In Section 5.6, we learned that triple bonds are stronger and shorter than single bonds. For example, a C- Csingle bond has an average bond energy of 347 k.J/mole, while a C C triple bond has an average bond energy of 837 k.J/mole. Use valence bond theory to explain why a triple bond is not simply three times as strong as a single bond.
A) The C C triple bond is an exception. According to valence bond theory, a triple bond is generally three times as strong as a single bond. B) Because according to valence bond theory, a triple bond is the sharing of four electron pairs.
C) Because according to valence bond theory, a triple bond is actually composed of two different kinds of bonds, one σ and two π
Answers
Answer:
Because according to valence bond theory, a triple bond is actually composed of two different kinds of bonds, one σ and two π
Explanation:
The respective bond a energies of C-C single, double and triple bonds are; 347KJmol-1, 681 KJmol-1 and 837 KJmol-1 respectively.
It can be seen that the bond energy of a triple bond is really not thrice the bond energy of a C-C single bond. This is because, a triple bond is composed of two different kinds of bonds. A C-C sigma bond and two C-C pi bonds. The C-C sigma bonds are stronger than the C-C pi bonds because sigma bonds result from a greater degree of overlap between the atomic orbitals involved in the bond.
Hence, the C-C triple bond energy is not simply three times that of the C-C single bond energy since there are actually two different kinds of bonds present as explained above.
A triple bond is generally three times as strong as a single bond because according to valence bond theory, a triple bond is actually composed of two different kinds of bonds, one σ and two π.
What is a Bond?This is the type of attraction which occurs between atoms, ions etc and facilitate the formation of new compounds. The respective bond energies of C-C single, double and triple bonds are given as 347KJmol-1, 681 KJmol-1 and 837 KJ mol-1 respectively.
A triple bond however consists of two different kinds of bonds. A C-C sigma bond and two C-C pi bonds in which the former is stronger which was why option C was chosen as the most appropriate choice.
Read more about Bond here https://brainly.com/question/1974529
Related Questions
A balloon that can hold 85L of air is inflated with 3.5 moles of gas at a pressure of 1.0 atmosphere.What is the tempreture in degree celcius of the balloon?
Answers
T
=
PV
nR
=
1.0 atm × 0.5 L
3.5 mol × 0.0821 L atm / (mol K)
=
1.74 K
We have that for the Question "A balloon that can hold 85L of air is inflated with 3.5 moles of gas at a pressure of 1.0 atmosphere.What is the temperature in degree Celsius of the balloon?" it can be said that the temperature in degree Celsius of the balloon is
T=296K
T=23 Celsius
From the question we are told
A balloon that can hold 85L of air is inflated with 3.5 moles of gas at a pressure of 1.0 atmosphere.What is the temperature in degree Celsius of the balloon?
Generally the equation for a is ideal Gas is mathematically given as
PV=nRT
Therefore
PV=nRT
1*85=3.5*0.082*T
T=296K
Hence
the temperature in degree Celsius of the balloon is
T=296K
T=23 Celsius
For more information on this visit
https://brainly.com/question/17756498
A compound is found to contain 9.227 % boron and 90.77 % chlorine by mass. What is the empirical formula for this compound?
Answers
Assuming a 100 g sample of the compound, we can convert the mass percentages to masses in grams:
- 9.227 g B
- 90.77 g Cl
Next, we need to convert these masses to moles using the atomic masses of the elements:
- B: 10.81 g/mol
- Cl: 35.45 g/mol
- 9.227 g B ÷ 10.81 g/mol = 0.853 mol B
- 90.77 g Cl ÷ 35.45 g/mol = 2.562 mol Cl
Now we need to divide both mole values by the smaller of the two, which is 0.853 mol:
- 0.853 mol B ÷ 0.853 mol = 1.000 mol B
- 2.562 mol Cl ÷ 0.853 mol = 3.000 mol Cl
This gives us a B:Cl ratio of 1:3. The empirical formula for the compound is therefore BCl3.
Answer:
Empirical formula of a compound means that it provides simplest ratio of whole number.
