opposite to that of the uncharged hence causing attraction
Answer:
d
Explanation:
The second object had a weaker charge.
The liver, kidneys, and brain arise from
which germ layer(s)
Answers
mesoderm is the muscle bone urinary tract and the kidneys.
How would a mutation affect how DNA is eventually read when it forms a protein
Answers
Answer:
Explanation:
A mutation in the DNA results in a change in the mRNA and, ultimately, to a different protein structure.
Answer:
A mutation in the DNA results in a change in the mRNA and, ultimately, to a different protein structure. The DNA sequence found within a gene controls protein synthesis. If the DNA sequence is altered, this can alter the amino acid sequence within a protein
Explanation:
Your DNA is the blueprint used to make proteins. Your DNA is used to make a strand of mRNA (Messenger RNA.) This is sent to a ribosome where it is read. The codons in the mRNA instruct the ribosome which amino-acid to attach to the end of the protein chain and when to let it go.
If your DNA mutates then the mRNA copied from it will be different. When that gets to the ribosome the effect depends on just what the altered codon(s) are. The ribosome will blindly follow the instructions it has been given. This might be to add a different amino-acid to the chain or to prematurely release it.
What is the electron structure of nitrogen?
o 1s 2s 2p 5
o 1s 22s22p3
O1s 328 32p
15 225 235 22p
Answers
Answer:
(1s)^2 (2s)^2 (2p)^3
Explanation:
Nitrogen has 7 electrons. Hence, the structure will be,
=>(1s)^2 (2s)^2 (2p)^3
Hope it helps:)
The electronic configuration is used to explain the orbitals of an atom and it helps to determine the physical and chemical properties of the elements. The electronic configuration of Nitrogen is 1s² 2s² 2p³. The correct option is B.
What is electronic configuration?The distribution of electrons in the atomic orbitals is given by the electronic configuration. It is a standard notation in which all the electrons holding atomic subshells are arranged in a sequence. Each element has a unique electronic configuration.
The electronic configuration of an element can be written in two ways, in standard notation, and in condensed form. In the case of elements with larger atomic numbers, the electronic configuration becomes lengthy in standard notation. So in such cases condensed form is generally used.
The electronic configuration of 'N' of atomic number 7 in standard form is 1s² 2s² 2p³ and in condensed form it is [He] 2s²2p³.
Thus the correct option is B.
To know more about electronic configuration, visit;
https://brainly.com/question/29757010
#SPJ7
A clinical trial was conducted to test the effectiveness of a drug for treating insomnia in older subjects. Before treatment, 16 subjects had a mean wake time of 104.0 min. After treatment, the 16 subjects had a mean wake time of 94.1 min and a standard deviation of 23.7 min. Assume that the 16 sample values appear to be from a normally distributed population and construct a 99% confidence interval estimate of the mean wake time for a population with drug treatments. What does the result suggest about the mean wake time of 104.0 min before the treatment? Does the drug appear to be effective? Construct the 99% confidence interval estimate of the mean wake time for a population with the treatment. min<μ
Answers
The mean wake time of 104.0 min before treatment is outside the 99% confidence interval of the mean wake time after treatment, it suggests that the drug is effective. This is further confirmed by the significant decrease in the mean wake time after treatment of 94.1 min. Therefore, it can be concluded that the drug is effective in treating insomnia in older subjects.
A clinical trial was conducted to test the effectiveness of a drug for treating insomnia in older subjects. Before treatment, 16 subjects had a mean wake time of 104.0 min.
After treatment, the 16 subjects had a mean wake time of 94.1 min and a standard deviation of 23.7 min.
Assume that the 16 sample values appear to be from a normally distributed population and construct a 99% confidence interval estimate of the mean wake time for a population with drug treatments.
The formula for the confidence interval of the mean is:
\($$\overline{X} \pm z_{\alpha/2} \frac{s}{\sqrt{n}}$$\)
Here,
\($z_{0.005} = 2.576$\) for a 99% confidence interval as
\($α/2 = 0.005$\)
and the degrees of freedom is 15 since \($n-1=15$\).
Now, substituting all the values:
\($$94.1 \pm 2.576 \times \frac{23.7}{\sqrt{16}}$$\)
The calculation gives a 99% confidence interval estimate of the mean wake time of 94.1 ± 15.4 min (rounded off to one decimal place).
The mean wake time of 104.0 min before treatment is not within the 99% confidence interval of the mean wake time after treatment. This indicates that there is a significant decrease in the mean wake time after treatment.
