In the figure displayed, what is the temperature and pressure of the critical point?Phase diagram. The x axis is labeled Temperature degrees Celsius, and increases by increments of 40, starting at negative 80 and ending at 760. The y axis is labeled Pressure (atm), and increases by increments of 1, beginning at 0 and ending at 15. There is a curved line that starts at -80, 0, which slopes gently upward and to the right, following an almost straight diagonal line until it reaches the point 600, 12, where it ends at a marked point. At the point where this line crosses 80, 2 another line starts that shoots upward almost vertically, but slightly off to the right. This line ends around the point 160, 15. The 2 lines previously mentioned divide the graph into 3 labeled sections. To the left of the almost vertical line, the graph is labeled solid. In between the line and the first mentioned curve, the graph is labeled liquid. To the right of the first mentioned curve, the graph is labeled gas. There is a dotted line that runs horizontally on y=7, from x=0 to x=760. On the far left this line is labeled A, and on the far right this line is labeled B.520°C and 7 atm80°C and 7 atm80°C and 2 atm600°C and 12 atm

Answers
Answer
600 °C and 12 atm
Explanation
Consider the typical phase diagram and use the information gather from it and let us it to answer your question.
Comparing the critical point in the typical phase diagram above, we can conclude that the temperature and pressure of the critical point in the give phase diagram are 600 °C and 12 atm.
Thus, the correct answer is given in the last option: 600 °C and 12 atm.

Related Questions
Suppose you are titrating an acid of unknown concentration with a standardized base. At the beginning of the titration, you read the base titrant volume as 1.12 mL. After running the titration and reaching the endpoint, you read the base titrant volume as 26.78 mL. What volume of base was required for the titration
Answers
Answer:
The volume of base required is = 25.66 mL
Explanation:
Volume of titrant required is given by:
Final titre value - Initial titre value
=26.78 - 1.12
= 25.66 mL
Note: repeat and average volumes for accuracy of result.
Rock is driven underground and changed by heat and pressure. This describes
what?
a. Igneous changing to sedimentary
b. Metamorphic changing to sedimentary
C. Sedimentary changing to metamorphic
d. Sedimentary changing to igneous
Answers
Answer:
Explanation:
metamorphic
If the percent (mass/mass) for a solute is 4% akd the mass of the solute is 200g, what is the mass of the solute in the solution?
Answers
Answer:
To find the mass of the solute in the solution, we first need to convert the percent to a decimal by dividing by 100. Since the percent is 4%, we can divide by 100 to get 0.04.
Next, we can multiply the decimal value by the total mass of the solute to find the mass of the solute in the solution. In this case, the total mass of the solute is 200g, so we can multiply 0.04 by 200g to get:
0.04 * 200g = 8g
Therefore, the mass of the solute in the solution is 8g.
what happens to the valency when we move down in a periodic table of non metal and metal
Answers
The valency of elements tends to decrease as we move down the periodic table of non-metal and metal.
The ability of an element to combine is referred to as valency. It is the number of electrons that an elemental atom loses, gains, or shares with another atom to form a stable configuration of electrons.
The occasional table is organized so that components with comparable valencies are set in a similar gathering. Components in a similar gathering have similar number of valence electrons, which decides their substance properties.
The valency of the elements tends to decrease as we move down a periodic table of metals and non-metals. This is because the number of electron shells, or energy levels, increases as we move down a group. The peripheral electrons in a particle are the valence electrons.
The expanded distance between the core and the peripheral electrons brings about more vulnerable fascination between them. As a result, the atom becomes more reactive and can lose or gain electrons more easily.
For instance, in bunch 1 of the occasional table, the valency of the components diminishes as we drop down the gathering. The valencies of lithium (Li), sodium (Na), and potassium (K) are, respectively, 1, 1, and 1.
This is due to the fact that each possesses one valence electron. Because the outermost electron is further from the nucleus and therefore more likely to be lost or gained, the valency decreases as we move down the group.
In conclusion, as we move down the periodic table, from non-metal to metal, the valency of elements tends to decrease. This is because the nucleus and the outermost electrons are less attracted to one another as the energy levels rise.
For more such questions on valency
https://brainly.com/question/30555742
#SPJ11
What is the specific heat capacity of gold if it requires 48.8 J to raise the temperature of 15 grams of gold 25oC?