Explanation:
Mass of boron and chlorine is 9.224% and 90.74%
3. How many grams of oxygen are required to completely react with 240g of C₂H6?
Answers
Approximately 766.08 grams of oxygen are required to completely react with 240g of C₂H₆.
To determine the amount of oxygen required to completely react with 240g of C₂H₆ (ethane), we need to set up a balanced chemical equation for the combustion of ethane.
The balanced equation for the combustion of ethane is as follows:
C₂H₆ + O₂ → CO₂ + H₂O
From the balanced equation, we can see that the stoichiometric ratio between C₂H₆ and O₂ is 1:3. This means that for every one mole of C₂H₆, three moles of O₂ are required for complete combustion.
To calculate the amount of oxygen required, we need to convert the given mass of C₂H₆ to moles using its molar mass, and then use the stoichiometric ratio to determine the moles of O₂ required. Finally, we can convert the moles of O₂ back to grams using the molar mass of oxygen.
The molar mass of C₂H₆ is calculated as follows:
(2 x atomic mass of carbon) + (6 x atomic mass of hydrogen)
(2 x 12.01 g/mol) + (6 x 1.01 g/mol) = 30.07 g/mol
Now, we can proceed with the calculation:
Calculate the moles of C₂H₆:
moles of C₂H₆ = mass of C₂H₆ / molar mass of C₂H₆
moles of C₂H₆ = 240 g / 30.07 g/mol ≈ 7.98 mol
Determine the moles of O₂ using the stoichiometric ratio:
moles of O₂ = moles of C₂H₆ x (3 moles of O₂ / 1 mole of C₂H₆)
moles of O₂ = 7.98 mol x 3 ≈ 23.94 mol
Convert moles of O₂ to grams:
mass of O₂ = moles of O₂ x molar mass of O₂
mass of O₂ = 23.94 mol x 32.00 g/mol = 766.08 g
For more such questions on oxygen visit:
https://brainly.com/question/28009615
#SPJ8
atomic size of an atom is decided by_________
Answers
Answer:
The edge of its orbital
Explanation:
The size of an atom is defined by the edge of its orbital. However, orbital boundaries are fuzzy and in fact are variable under different conditions
Calculate the ΔH for the reaction 4 CO2 (g) + 2 H2O (g) → 2 C2H2 (g) +5 O2 (g), from the following:C2H2 (g) + 2 H2 (g) → C2H6 (g)ΔH = – 94.5 kJH2O (g) → H2 (g) + ½ O2 (g)ΔH = + 241.8 kJC2H6 (g) + 3 ½ O2 (g) → 2 CO2 (g) + 3 H2O (g)ΔH = – 283 kJ

Answers
Answer:
Explanation:
Here, we want to calculate the change in enthalpy of the given reaction
To get that, we are going to use the 3 related equations below it
We proceed as follows:
\(\begin{gathered} We\text{ multiply equation ii by 3} \\ The\text{ heat becomes } \\ 3\text{ }\times\text{ 241.8 = +725.4 KJ} \end{gathered}\)We arrange equation 1 in the opposite direction
This makes the heat become:
\(+94.5\text{ KJ}\)For equation iii, we arrange in opposite direction too
The heat becomes:
\(-725.4\text{ KJ}\)We can now begin to make additions and subtractions as follows:
\(undefined\)Why are the united nation members upset with Wakanda?
Answers
The "Wakanda speech," six weeks have passed, and the world is still in shock.
Thus, Global leaders and analysts were taken aback by King T'Challa's declaration at the United Nations General Assembly that the Kingdom of Wakanda is not a developing country of textiles, farms, and shepherds with a GDP per person of roughly $760 but rather a technological superpower and Wakanda speech.
The country's widespread employment of cutting-edge magnetic levitation trains, flying machines, opaque holograms, and spinal cord-healing beads has led to the coining of the phrase "uber-developed" nation.
The most watched video ever is currently "Welcome to the Future," an introduction video created by Wakanda's recently established Ministry of Foreign Affairs.