Learn more about mean wake time from the given link:
https://brainly.com/question/16032908
#SPJ11
ann swims the length of a 50 meter swimming pool in 25 seconds. what is her average speed.
this is science
Answers
Answer:
2m/s
Explanation:
2meters/second
Home can use ____to distribute warm air from a sunroom
Answers
Answer:
Solarpannels? I honestly don't know I'm just trying to be helpful.
how many iron atoms are present in 0.552 mol of iron?
Answers
Answer:
3.32 × 10²³ atomsExplanation:
The number of atoms can be found by using the formula
N = n × L
where n is the number of moles
N is the number of entities
L is the Avogadro's constant which is
6.02 × 10²³ entities
From the question we have
N = 0.552 × 6.02 × 10²³
We have the final answer as
3.32 × 10²³ atomsHope this helps you
calculate the molarity of 1.75l o2 in 0.375l h2o.
Answers
It is not possible to calculate the molarity of oxygen in water based on the given information.
To calculate the molarity of a solute in a solution, we need to know the number of moles of the solute and the volume of the solution.The problem statement provides the volume of oxygen gas (1.75 L) but does not provide information on the number of moles of oxygen gas or the volume of water.
Additionally, we would need to know if any oxygen gas has actually dissolved in the water to form a solution.Therefore, we cannot calculate the molarity of oxygen in water based on the given information.
To know more about molarity refer here
brainly.com/question/31545539#
#SPJ11
What is the electron group geometry and hybridization state of the carboxyl carbon in a carboxylate ion?
a. linear, sp3 b. tetrahedral, sp3
c. trigonal planar, sp d. tetrahedral, sp e. linear, sp f. linear, sp2 g. trigonal planar, sp h. tetrahedral, sp2 i. trigonal planar, sp
Answers
The electron group geometry of the carboxyl carbon in a carboxylate ion is tetrahedral. The hybridization state of the carbon is sp3.sp3. Therefore, the correct answer is (b) tetrahedral, sp3.
The electron group geometry and hybridization state of the carboxyl carbon in a carboxylate ion is: Trigonal planar, sp2
The concept of hybridization is defined as the process of combining two atomic orbitals to create a new type of hybridized orbitals. This intermixing typically results in the formation of hybrid orbitals with completely different energies, shapes, and so on. Hybridization is primarily carried out by atomic orbitals of the same energy level. However, both fully filled and half-filled orbitals can participate in this process if their energies are equal. The concept of hybridization is an extension of valence bond theory that helps us understand bond formation, bond energies, and bond lengths.
Visit here to learn more about hybridization : https://brainly.com/question/6986666
#SPJ11
why hydrogen and oxygen atoms are more stable when they form bonds in a water molecule?
Answers
The outer shell of electrons is full, so there is no need to gain or lose electrons.
The hydrogen and oxygen atoms are more stable in water molecules as they get a complete valence shell when they bonded with other atoms.
What is the reaction to the formation of water?In a chemical reaction, two or more reactants react with each other under the chemical condition to form two or more products. The Chemical reaction is defined as a reaction two or more reactant components react with each other to form two or more products.
The water is formed by reacting hydrogen and oxygen. The chemical reaction of water formation is shown below.
\(H_2(g)+O_2(g)\longrightarrow H_2O(l)\)
The balanced chemical equation can be defined as a reaction where the number of moles of reactants is equal to the number of moles of products.
\(H_2(g)+ \frac{1}{2}O_2(g)\longrightarrow H_2O(l)\)
The hydrogen atoms have one electron and the oxygen has two electrons less than the complete valence shell. But in the water molecule, every atom has a complete valence shell so atoms become more stable.
Learn more about water formation, here:
https://brainly.com/question/16046990
#SPJ2
Use the periodic to fill in the numbers in the electron configurations shown below.
B: 1s2 2sA2pB
A =
2
B =
1
Na: 1s22sC2pD3sE
C =
D =
E =
Answers
Answer:
B: 1s²2s²2p¹
Na: 1s²2s²2p⁶3s¹
Explanation:
A = 2
B = 1
C = 2
D = 6
E = 1
10 pts
Which elements shares similar characteristics to lithium (Li) and potassium (K)?