Answers
Answer:
0.13 J/gC i believe.
Explanation:
Answer:
130.133 J/(kg·K)
Explanation:
Result in other units:
0.130133 J/(g·K)
31.0876675695 cal/(kg·K)
0.0310876675695 kcal/(kg·K)
0.130133 J/(g·°C)
at a certain temperature nitrogen dioxide gives dinitrogen tetroxide in a second order reaction. the rate constant for this reaction is 4.50/M min. what will be the concentration of nitrogen dioxide after 0.355 min, if one starts with an initial concentration of 0.281 M
Answers
Answer:
memes
Explanation:
memes are the answers, ma doods
The concentration of nitrogen dioxide after 0.355 min is equal to 0.194 M.
What is the second-order reaction?Second order reactions can be described as chemical reactions in which the sum of the exponents in the rate law of the chemical reaction is equal to two.
The rate of a second-order reaction can be written as:
r = k[A]², or as r = k[A][B].
The concentration of reactant can be calculated at time 't':
kt = 1/[A] -1/[A₀]
Given the initial concentration of nitrogen dioxide, [A₀] = 0.281 M
The time 't' for the final concentration, t = 0.355 min
The rate constant of the reaction, k = 4.50 M⁻¹min⁻¹
The concentration of the nitrogen dioxide after 0.355 min is :
4.50 × 0.355 = 1/[A] - (1/0.281)
1.5975 = 1/[A] - 3.559
1/[A] = 5.156
[A] = 0.194 M
Therefore, the concentration of nitrogen dioxide after 0.355 min, is 0.194 M.
Learn more about second order reaction, here:
https://brainly.com/question/12446045
#SPJ2
if 650J heat is absorbed by a system and 450J work is done on the system, then find the change in internal energy of the system
Answers
Answer: 380 J. Please mark
Explanation:
Of the following regions of the electromagnetic spectrum, which one has the shortest wavelength?
a.
gamma rays
b.
infrared
c.
radio waves
d.
X rays
e.
microwaves
f.
ultraviolet
Answers
Answer:
A ---->gamma ray
Explanation:
Gamma rays have the highest frequencies among all electromagnetic waves and therefore have the shortest wavelengths.
URGENT!! WILL MARK ANYONE WITH ALL ANSWERS AS BRAINLIEST!!!




Answers
Answer:
1) 9 moles
2) 8.75 moles
3) 1.76 moles
4) 10.2 moles
Explanation:
Okay so mole ratio is 2:1
So, 9 moles of HI is required for 4.5 moles of Iodine gas
Mol ratio of water to CaCl2 is 2:1
So, 17.5 moles of water produced is (17.5/2) moles of CaCl2 i.e. 8.75 moles
Okay so mol ratio of Hydrogen to NH3 is 3:2
So, 2.64 moles of hydrogen is (2.64 * 2)/3 moles of NH3 i.e. 1.76 moles
Once again, mol ratio of Hydrogen to NH3 is 3:2
When 15.3 moles of hydrogen is used, (15.3 * 2)/3 moles of NH3 i.e. 10.2 moles
Hope this helps and be sure to mark this as brainliest! :)
what is the answer please

Answers
Answer:
A.
Explanation:
A decomposition reaction is when one reactant (the substances to the left of the arrow in the reaction) breaks apart into two or more products (the substances to the right of the arrow).
Because KClO3 is breaking down into KCl and O2, it's a decomposition reaction.
According to the activity series, which of these metals will react with most acids to produce H2 gas?
Mg
Li
Hg
Answers
When many metals are introduced to a solution containing a potent acid, hydrogen gas is created. Magnesium and zinc are the two most often used materials.
Most hydrogen gas is created in what way?In the United States, the majority of the hydrogen produced each year is created by natural gas reforming with steam. A pressurized gasifier can also produce synthesis gas by combining high-temperature steam, oxygen, and coal or biomass.
HCl and Copper do they react?In the reactivity sequence, copper is placed below hydrogen. This indicates that copper cannot remove hydrogen from the acidic solution because it is less reactive than hydrogen. Copper does not react with hydrochloric acid because of this.
To know more about metals visit:-
brainly.com/question/14072179
#SPJ1
2C +2H yield C2H4 Delta H=+52.4 kj/mol
What is the kj of energy absorbed for every mole of carbon reacted
Answers
The kJ of the energy absorbed for the every mole of the carbon reacted is 104.8 kJ.