Thus, The "Wakanda speech," six weeks have passed, and the world is still in shock.
Learn more about Wakanda, refer to the link:
https://brainly.com/question/31162803
#SPJ1
Which of these represents a wave with the highest energy?
Answers
Answer:Gamma rays have the highest energy and shortest wavelength. Then come X-rays, ultraviolet light, visible light, infrared radiation and microwave radiation.
Explanation:
3. Given 20g of Barium Hydroxide, how many grams of
Ammonium Nitrate would it take for all the Barium Hydroxide
to react? Balance the chemical equation first
Answers
The number of grams of ammonium nitrate (NH₄NO₃) it would take for all the barium hydroxide to react is 18.7g
First, we will write the balanced chemical equation for the reaction
The balanced chemical equation for the reaction is
Ba(OH)₂ + 2NH₄NO₃ → 2NH₄OH + Ba(NO₃)₂
This means, 1 mole of barium hydroxide is required to react with 2 moles of ammonium nitrate
Now, we will calculate the number of moles of barium hydroxide present.
Mass of barium hydroxide (Ba(OH)₂) = 20 g
Using the formula
\(Number\ of\ moles = \frac{Mass}{Molar\ mass}\)
Molar mass of Ba(OH)₂ = 171.34 g/mol
∴ Number of moles of Ba(OH)₂ present =\(\frac{20}{171.34}\)
Number of moles of Ba(OH)₂ present = 0.116727 mole
Now,
Since 1 mole of barium hydroxide is required to react with 2 moles of ammonium nitrate
Then,
0.116727 mole of barium hydroxide will react with 2 × 0.116727 mole of ammonium nitrate
2 × 0.116727 = 0.233454 mole
∴ Number of moles of NH₄NO₃ required is 0.233454 mole
Now, for the mass of ammonium nitrate (NH₄NO₃) required
From the formula
Mass = Number of moles × Molar mass
Molar mass of NH₄NO₃ = 80.043 g/mol
∴ Mass of NH₄NO₃ required = 0.233454 × 80.043
Mass of NH₄NO₃ required = 18.68636 g
Mass of NH₄NO₃ required ≅ 18.7g
Hence, the number of grams of ammonium nitrate (NH₄NO₃) it would take for all the barium hydroxide to react is 18.7g
Learn more on determining mass of reactant required here: https://brainly.com/question/11232389
does anyone know this??

Answers
Answer: C2H6O
Explanation: It is C2H6O with a molar mass of 46.07 g/mol.
How many Moles of glucose are produced from 12 moles of CO2 in photosynthesis?
Answers
Answer:
2 MOLES OF GLUCOSE
Explanation:
How many Moles of glucose are produced from 12 moles of CO2 in photosynthesis?
6CO2 +6H20-------------->C6H12O6 -+ 6O20
(IN THE PRESENCE OF CHLOROPHYLL AND UV FROM )
)
6 MOLES OF CO2 MAKE 1 MOLE OF GLUCOSE
12 MOLES THEN MAKE 2 MOLES OF GLUCOSE
In the photosynthesis process, plants can use 12 moles of carbon dioxide to form 2 moles of glucose.
What is photosynthesis?It is the process by which green plants and some other organisms convert light energy into chemical energy.
Let's consider the balanced equation for the synthesis of glucose through photosynthesis.
6 CO₂ + 6 H₂O ⇒ C₆H₁₂O₆ + 6 O₂
The molar ratio of CO₂ to C₆H₁₂O₆ is 6:1. The moles of glucose formed from 12 moles of carbon dioxide are:
12 mol CO₂ (1 mol C₆H₁₂O₆/6 mol CO₂) = 2 mol C₆H₁₂O₆
In the photosynthesis process, plants can use 12 moles of carbon dioxide to form 2 moles of glucose.
Learn more about photosynthesis here: https://brainly.com/question/3529377
PLEASE HELP ME THERES 100 POINTS ON THE LINE


Answers
Answer:
1.93 2.86
Explanation:
exactly the reason for the above text
Which phrase describes one type of freshwater wetland?