Answers
Answer:
sodium, rubidium, caesium, francium
Explanation:
all are group I elements, so they have the similar properties
7. Sodium + Oxygen
8. Lithium + Oxygen
9. Barium + Sulfur
10. Sodium + lodine
11. Strontium + Chlorine
12. Aluminum + Phosphorus
13. Magnesium + Sulfur
14. Magnesium + Oxygen
15. Potassium + lodine
16. Lithium + Chlorine
17 Aluminum + Astatine
18. Silver + Selenium
19 Zinc + Tellurium
20. Aluminum + Arsenic
21 What classifies the above compounds as "Type 1" compounds?
help me bukas na pasahan nito
yong formula
Answers
Answer: Burger king
Explanation: Yes
The acid dissociation constant of alloxanic acid is. Calculate the ph of a solution of alloxanic acid. Round your answer to decimal place
Answers
Answer:
The pH of a 4.4 M solution of boric acid is 4.3
Explanation:
at t=0 cM 0 0
at eqm
So dissociation constant will be:
Give c= 4.4 M and = ?
Putting in the values we get:
Also
Thus pH of a 4.4 M solution is 4.3
QUESTION 1 Before there was evidence from rocks and fossils, many scientists theorized that the continents were once joined together. Using only maps, these scientists observed that -
Answers
Using only maps, scientists observed several lines of evidence that led them to theorize that the continents were once joined together before there was evidence from rocks and fossils.
One key observation was the remarkable fit between the coastlines of different continents, such as South America and Africa. They noticed that the shapes of these continents seemed to match like puzzle pieces, suggesting they were once connected.
Additionally, scientists observed similar geological features across continents, such as mountain ranges and rock formations, that extended across apparent continental boundaries. They also noticed the distribution of certain plant and animal species that were found on different continents but had no means of natural dispersal.
These observations, made solely through maps, led scientists to propose the concept of continental drift, which was later supported by geological and paleontological evidence found in rocks and fossils.
Therefore, using only maps, scientists observed several lines of evidence that led them to theorize that the continents were once joined together before there was evidence from rocks and fossils.
for more such question on fossils
https://brainly.com/question/30503344
#SPJ8
in a complete paragraph (5+ sentences) rank the strength of attraction between the protons and valence electrons of atoms of Nitrogen, Gallium, and Arsenic. Fully EXPLAIN why you ranked them in the way you did, comparing and explaining the differences in their Coulombic attraction as you learned yesterday.
Answers
Answer:
N> As> Ga
Explanation:
There are two important factors that come into play when we are discussing the strength of attraction between protons and valence electrons;
1) distance of the valence electrons from the nucleus
2) size of the nuclear charge
If we consider the elements; nitrogen, arsenic and gallium, nitrogen is in period 2. This implies that it has only two shells. The valence electrons are closer to the nucleus and are more strongly attracted to the protons in the nucleus.
Arsenic and gallium are both in period 4. This implies that they both contain a total of 4 electron shells. However, arsenic has a nuclear charge of 33 protons while gallium has a nuclear charge of 31 protons. Hence arsenic has a greater size of nuclear charge than gallium. The attraction between protons and valence electrons in arsenic is therefore greater than it is in gallium.
what is a polar zone
Answers
Answer:
polar zone - the part of the Earth's surface forming a cap over a pole; characterized by frigid climate. Frigid Zone, polar region. climatic zone - any of the geographical zones loosely divided according to prevailing climate and latitude.
Explanation:
The polar regions, also called the frigid zones, of Earth are the regions of the planet that surround its geographical poles, lying within the polar circles.
When referring to immunity, what does the term innate imply?
the mechanism will provide defense against many different types of pathogens
the mechanism will develop based upon exposure to specific pathogens
the mechanism will be built-in and present at birth
the mechanism will be acquired over an individual's lifetime
Answers
Answer:
The answer should be A. the mechanism will provide defense against many different types of pathogens :)
Have an amazing day!!
Please rate and mark brainliest!!
Is the following an element, compound, homogeneous mixture, or heterogeneous mixture?
Coca-cola
Answers
Answer:
CO2 is classified as a compound
Explanation:
Who was the Russian chemist that arranged the elements of the periodic table in order of increasing atomic mass?
Answers
Answer:Dmitri Mendeleev
Explanation:
Salt is a compound made from sodium (Na) and chlorine (Cl). Salt _____.
has properties very similar to sodium
has properties very similar to chlorine
has very different properties from sodium and chlorine
Answers
Answer:
has very different properties from sodium and chlorine
Explanation:
We know that salt (chemical name: Sodium Chloride) is made from two elements, Sodium (Na) and Chlorine (Cl). But the properties of the compound of sodium and chlorine has completely different properties than those of its elements: Sodium and Chlorine.