The chemical equation is as :
2C + 2H ---> C₂H₄ , ΔH = + 52.4 kJ/mol
The ΔH is the enthalpy change that is determined by the subtracting the energy of the reactants to the products.
The ΔH = energy of the products - energy of the reactants
The expression for the energy is as :
q = n ΔH
Where,
n = number of the moles
ΔH = enthalpy change
The kJ of the energy absorbed for the every mole of the carbon reacted :
q = 2 mol × 52.4 kJ/mol
q = 104.8 kJ
To learn more about energy here
https://brainly.com/question/12716509
#SPJ4
Calculate the pH of a solution that has a hydronium ion concentration, [H3O+], [ H 3 O + ] , of 2.56×10−6 M.
Answers
The pH of a solution with a hydronium concentration of 2.56 x \(10^{-6\) M would be 5.59.
What is pH?The pH of a substance is the degree of acidity or alkalinity of the substance. It is simply a measure of how acidic or basic the substance is.
The pH of any substance is mathematically expressed as:
pH = -log \([H^+]\)
where \([H^+]\) is the hydrogen ion concentration.
pH = 14 - pOH
Also,
pH = - log \([H_3O^+]\)
Where \([H_3O^+]\) is the hydronium ion concentration.
In this case, the hydronium ion concentration is given as 2.56 x \(10^{-6\)M
Thus,
pH = -log 2.56 x \(10^{-6\)
= 5.59
In other words, the pH of a solution that has a hydronium ion concentration of 2.56 x \(10^{-6\) M is 5.59.
More on pH can be found here: https://brainly.com/question/15289741
#SPJ1
Express the density of mecury in lb/ft³
Answers
Answer:
what is the difference between a example
Which of the following reasons correctly explains the color changes that take place when ethylenediamine (C2N2H8) is added to the solution of cobalt(II) chloride?
a. Addition of the liquid ethylenediamine dilutes the concentration of cobalt(II) chloride in the solution resulting in a color change.
b. The ethylenediamine is oxidized and the resulting solution is deeply colored.
c. The water ligands surrounding the cobalt metal center are being replaced by ethylenediamine and chloride ligands which results in a different crystal field splitting. Thus, the energy associated with electron transitions between the do-orbitals will differ for the two compounds showing a color change.
Answers
Answer:
The water ligands surrounding the cobalt metal center are being replaced by ethylenediamine and chloride ligands which results in a different crystal field splitting. Thus, the energy associated with electron transitions between the do-orbitals will differ for the two compounds showing a color change.
Explanation:
The five d-orbitals are usually degenerate. Upon approach of a ligand, the d-orbitals split into two sets of orbitals depending in the nature of the crystal field.
The magnitude of crystal field splitting is affected by the nature of the ligand. Ligands having filled p-π orbitals such as ethylenediamine lead to greater crystal field splitting.
The change in the colour that takes place when ethylenediamine is added to the solution of cobalt(II) chloride occurs due to a different crystal field splitting pattern. Thus, the energy associated with electron transitions between the d-orbitals now differ for the two compounds showing a color change.
4- The standard potential of cell: Sn/Sn²+||Cr³+/Cr is −0.60V.what is the standard
reduction potential of the Cr³+/Crelectrode? Es = -0.14V
Sn²+
(b) +0.74V
(c) -0.88V
(d) -0.74V
(a) +0.88V
Answers
The standard reduction potential of the Cr³+/Cr electrode is -0.46V. None of the option is correct.
To determine the standard reduction potential of the Cr³+/Cr electrode, we can use the Nernst equation, which relates the standard reduction potential to the cell potential under non-standard conditions. The Nernst equation is given by:
E = E° - (0.0592/n) * log(Q)
where E is the cell potential, E° is the standard reduction potential, n is the number of electrons transferred in the half-reaction, and Q is the reaction quotient.
In this case, we have the standard potential of the cell as −0.60V. We know that the standard reduction potential of the Sn/Sn²+ electrode is -0.14V. Therefore, the reduction potential of the Cr³+/Cr electrode can be calculated as:
E = -0.60V - (-0.14V)
E = -0.60V + 0.14V
E = -0.46V
Therefore, the standard reduction potential of the Cr³+/Cr electrode is -0.46V.