2
acidic areas found in northern climates
reservoirs created by dams at the end of rivers
areas of high elevation that feed into river systems
small streams that flow into larger streams and wetlands
Answers
Answer:
The correct answer is D
Explanation:
Wetlands are environments that are either submerged under water or are frequently wet. Marshes, swamps, bogs, and fens are examples of freshwater wetlands. Here small streams flow into larger streams and wetlands. The correct option is D.
Ecosystems in freshwater wetlands are impacted by transient or ongoing flooding. They are essential habitats for fauna (including migratory species), play a significant role in the regulation of water flow and water quality to entire catchments, and offer shelter for fauna during droughts.
In tropical and subtropical areas, mangrove swamps are coastal wetlands. In brackish to saline tidal waters, they are distinguished by halophytic (salt-loving) trees, shrubs, and other plants.
Thus the correct option is D.
To know more about wetlands, visit;
https://brainly.com/question/11438518
#SPJ6
If you evaporated 150. mL of a 3.5 M solution of iron (II) nitrite, how many moles of iron (II) nitrite would you recover?
Answers
Taking into account the definition of molarity, you would recover 0.525 moles of iron (II) nitrite.
Definition of molarityMolar concentration or molarity indicates the number of moles of solute that are dissolved in a given volume.
The molarity of a solution is calculated by dividing the moles of solute by the volume of the solution:
molarity= number of moles÷ volume
Molarity is expressed in units moles/L.
Moles of iron (II) nitriteIn this case, you have:
Molarity= 3.5 MNumber of moles= ?Volume= 150 mL= 0.150 L (being 1000 mL= 1 L)Replacing in the definition of molarity:
3.5 M= number of moles÷ 0.150 L
Solving:
3.5 M× 0.150 L= number of moles
0.525 moles= number of moles
Finally, you would recover 0.525 moles.
Learn more about molarity:
brainly.com/question/9324116
#SPJ1
Why is there no isotactic or syndiotactic form ofpolyethylene?
Answers
Answer: There is no isotactic or syndiotactic form of polyethylene.