Sodium is quite a low density but highly reactive silvery white metal and Chlorine is a highly reactive greenish yellow gas. While salt (NaCl) is a crystalline material, white in color, which readily dissolves in water and is used as a condiment to add flavor to food.
plz mark me as a brainlest
Calculate the amount of heat needed to convert 230.0g of ice at -10C to water at 0C
Answers
Answer:
It would take approximately 81.65 kJ (or 81,650 J) of heat to convert 230.0g of ice at -10C to water at 0C.
Explanation:
To calculate the amount of heat needed to convert ice at -10°C to water at 0°C, we need to consider two steps:
1. Heating the ice from -10°C to 0°C (heat required to raise the temperature of ice)
2. Melting the ice into water at 0°C (heat required to change the state of ice)
Let's first calculate the heat required for step 1:
Q1 = m × c × ΔT
where Q1 is the heat required, m is the mass of the ice, c is the specific heat capacity of ice, and ΔT is the change in temperature.
The specific heat capacity of ice is 2.09 J/g°C, and ΔT is (0°C - (-10°C)) = 10°C.
So, Q1 = 230.0 g × 2.09 J/g°C × 10°C = 4827 J
Now, let's calculate the heat required for step 2:
Q2 = m × Lf
where Q2 is the heat required, m is the mass of the ice, and Lf is the latent heat of fusion of ice.
The latent heat of fusion of ice is 334 J/g.
So, Q2 = 230.0 g × 334 J/g = 76820 J
Therefore, the total amount of heat needed to convert 230.0g of ice at -10C to water at 0C is:
Qtotal = Q1 + Q2 = 4827 J + 76820 J = 81647 J
Therefore, it would take approximately 81.65 kJ (or 81,650 J) of heat to convert 230.0g of ice at -10C to water at 0C.
Use the equation to answer the question.
H2O(l) + heat ⇄ H2O(g)
A sample of water is at equilibrium at 100°C. Which statement best describes what will happen if liquid water is added to the system?
-More liquid water molecules will change to water vapor until a new equilibrium is reached.
-All of the liquid water molecules that are added will become water vapor.
-More water vapor molecules will change to liquid water until a new equilibrium is reached.
-All of the liquid water molecules that are added will remain liquid water.
I need help on this quick check ASAP!
Answers
Answer:
A: More liquid water molecules will change to water vapor until a new equilibrium is reached.
Explanation:
I'm not good at explaining but I took the quick check and this answer was correct b)
The increase in liquid water thus results in the more formation of water vapor, for reaching new equilibrium. Thus, option A is correct.
What is an equilibrium?Equilibrium is the condition of the equal concentration of the product and reactant in the chemical equation. The reaction at equilibrium has constant rate.
The increase in the concentration of the product or reactant results in the distortion of the equilibrium condition, and the reaction will be processes in the forward or backward direction, according to equilibrium principle.
The addition of liquid water molecules to the reaction results in the increase in the concentration of the reactants. The reaction starts approaching the equilibrium until the concentration of liquid water, and gaseous water become same.
The increase in liquid water thus results in the more formation of water vapor, for reaching new equilibrium. Thus, option A is correct.
Learn more about equilibrium, here:
https://brainly.com/question/13524990
Which of the following terms best describes body fossils?
Answers
Answer:
Rare.
Explanation:
The pressure and temperature inside a bike tire is 10 atm and 10 K respectively. What will the pressure become in the tire when the temperature is increased 20 k?
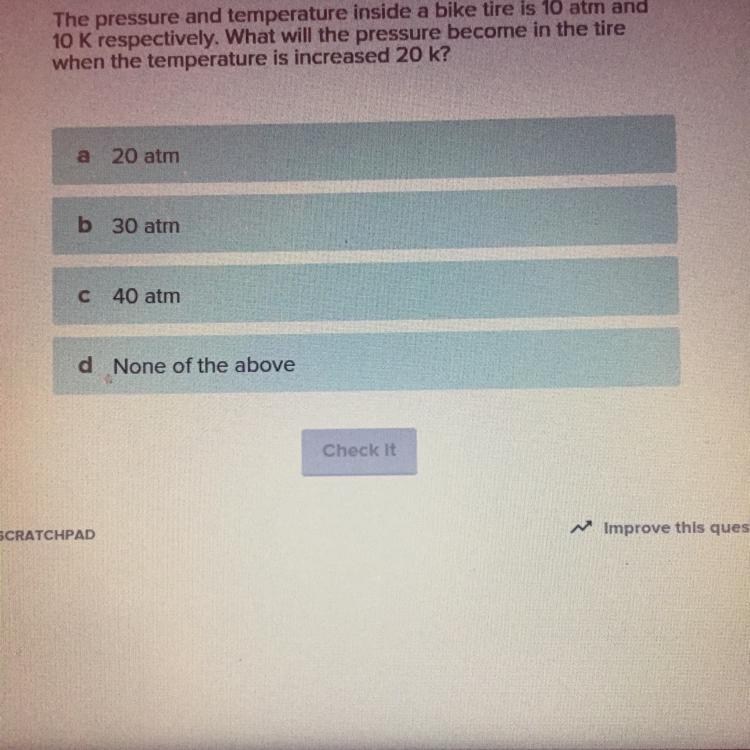
Answers
Answer:20 atm
Explanation:
write the chemical symbol of the element that makes up most of earth's core.