For more such question on standard reduction potential visit;
https://brainly.com/question/31482299
#SPJ8
Use the information in table to find standard enthalpy of the combustion of methane and also create a hess enthalpy diagram for reaction.
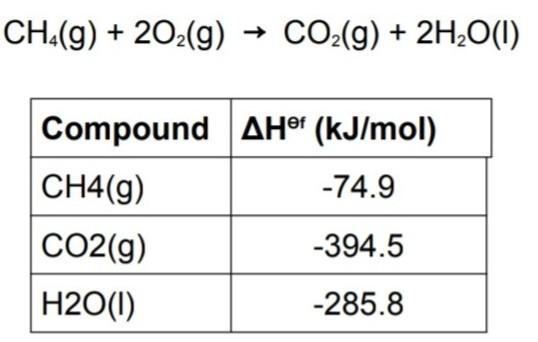
Answers
The combustion of methane can be represented by the equation CH4(g) + 2O2(g) → CO2(g) + 2H2O(g)We are to find the standard enthalpy of combustion of methane.
We are also to create a Hess enthalpy diagram for the reaction. Standard enthalpy of combustion is the amount of energy released when one mole of a substance is completely burned in excess oxygen at standard temperature and pressure. We can use the data in the table to calculate the standard enthalpy of combustion of methane.The table gives us the standard enthalpies of formation of the reactants and products. The standard enthalpy of formation is the amount of energy change that occurs when one mole of a compound is formed from its constituent elements in their standard states (usually at 25°C and 1 atm).We can use the standard enthalpies of formation to calculate the standard enthalpy of combustion of methane. The standard enthalpy of combustion is given by the following equation:ΔH°c = ΣΔH°f(products) – ΣΔH°f(reactants)We can calculate the standard enthalpy of combustion of methane using the standard enthalpies of formation from the table as follows:ΔH°c = [ΔH°f(CO2(g)) + 2ΔH°f(H2O(g))] – [ΔH°f(CH4(g)) + 2ΔH°f(O2(g))]Substituting the values from the table:ΔH°c = [(–393.5 kJ/mol) + 2(–241.8 kJ/mol)] – [(–74.8 kJ/mol) + 2(0 kJ/mol)]ΔH°c = (–890.2 kJ/mol) – (–74.8 kJ/mol)ΔH°c = –815.4 kJ/mol. Therefore, the standard enthalpy of combustion of methane is –815.4 kJ/mol.We can create a Hess enthalpy diagram for the reaction as shown below: The Hess enthalpy diagram is used to show how the standard enthalpy of the reaction can be calculated using the standard enthalpies of formation of the reactants and products. The diagram shows the two steps involved in the reaction. The first step involves the breaking of the bonds in methane and oxygen to form the reactants. The second step involves the formation of the products from the reactants. The standard enthalpy of combustion is the difference between the standard enthalpies of formation of the products and reactants. The Hess enthalpy diagram shows that the same standard enthalpy of combustion can be obtained regardless of the pathway taken to get from reactants to products.
for such more questions on equation
https://brainly.com/question/26694427
#SPJ8
Why do we need to identify matter?
Answers
Answer:
It's important for scientists to know the properties of matter because all things are made up of matter. Each type of matter has different physical characteristics and scientists need to know and understand these characteristics to make calculations. ... The main phases of matter are solid, liquid, and gas
Explanation:
^^
write the atomicity of oxygen
Answers
True or false, rewrite it to make it true.
To balance a chemical equation, only the subscripts of a reactant and product can be changes
Answers
Answer:
false
Explanation:
What is the wavelength of the yellow sodium emission, which has a frequency of 5.09 x 10^14/s?
Answers
Answer:
5.89×10^-7 meters
Explanation:
wavelength= speed of light/frequency
3.00×10^8m/s÷5.09×10^14s=5.89×10^-7 meters
Read the given equation.
2Na+ 2H₂O 2NaOH + H₂
During a laboratory experiment, a certain quantity of sodium metal reacted with water to produce sodium hydroxide and hydrogen gas. What was the initial quantity of
sodium metal used if 7.80 liters of H₂ gas were produced at STP?