Explanation: Polyethylene is a type of polymer that is made up of repeating units of ethylene monomers. The polymer chains in polyethylene are arranged randomly, without any specific stereochemistry, which means that there is no isotactic or syndiotactic arrangement of the polymer chains. The lack of stereochemistry in polyethylene results in a material that is amorphous and has low crystallinity, which gives it unique mechanical and physical properties that make it useful for a wide range of applications.
The alkyl halide shown below undergoes an elimination reaction. Select all possible elimination products from the given structures provided below. (1 pts)}
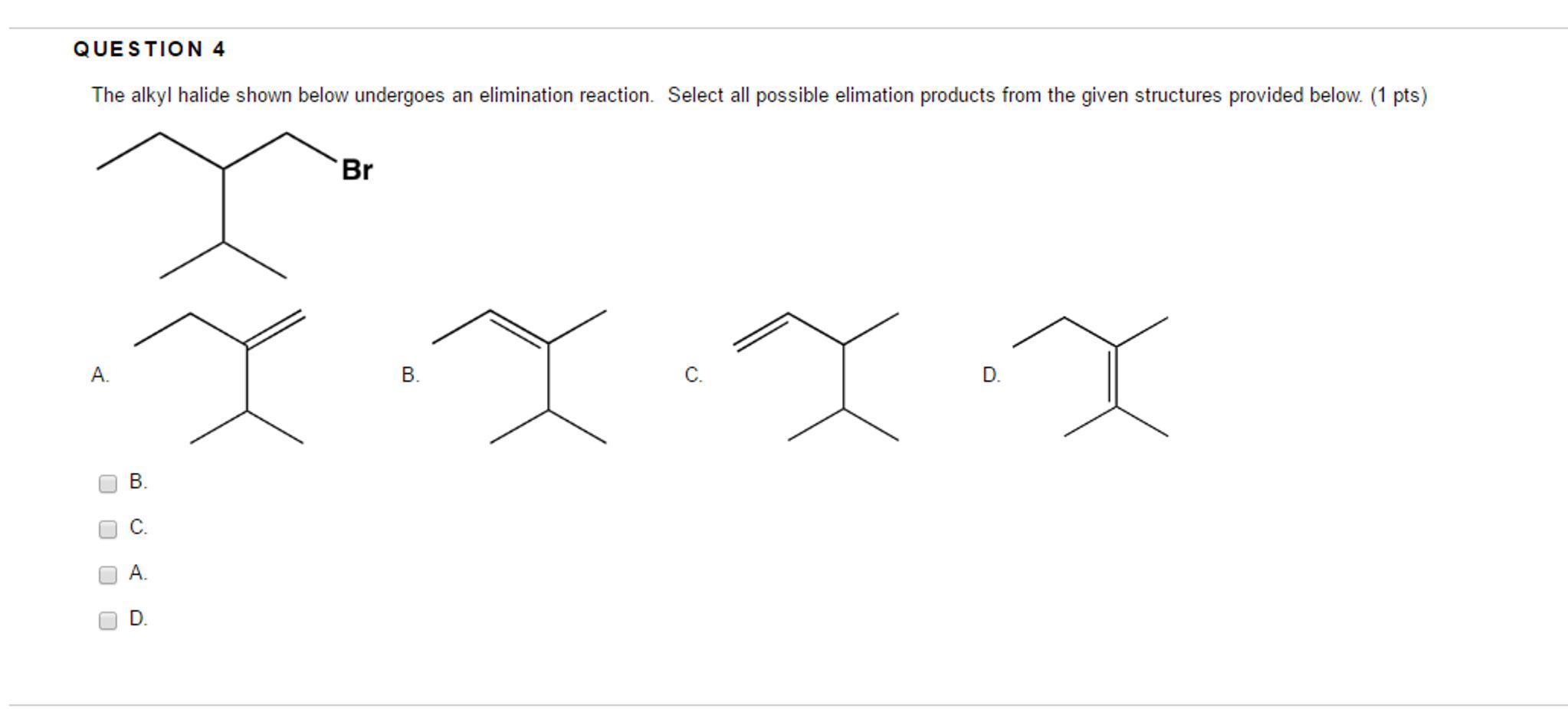
Answers
OPTION A and OPTION D are the possible products from the given structures provided.
Alkyl Halides: What Are They?
Alkyl halides are chemical compounds that are frequently generated from alkanes that include one or more halogens. They are also known as haloalkanes or halogenoalkanes. Alkyl halides are also a part of the larger category of halocarbons.What does alkyl halide mean?
An alkyl halide, as its name indicates, is a hydrocarbon molecule in which halogens stand in for one or more hydrogen atoms. Fluorine, chlorine, bromine, and iodine are among them. R-X, where X is a halogen and R is an alkyl group, is the general structure.What varieties of alkyl are there?
Make sure you understand the definitions of and use for the essential phrases listed below.Alkyl group, methyl group, isobutyl group, sec-butyl group, tert-butyl group, primary carbon, and secondary carbon.To know more about alkyl checkout this link:
https://brainly.com/question/6419720
#SPJ4
The following series of reactions were carried out.
PbCO3(s) + 2HNO3(aq) → Pb(NO3)2(aq) + H₂O(1) + CO₂(g)
Pb(NO3)2(aq) + 2HBr(aq) → 2HNO3(aq) + PbBr2(s)
(a) If a student starts with 2.457 g of lead(II) carbonate for the first reaction and all
other reagents are added in excess, what is the theoretical yield of lead(II) bromide
solid?
Answers
First, let's calculate the molar mass of PbCO3:
PbCO3: Pb (207.2 g/mol) + C (12.0 g/mol) + 3O (16.0 g/mol) = 267.2 g/mol
Next, we can calculate the number of moles of PbCO3:
moles = mass / molar mass = 2.457 g / 267.2 g/mol ≈ 0.00919 mol
From the balanced equation, we can see that the mole ratio between PbCO3 and PbBr2 is 1:1. Therefore, the moles of PbBr2 formed will be the same as the moles of PbCO3 used.