Answers
The chemical symbol for the element that makes up most of Earth's core is Fe, which stands for iron. Iron makes up approximately 85% of the Earth's core, with the remainder being nickel and other trace elements.
The high abundance of iron in the Earth's core is responsible for the planet's magnetic field, which is essential for life on Earth as it helps to protect us from harmful solar radiation. The study of the Earth's core and its composition is important for understanding the planet's history and evolution, as well as for predicting future changes in the Earth's magnetic field. The element that makes up most of Earth's core is iron. Its chemical symbol is Fe, which is derived from the Latin word "ferrum." Iron is the primary component of the inner and outer core, accounting for about 85% of its composition. This element plays a vital role in Earth's geophysics, including the generation of the planet's magnetic field.
To know about nickel:
https://brainly.com/question/3039765
#SPJ11
Use the model for double replacement reactions to predict the products for the following reactants. Circle the ionic solution and underline the other compound.
Ca(OH2(aq) + 2HCl(aq) —->
Answers
The double replacement reaction generally results in the formation of precipitate. Here the products of the given reaction are CaCl₂ and H₂O and the ionic solution is CaCl₂.
What is double replacement reaction?The chemical reaction in which two chemical substances react by exchanging the ions to form two new molecules. In this reactions, positive ions exchange negative ions. A double displacement is also called the double decomposition reaction.
In general the reaction can be given as:
AB + CD → AD + CB
The reaction of Ca (OH)₂ with HCl is:
Ca (OH)₂ + 2HCl → CaCl₂ + 2 H₂O
Thus here the given reaction is double displacement, CaCl₂ is the ionic compound and other compound is water.
To know more about double displacement, visit;
https://brainly.com/question/13870042
#SPJ1
What happens to particles when their energy levels decrease
ASAP
Answers
Your answer ✌
Answer:
The kinetic theory of matter can be used to explain how solids, liquids and gases are interchangeable as a result of increase or decrease in heat energy. If it is cooled the motion of the particles decreases as they lose energy.
Explanation:
This is the last one I need. Just want to make sure I did it right.

Answers
To combine ions to form ionic compounds, we need the combine in such a way that it gets neutral charge.
We can combine each anion with each cation to get the 4 compounds we need.
To combine SO₄²⁻ with Pb⁴⁺ we first find the Least Common Multiple of their charges, 2 and 4.
They have the factor 2 in common, so the LCM is 4. This is the final charge of each that will cancel out.
To get 4+, we only need 1 Pb⁴⁺.
To get 4-, we need 2 SO₄²⁻.
So, the formula is:
Pb(SO₄)₂
To combine SO₄²⁻ with NH₄⁺ is easier because one of them has single charge. In this case, we can simply pick one of the multiple charge ion and the same amount that will cancel its charge of the single charged one.
So, we picke 1 SO₄²⁻, ending with 2-.
And we picke 2 NH₄⁺, ending with 2+.
The formula:
(NH₄)₂SO₄
To combine C₂H₃O₂⁻ with Pb⁴⁺ we do the same, because the anion is single charged.
Pick 1 Pb⁴⁺, ending with 4+.
Pick 4 C₂H₃O₂⁻, ending with 4-.
The formula:
Pb(C₂H₃O₂)₄
To combine C₂H₃O₂⁻ with NH₄⁺, both have same charge, so we just need one of each and their charges will cancel out.
The formula:
NH₄C₂H₃O₂
So, the formulas are:
Pb(SO₄)₂
(NH₄)₂SO₄
Pb(C₂H₃O₂)₄
NH₄C₂H₃O₂