07:29 grams
09.30 grams
12.2 grams
16.0 grams
Answers
How many mL of 2.25M H2SO4 are needed to react completely with 69.9g BaO2

Answers
Answer:
4 millllllermeeters jb
Question 4 (1 point)
If the decomposition of (NH4)2(CO3) is a first-order process with a rate constant of
0.196 s-1, how much ammonium carbonate would remain after 39.0 s, starting from
a concentration of 0.957 M?
Your Answer in units:
Answers
The final concentration of the reactant of a first order reaction can be determined from the rate constant equation. The concentration of ammonium carbonate after 39 s will be 0.003 M.
What is rate constant?Rate constant of a reaction is the rate of reaction when one molar concentration of the reactant is involved in the reaction. The expression for the rate constant k for first order reaction is :
k = 1/t ln (C0/Ct)
Where C0 be the initial concentration and Ct be the concentration after t seconds.
Given that C0 of ammonium nitrate = 0.957 M
rate constant = 0.196 /s
t = 39 s.
The concentration after 39 seconds is calculated as follows:
0.196 /s = 1/39s ln (0.957 M / Ct)
Ct = 0.957 / (ln⁻¹ (0.196 × 39))
= 0.003 M.
Therefore, the concentration of ammonium carbonate after 39 seconds will be 0.003 M.
To find more on rate constant, refer here:
https://brainly.com/question/20305871
#SPJ1
Balance this equation Hgo —> Hg + O2

Answers
Answer:
2Hgo —>2Hg + O2
Explanation:
HQ5.40
Homework Answered Due Today, 11:59 PM
The reaction 3H₂(g) + N₂(g) → 2NH3(g) has an enthalpy of reaction of -92.6 kJ/mol. If 1 g of hydrogen and 2 g of nitrogen are
reacted, how much heat is produced (kJ)?
Answers
The amount of heat energy produced when 1 g of hydrogen and 2 g of nitrogen are reacted, is -6.61 KJ
How do i determine the heat energy produced?First, we shall obtain the limiting reactant. Details below:
3H₂ + N₂ -> 2NH₃
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 g Molar mass of H₂ = 2 g/molMass of H₂ from the balanced equation = 3 × 2 = 6 gFrom the balanced equation above,
28 g of N₂ reacted with 6 g of H₂
Therefore,
2 g of N₂ will react with = (2 × 6) / 28 = 0.43 g of H₂
We can see that only 0.43 g of H₂ is needed in the reaction.
Thus, the limiting reactant is N₂
Finally, we the amount of heat energy produced. Details below:
3H₂ + N₂ -> 2NH₃ ΔH = -92.6 KJ
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 gFrom the balanced equation above,
When 28 grams of N₂ reacted, -92.6 KJ of heat energy were produced.
Therefore,
When 2 grams of N₂ will react to produce = (2 × -92.6) / 28 = -6.61 KJ
Thus the heat energy produced from the reaction is -6.61 KJ
Learn more about heat energy:
https://brainly.com/question/31429264
#SPJ1
What does the pH of a solution represent?
A. The pH indicates how acidic or basíc a solution is,
B. The pH represents the partial pressure of hydrogen gas.
O.C. The pH tells how quickly a reaction reaches equilibrium
D. The pH is an indicator of the salt content of a solution
Answers
Answer:
a
Explanation:
List the 2 pKa's for H2SO4
Answers
Table salt is 40% sodium by mass. What is true about all sodium chloride?
A. The mass of sodium is sometimes 30% by mass.
B. Not all sodium chloride will contain 40% sodium by mass.
C. All sodium chloride will contain 40% sodium by mass.
D. The ratio of sodium to chlorine in sodium chloride will vary.
Answers
Answer:
C. All sodium chloride will contain 40% sodium by mass.
Explanation:
It is definitely true that all sodium chloride will contain 40% sodium by mass. Table salt is the common name of sodium chloride which is the chemical name.
The formula is designated as NaCl.
According to the Law of constant composition or definite proportions; "all pure samples of the same compound contain the same proportions of the elements by mass".
This implies that every time a particular compound forms, it forms in the same percentage composition. It forms in the same percentage composition.
So, we see that all sodium chloride will contain 40% sodium by mass.
Option A, B and D are in contradiction with the law of constant composition.
An ion of a single pure element always has an oxidation number of ________.
Answers
Answer: An ion of a single pure element always has an oxidation number of
zero.
Explanation:
Answer : zero