The molar mass of PbBr2 is:
PbBr2: Pb (207.2 g/mol) + 2Br (2 × 79.9 g/mol) = 366.0 g/mol
Now, we can calculate the theoretical yield of PbBr2:
theoretical yield = moles of PbBr2 × molar mass of PbBr2
= 0.00919 mol × 366.0 g/mol
≈ 3.36 g
Therefore, the theoretical yield of lead(II) bromide (PbBr2) solid is approximately 3.36 grams.
]All organic compounds contain the element carbon but, not all compounds containing the element “carbon”are organic .Justify this statement.
Answers
The statement "All organic compounds contain the element carbon, but not all compounds containing the element 'carbon' are organic" can be justified based on the definition and characteristics of organic compounds.
Organic compounds are compounds primarily composed of carbon and hydrogen atoms, often with other elements like oxygen, nitrogen, sulfur, and phosphorus. These compounds are typically associated with living organisms and are known for their unique properties and behavior, including the ability to form complex structures, exhibit covalent bonding, and undergo organic reactions.
On the other hand, there are compounds that contain carbon but are not classified as organic. One notable example is carbon dioxide (\(CO_{2}\)), which is a simple inorganic compound composed of carbon and oxygen. Carbon dioxide does not possess the characteristic properties of organic compounds, such as the ability to form long chains or undergo organic reactions.
Additionally, there are inorganic compounds like carbonates (such as calcium carbonate) and carbides (such as calcium carbide) that contain carbon but are not considered organic. These compounds have distinct chemical and physical properties different from those of organic compounds.
In summary, while all organic compounds contain carbon, not all compounds containing carbon are organic. The classification of a compound as organic or inorganic depends on its overall molecular structure, bonding, and characteristic properties.
Know more about molecular structure here:
https://brainly.com/question/27789666
#SPJ8
Photoelectric effect will occur only if frequency of light striking an electron in a metal is above a certain threshold frequenci
Answers
The statement is correct. The photoelectric effect refers to the phenomenon where electrons are ejected from the surface of a material when it is exposed to light. The frequency of light striking an electron in a metal must be above the threshold frequency in order for the photoelectric effect to occur.
The statement is correct. The photoelectric effect refers to the phenomenon where electrons are ejected from the surface of a material when it is exposed to light. However, for the photoelectric effect to occur, the frequency of the incident light must be above a certain threshold frequency.
The threshold frequency is the minimum frequency of light required to dislodge electrons from the material. Below this threshold frequency, regardless of the intensity or duration of the light, no electrons will be emitted.
This behavior can be explained by the particle-like nature of light, where light is composed of discrete packets of energy called photons. The energy of a photon is directly proportional to its frequency. Only photons with energy greater than or equal to the binding energy of the electrons in the material can dislodge them.
Therefore, the frequency of light striking an electron in a metal must be above the threshold frequency in order for the photoelectric effect to occur.
For more question on photoelectric
https://brainly.com/question/1458544
#SPJ8
List the three subatomic particles. Give the relative masses and charges of each (sorry for putting it in college TvT)
Answers
Answer:
Explanation:
The electron is subatomic particle that revolve around outside the nucleus and has negligible mass. It has a negative charge.
relative charge = -1
Relative mass = 1.1836
It was discovered by j. j. Thomson in 1897 during the study of cathode ray properties.
He constructed the glass tube and create vacuum in it. He applied electric current between electrodes. He noticed that a ray of particles coming from cathode to wards positively charged anode. This ray was cathode ray.
Properties of cathode ray:
The ray is travel in straight line.
The cathode ray is independent of composition of cathode.
When electric field is applied cathode ray is deflected towards the positively charged plate.
Hence it was consist of negatively charged particles.
While neutron and proton are present inside the nucleus. Proton has positive charge while neutron is electrically neutral. Proton is discovered by Rutherford while neutron is discovered by James Chadwick in 1932.
Relative mass of proton= 1
Relative mass of neutron = 1
Relative mass of proton = +1
Relative charge of neutron = 0
The number of electron or number of protons are called atomic number while mass number of an atom is sum of protons and neutrons. The umber of protons and electrons are always equal to make the atom electrically neutral.
Select the correct answer
What determines the average kinetic energy of the particles in a gas?
ОА
the number of collisions
OB.
the number of particles
OC. the size of the particles
OD. the temperature
Answers
Answer:
Temperature
Explanation:
Kinetic energy of gass molecules is directly propotional to the temperature.
What volume of CO2(g), measured at STP is produced if 15.2 grams of CaCO(s) is heated?
Answers
Answer:
Volume = 3.4 L
Explanation:
In order to calculate the volume of CO₂ produced when 15.2 g of CaCO₃ is heated, we need to first write out the balanced equation of the thermal decomposition of CaCO₃:
CaCO₃ (s) + [Heat] ⇒ CaO (s) + CO₂ (g)
Now, let's calculate the number of moles in 15.2 g CaCO₃:
mole no. = \(\mathrm{\frac{mass}{molar \ mass}}\)
= \(\frac{15.2}{40.1 + 12 + (16 \times 3)}\)
= 0.1518 moles
From the balanced equation above, we can see that the stoichiometric molar ratios of CaCO₃ and CO₂ are equal. Therefore, the number of moles of CO₂ produced is also 0.1518 moles.
Hence, from the formula for the number of moles of a gas, we can calculate the volume of CO₂:
mole no. = \(\mathrm{\frac{Volume \ in \ L}{22.4}}\)
⇒ \(0.1518 = \mathrm{\frac{Volume}{22.4}}\)
⇒ Volume = 0.1518 × 22.4
= 3.4 L
Therefore, if 15.2 g of CaCO₃ is heated, 3.4 L of CO₂ is produced at STP.
Explain what you discovered from the Simulation about why food coloring spreads faster in warmer water.
Answers
Answer:
the water molecules have more energy and are moving faster than the molecules of cold water. This makes it easier for the dye to get mixed throughout the hot water.
Explanation:
Answer:
Because particles moves more by kinetic energy and diffusion will spread with the particles
Explanation:
Consider a hypothetical gas which has the following Van der Waals constants:
a = 2.34 L2 bar mol-2
b = 0.0 L mol-1
Under conditions of very high pressures, what would you predict about the experimental volume of the gas?
The volume would be lower than predicted by the Ideal Gas Law
The volume would be higher than predicted by the Ideal Gas Law
The volume would be exactly the same as predicted by the Ideal Gas Law
Answers
Under conditions of very high pressures, the volume would be lower than predicted by the Ideal Gas Law.
According to the kinetic theory of gases, a gas spreads out to fill the volume of the container holding it. Hence a gas does not have a definite volume due to the fact that there is no inter-molecular interaction between gas molecules.
When the gas is subjected to very high pressure, inter-molecular interactions become significant leading to a decrease in the volume of the gas.
Therefore, when subjected to high pressure, the experimental volume would be lower than predicted by the Ideal Gas Law.
Learn more: https://brainly.com/question/21443108
0.2g of sand in two-third in liter of ethanol . What is the concentration in g per dm cube
Answers
The mass concentration of sand in the ethanol solution is 0.299 g/dm³.
What is the concentration in grams per dm³?To find the concentration in grams per cubic decimeter (g/dm³), we first need to convert the volume from liters to cubic decimeters (dm³). Since 1 liter is equal to 1 cubic decimeter, we can directly convert the volume.
Given:
Mass of sand = 0.2 g
Volume of ethanol = two-thirds liter
Converting volume to dm³:
1 liter = 1 cubic decimeter
two-thirds liter = (2/3) cubic decimeter = 0.67 dm³ (rounded to two decimal places)
Now we can calculate the concentration in g/dm³ by dividing the mass of sand by the volume in dm³:
Concentration = Mass / Volume
Concentration = 0.2 g / 0.67 dm³
Concentration ≈ 0.299 g/dm³ (rounded to three decimal places)
Learn more about mass concentration at: https://brainly.com/question/23437000
#SPJ1
2.59 Using the periodic table to guide you, predict the chemical formula and name of the compound formed by the following elements: (a) Ga and F, (b) Li and H, (c) Al and I, (d) K and S.
Answers
Answer:
(a) GaF3, gallium(III) fluoride
(b) LiH, lithium hydride
(c) AlI3, aluminum(III) iodide
(d) K2S, potassium sulfide
2 Al(s) + Fe2O3(aq) - AlO3(aq) + 2 Fe(s)
You react 20.00 grams of aluminum with iron(III) oxide. How many grams of iron should you produce?
What is the percent yield if the experimental yield is 32.67 grams of iron?
Answers
Answer:
78.8%
Explanation:
The equation of the reaction is;
2 Al(s) + Fe2O3(aq) ------> Al2O3(aq) + 2Fe(s)
Number of moles in 20g of Al= 20g/27 g/mol = 0.74 moles
From the reaction equation;
2 moles of Al yields 2 moles of Fe
0.74 moles of Al yields 0.74 moles of Fe
Hence;
Mass of Fe produced = 0.74 moles of Fe * 56 g/mol
Mass of Fe produced = 41.44 g of Fe (This is the theoretical yield of Fe)
percent yield = actual yield/ theoretical yield * 100
actual yield = 32.67 grams of iron
percent yield = 32.67 g/41.44 g * 100
percent yield = 78.8%
Part H Why is the number of hydrogen atoms and oxygen atoms the same in both the reactants and the products?
Answers
Answer:
It's because they aren't destroyed they're just rearranged differently to create different products. There's like a name for this. It's something like the law of conservation energy. But the reason is mass cannot be created nor can it be destroyed.
Answer:
Atoms don’t appear or disappear during the reaction. Instead, they get rearranged.
Explanation:
Exact answer from Plato/Edmentum!! :)
How many H atoms are in 0.170 mole of ammonium sulfate?
Answers
Answer:
8
Explanation:
Which process is most likely responsible for the formation of limestone caves?(1 point)
Responses
abrasion
abrasion
carbonation
carbonation
hydrolysis
hydrolysis
oxidation
Answers
1- Unit of life is _______,
2- __________ with similar structure and function are called _______________.
3-Similar ___________ when working together they make up ________________.
4- when similar ___________ are processing the same type of molecules are called ______________ .
5- different ____________ together make up a ___________________.
Word bank: body systems human body cells organs body systems 3- list different types of body systems learned in this unit along with their functions. 4- Cellular respiration
Cellular respiration happens in ________________ of our _____________ cells.
During cellular respiration ___________ and ___________ molecules are used, and _____________, __________________, and _________________ gas are produced.
5- Why does the process of cellular respiration happen continuously in our bodies?
6- What cells are needed for cellular growth and repair, and where energy comes from?
Complete the passage to describe how electrons are represented in electron dot diagrams.
The maximum number of dots an electron dot diagram can have is
. The maximum number of dots drawn on a side of a chemical symbol in an electron dot diagram is
.
Answers
The maximum number of dots an electron dot diagram can have is 8.
The maximum number of dots drawn on the side of a chemical symbol in an electron dot diagram is 2.
Electron dot diagramsThe electron dot diagrams is otherwise known as Lewis electron dot diagrams, Lewis structure, or simple Lewis diagram. It is a diagrammatic representation of the valence electrons of atoms using dots to represent electrons around the symbols of atoms.
The valence electron of an atom is the number of electrons present in the outermost shell of the atom. According to the rules, the first shell of an atom can take a maximum of 2 electrons while the remaining shells can take a maximum of 8 electrons each.
Atoms with 8 electrons in their outermost shells are said to be in an octet state.
The number of dots an atom will have around it will, therefore, depend on the number of valence electrons it has. As a rule of thumb, each side of an atom can take a maximum of 2 electrons. Thus, an atom with 8 valence electrons will have 2 dots each on its 4 sides.
More on Lewis dot structure can be found here: https://brainly.com/question/20300458
#SPJ1
Answer:
the first is 8 an the second one is 2
Explanation: