In the notations C-12 and C-14, what do the numbers after the element
symbol and hyphen represent?
Answers
Related Questions
5.00 ml of 5.83 m fe(no3)3 is combined with 1.00 ml of 0.50 m hclo4 and 4.00 ml of 2.00 × 10–2 m kscn. what is the concentration of fe3 in the solution after the other reactants are added?
Answers
Since the stoichiometric ratio between Fe(NO₃)₃ and Fe(SCN)₃ is 1:1, the amount of Fe(SCN)₃ produced is also 0.02915 moles.
To find the concentration of Fe³⁺ in the solution after the other reactants are added, we need to determine the limiting reagent and calculate the amount of Fe³⁺ produced.
First, let's calculate the amount of Fe³⁺ produced from the reaction between Fe(NO₃)₃ and KSCN:
Fe(NO₃)₃ + 3KSCN → Fe(SCN)₃ + 3KNO₃
From the balanced equation, we can see that 1 mole of Fe(NO₃)₃ reacts with 3 moles of KSCN to produce 1 mole of Fe(SCN)₃.
The initial concentration of Fe(NO₃)₃ is 5.83 M and the volume used is 5.00 ml (which is equivalent to 0.00500 L). Thus, the amount of Fe(NO₃)₃ used is:
Amount of Fe(NO₃)₃ = concentration × volume
= 5.83 M × 0.00500 L
= 0.02915 moles
Know more about stoichiometric ratio here:
https://brainly.com/question/6907332
#SPJ11
What is the ground state configuration of calcium (Ca)?
Answers
The ground state configuration of calcium (Ca) is 1s2 2s2 2p6 3s2 3p6 4s2. The ground state configuration is the lowest energy state that an atom or molecule can occupy.
It is the electron configuration of an atom or molecule in its most stable form, corresponding to the minimum energy state. The least energetic and most stable configuration is the ground state configuration. An excited state configuration is a higher energy configuration (it requires energy input to create an excited state). The electrons used for bonding are called valence electrons. The primary quantum number (n), the orbital (s, p, d, or f), and the total number of electrons are used to represent electron configurations. The total number of electrons is expressed as a superscript.
To learn more about electron configuration click here https://brainly.com/question/29757010
#SPJ4
suppose that the power used by a light bulb in a circuit is 16 W, and the bulb has a resistance of 4 ohms. Calculate the current (in amps) flowing through it.
Answers
Answer:
2 A
Explanation:
The power flowing in a circuit is given by;
P= I^2 R
Where;
I= current = the unknown
R= resistance= 4 ohms
P= power=16 W
I= √P/R
I= √16/4
I= 2 A
If the bond angle between two adjacent hybrid orbitals is 109.5°, which is the hybridization?
a. Sp2
b. Sp3d
c. Sp3
d. Sp
Answers
If the bond angle between two adjacent hybrid orbitals is 109.5°, the hybridization is sp³. ore about
The hybridization is sp³ for the bond angle between two adjacent hybrid orbitals is 109.5°. the electrons groups are involved in this process. the four orbitals involves in the sp³ hybridization , the one s orbital and the p orbitals are involve and the hybrid orbital is formed is as sp³ hybridization. the geometry is tetrahedral. the example of the sp³ is the methane.
Thus, for the sp³ hybridization the bond angle between two adjacent hybrid orbitals is 109.5° and the geometry is tetrahedral.
To learn more about hybridization here
https://brainly.com/question/29729198
#SPJ4
what is the equilibrium constant for the following reaction? be sure your answer has the correct number of significant digits.
Answers
The equilibrium constant for the reaction 2SO2(g) + O2(g) → 2SO3(g) is Kc = 0.113.
This value is determined using the reaction quotient equation, which calculates the ratio of product concentrations to reactant concentrations at equilibrium.
The number of significant digits in the equilibrium constant is dependent on the number of significant digits in the concentrations of the reactants and products.
In this case, the concentration for each reactant and product is only known to two significant digits, so the equilibrium constant is also only known to two significant digits. As a result, the equilibrium constant for this reaction is Kc = 0.113.
Know more about significant digits here
https://brainly.com/question/1658998#
#SPJ11
What is theoretical yield in moles of oxygen when there are 18.3 moles of hydrogen ?
2H2 + O2 --> 2H2O
Answers
The theoretical yield of the water in the reaction is 329.4 g.
What is the theoretical yield?
We know that we can be able to obtain the theoretical yield when we look at the stoichiometry of the reaction that we have here. In this case, it is clear that would need to rely on the reaction equation and it would be our guide.
We have that;
2 moles of hydrogen would produce 2 moles of water
18.3 moles of hydrogen would also produce 18.2 moles of water.
Theoretical yield of water would now be;
18.3 * 18 g/mol
= 329.4 g
Learn more about theoretical yield:https://brainly.com/question/14966377
#SPJ1
Write all each equation with absolute without absolute value given for the conditions y=|x + 5| if x<-5
Answers
Without absolute value , for y=|x + 5| for x <-5, y = -2x
If x < -5, then x + 5 < 0. Therefore, y = |x + 5| = -(x + 5).
y = -(x + 5)
Note that this equation is only valid when x < -5. For values of x greater than or equal to -5, the absolute value of (x + 5) becomes positive, and y = |x + 5| = x + 5. Therefore, the equation for the full domain of y = |x + 5| is:
y = -(x + 5) for x < -5
Therefore | x -5 | should be changed to 5 - x to get a positive value.
Also | x - (-5) | should be changed to -5 -x to get a positive value.Therefore y = 5 - x + -5 - x = -2xwithout absolute value , for x <-5, y = -2x
The term "absolute value" is not commonly used. However, there is a related concept called "absolute configuration." Absolute configuration refers to the spatial arrangement of atoms or groups of atoms around a chiral center in a molecule. A chiral center is a carbon atom that has four different groups attached to it.
The absolute configuration of a chiral center can be determined using the Cahn-Ingold-Prelog (CIP) rules, which assign priority to the four different groups based on their atomic numbers. By following these rules, we can determine whether the chiral center has an R or S configuration. Knowing the absolute configuration of a chiral center is important because it determines the molecule's biological activity and the way it interacts with other molecules in a chemical reaction.
To learn more about Absolute value visit here:
brainly.com/question/4691050
#SPJ4
patterns of reactivity quick check
Answers
Reactivity decreases as you move from left to right. The farther to the left and down the periodic chart you move, easier it is for electrons to be given or taken away, hence resulting in higher reactivity.
What is the pattern of reactivity in periodic table?Reactivity decreases as we move down the column. As you learn more about the table, you will be able to find that this pattern is true for other families.
The atoms get bigger, as the atomic number increases. The chemical properties change slightly when compared to the element right above them on the table. The non-metal elements in Group 7 that are known as the halogens, get less reactive as you move to the down of the group.
To know more about pattern of reactivity, refer
https://brainly.com/question/6759644
#SPJ4
cars run on gasoline, where octane (c8h18) is the principle component. this combustion reaction is responsible for generating enough energy to move a vehicle, or do other work. how much co2 and h2o (in grams) are produced in the combustion of 0.87 gallons of octane? (density of octane
Answers
The combustion of 0.87 gallons of octane produces approximately 6.98 kg of CO₂ and 3.21 kg of H₂O.
To calculate the amount of CO₂ and H2O produced in the combustion of octane, we need to first convert the volume of octane from gallons to moles using its density and molar mass.
The density of octane is around 0.703 g/mL and its molar mass is 114.23 g/mol. One gallon is approximately 3.785 liters.
So, the amount of moles of octane in 0.87 gallons is:
moles of octane = (0.87 gallons) x (3.785 L/gallon) x (0.703 g/mL) / (114.23 g/mol) = 19.8 moles
The balanced chemical equation for the combustion of octane is:
2 C₈H₁₈ + 25 O₂ → 16 CO₂ + 18 H₂O
From this equation, we see that 2 moles of octane reacts with 25 moles of oxygen to produce 16 moles of CO₂ and 18 moles of H₂O.
Using stoichiometry, we can calculate the amount of CO₂ and H₂O produced from 19.8 moles of octane:
moles of CO₂ produced = 16/2 x 19.8 moles = 158.4 molesmoles of H₂O produced = 18/2 x 19.8 moles = 178.2 molesTo convert moles to grams, we can use the molar mass of each compound:
mass of CO₂ produced = 158.4 moles x 44.01 g/mol = 6,979 g or 6.98 kg (rounded to 2 decimal places)mass of H₂O produced = 178.2 moles x 18.02 g/mol = 3,209 g or 3.21 kg (rounded to 2 decimal places)Therefore, the combustion of 0.87 gallons of octane produces approximately 6.98 kg of CO₂ and 3.21 kg of H₂O.
Learn more about combustion on:
https://brainly.com/question/10458605
#SPJ11
what is metric and dimensional analysis
Answers
Answer:
Metrics are measures of quantitative assessment commonly used for assessing, comparing, and tracking performance or production.
Dimensional analysis is the analysis of the relationships between different physical quantities by identifying their base quantities and units of measure and tracking these dimensions as calculations or comparisons are preformed
Calculate the total pressure in a mixture of 8 g of dioxygen and 4 g of dihydrogen confined in a vessel of 1 dm8 at 27 ∘C. (R = 0.083 bar dm8 K−1 mol−1
Answers
Answer:
Total pressure = 56.77 bar
Explanation:
Given data:
Mass of dioxygen = 8 g
Mass of dihydrogen = 4 g
Volume of vessel = 1 dm³
Temperature = 27°C (27+273 = 300 K)
R = 0.083 bar.dm³ / mol.K
Total pressure = ?
Solution:
Number of moles of dioxygen:
Number of moles = mass/molar mass
Number of moles = 8 g/ 32 g/mol
Number of moles = 0.25 mol
Pressure of dioxygen:
PV = nRT
P = nRT/V
P = 0.25 mol × 0.083 bar.dm³ / mol.K × 300 K / 1 dm³
P = 6.97 bar.dm³ /1 dm³
P = 6.97 bar
Number of moles of dihydrogen:
Number of moles = mass/molar mass
Number of moles = 4 g/ 2 g/mol
Number of moles = 2 mol
Pressure of dihydrogen:
PV = nRT
P = nRT/V
P = 2 mol × 0.083 bar.dm³ / mol.K × 300 K / 1 dm³
P = 49.8 bar.dm³ /1 dm³
P = 49.8 bar
Total pressure of mixture in a vessel:
Total pressure = P (O₂) + P(H₂)
Total pressure = 6.97 bar + 49.8 bar
Total pressure = 56.77 bar
how does the subunits of a nucleic acid affect its structure
Answers
The subunits of a nucleic acid determine the encoded biological information and affect the structure as they dictate the structural stability of the molecule.
Nucleic acids are basically macromolecules which are very essential for life. They contain simpler subunits which are able to dictate the biological information which is being coded.
These subunits are basically the building blocks and hence they also dictate the structural stability of the nucleic acid. If there is any change in these subunits, it will lead to a change in the biological product that is encoded and also bring about a change in the structure stability of the nucleic acid.
To know more about nucleic acids here
https://brainly.com/question/26205149
#SPJ4
Which of the following BEST states the Law of Conservation of Matter? *
10 points
A. The total mass of the reactants in a reaction equals the total mass of the product(s).
B. The total mass of the reactants in a reaction is double the total mass of the product(s).
C. The total mass of the reactants in a reaction is less than the total mass of the product(s).
D. The total mass of the reactants in a reaction is greater than the total mass of the product(s).
Answers
Answer:
A. The total mass of the reactants in a reaction equals the total mass of the product(s).
Explanation:
The law of conversation of matter tells us that in a chemical reaction, matter is never created or destroyed, it's simply converted from one form to another. So the mass of reactants should always equal the mass of the products in a chemical reaction.
Which statement describes the endothermic reaction represented by this
graph?

Answers
Answer:
Option A, describes the endothermic reaction represented by this graph
Explanation:
In this graph the energy released (or the potential energy) at the time of product formation is lower than the potential energy of the reactants. Hence, additional energy input will be required to form the product. Thus, energy is absorbed during the reaction.
Option A is correct
Answer: energy is given off by the reaction
Explanation:
Convert 1.05 x 1026 molecules Ni to number of moles.
Answers
Answer:
1077.3 molecular in 1.05*1026 molecules
How many calories are there in 32 Calories?
a.64,000
b.16,000
C.32,000
D..032
Answers
Answer:
32
Explanation:
There cannot be more in a number than the number. Therefore, the answer has to be D, or 32.
Hope this helps! :)
a class of biochemical compounds including norepinephrine and dopamine is called:
Answers
The class of biochemical compounds including norepinephrine and dopamine is called catecholamines. Catecholamines are a group of neurotransmitters and hormones that play crucial roles in the central nervous system and the body's stress response.
Catecholamines are derived from the amino acid tyrosine through a series of enzymatic reactions. Norepinephrine, also known as noradrenaline, is primarily synthesized in nerve cells and functions as both a neurotransmitter and a hormone.
It is involved in regulating various physiological processes such as mood, attention, and stress response. Norepinephrine is released by nerve cells in response to stress or danger, leading to increased heart rate, blood pressure, and alertness.
Dopamine is another important catecholamine that acts as a neurotransmitter in the brain. It plays a crucial role in reward-motivated behavior, movement control, and regulation of mood and emotions. Dopamine is associated with pleasurable sensations and is involved in the brain's reward pathway, which is implicated in addiction and motivation.
To know more about Catecholamines refer:
https://brainly.com/question/30925048
SPJ11
define group of periodic tables
Answers
How many coulombs of positive charge are there in 4.00 kg of plutonium, given its atomic mass is 244 and that each plutonium atom has 94 protons?
Answers
Approximately 1.489 x 10^6 coulombs of positive charge exist in 4.00 kg of plutonium, calculated using the number of plutonium atoms, protons per atom, and the charge of each proton.
To find the number of coulombs of positive charge in 4.00 kg of plutonium, we need to use the following information:
1. Atomic mass of plutonium (Pu) = 244
2. Number of protons in each plutonium atom = 94
First, we need to calculate the number of plutonium atoms in 4.00 kg of plutonium. To do this, we'll use Avogadro's number (6.022 x 10^23 atoms/mole).
1 mole of plutonium = 244 grams (atomic mass of plutonium)
1 kg of plutonium = 1000 grams
So, 4.00 kg of plutonium is equal to (4.00 kg) / (244 g/mol) * (6.022 x 10^23 atoms/mol) = 9.877 x 10^23 atoms
Next, we need to calculate the total positive charge of these atoms. Since each plutonium atom has 94 protons, the total positive charge is equal to the number of atoms multiplied by the number of protons.
Total positive charge = (9.877 x 10^23 atoms) * (94 protons/atom)
= 9.284 x 10^25 protons
Finally, we need to convert the number of protons to coulombs. Each proton has a charge of approximately 1.602 x 10^-19 coulombs.
Total positive charge = (9.284 x 10^25 protons) * (1.602 x 10^-19 coulombs/proton)
= 1.489 x 10^6 coulombs
Therefore, there are approximately 1.489 x 10^6 coulombs of positive charge in 4.00 kg of plutonium.
Learn more About positive charge from the given link
https://brainly.com/question/28233928
#SPJ11
as atomic radius decreases, both ionization energy and electronegativity a increases b decrease c stay the same
Answers
Answer: B!!!
Explanation:
if you had 8.39 x 10^15 carbon atoms how many g K2CO3 total would be there?
Answers
With 8.39 x \(10^1^5\) carbon atoms, the amount of \(K_2CO_3\) would be 0.000001862 grams.
Percent compositionThe percent composition of carbon atoms in \(K_2CO_3\) is calculated as follows:
Percent composition of carbon = mass of carbon/mass of \(K_2CO_3\)
= 12/138 = 8.70%
6.022 x \(10^{23\) atoms = 1 mole of a substance
8.39 x \(10^1^5\) carbon atoms = 8.39 x \(10^1^5\)/6.022 x \(10^{23\)
= 1.35 x \(10^{-8\) mol
Mass of 1.35 x \(10^{-8\) mol carbon = 1.35 x \(10^{-8\) x 12
= 1.62 x \(10^{-7\) grams
If 8.70% = 1.62 x \(10^{-7\) grams
100% = 1.62 x \(10^{-7\) x 100/8.7
= 0.000001862 grams of \(K_2CO_3\)
In other words, if I have 8.39 x \(10^1^5\) carbon atoms, that would be equivalent to 0.000001862 grams of \(K_2CO_3\).
More on percent composition can be found here: https://brainly.com/question/17505281
#SPJ1
Explain why the Earth has four different seasons
Answers
Answer:
Earths rotation
Explanation:
Answer:
because if there was not four diferent season we would not have a day or a night because we would not be moving
Explanation:
calculate the liters in 89.51 grams of KCl4O
Answer in four sig figs
Answers
Answer:
89.51g of KClO4 is 0.03566 Liters (4 sig figs)
Explanation:
o calculate the liters in 89.51 grams of KClO4, we need to use the density of the substance. The density of KClO4 is 2.52 g/mL. To convert from grams to milliliters, we can use the formula:
Volume (mL) = mass (g) / density (g/mL)
Now we can plug in the given values:
Volume (mL) = 89.51 g / 2.52 g/mL = 35.66 mL
To convert mL to Liters we divide by 1000, so the volume of 89.51g of KClO4 is 0.03566 Liters (4 sig figs)
URGENT! Please help! Hi, I have to do a titration lab report using the Royal Society of Chemistry online titration lab. Please help me answer the following questions using the observation table I think?


Answers
Answer:
I'm sorry, but I cannot see the observations or the data table you mentioned in your question. However, I can still provide you with some general guidance on how to approach the calculations and answer the questions based on the given information.
4. To calculate the concentration of the NaOH solution, you need to know the mass of NaOH used and the volume of the solution. The formula to calculate concentration is:
Concentration (in mol/L) = (Mass of NaOH (in grams) / molar mass of NaOH) / Volume of solution (in L)
Make sure to convert the mass of NaOH to moles by dividing it by the molar mass of NaOH. The molar mass of NaOH is the sum of the atomic masses of sodium (Na), oxygen (O), and hydrogen (H).
5. The balanced equation for the neutralization reaction between NaOH and HCl is:
NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(l)
(aq) represents an aqueous solution, and (l) represents a liquid.
6a. To calculate the average concentration of HCl in the sample from site B, you need to know the volumes and concentrations of the NaOH and HCl solutions used in the titration. Use the formula:
Concentration of HCl (in mol/L) = (Volume of NaOH solution (in L) * Concentration of NaOH (in mol/L)) / Volume of HCl solution (in L)
Multiply the volume of NaOH solution used by its concentration to find the amount of NaOH used. Then, divide this amount by the volume of HCl solution used to find the concentration of HCl.
6b. To determine the pH of the water at site B, you need to know the concentration of HCl from the previous calculation. The pH can be calculated using the formula:
pH = -log10[H+]
Since HCl is a strong acid, it dissociates completely into H+ ions. Therefore, the concentration of H+ ions is equal to the concentration of HCl. Take the negative logarithm (base 10) of the H+ concentration to find the pH.
To check if the water is safe, compare the calculated pH value to the range provided (pH 4.5-7.5). If the pH falls within this range, the water is considered safe for plant and animal reproduction in an aquatic environment.
6c. Use a similar calculation as in 6a to determine the average concentration of HCl in the sample from site C.
6d. Use the concentration of HCl from 6c to calculate the pH using the formula in 6b. Follow the same procedure to check if the water is safe based on the pH range.
7. To find the most current pH value for the Grand River, you can search for the latest data from reliable sources such as environmental agencies, research institutions, or government websites. Compare this pH value to the pH values obtained in the experiment to assess the difference between them.
Remember, without the specific data and observations, the calculations and comparisons provided here are only general guidelines. It's important to use the actual data from your experiment to obtain accurate results and conclusions.
Please mark as Brainliest
Explain specifically how an electron gives off light in an atom.
Answers
Answer:
Then, at some point, these higher energy electrons give up their "extra" energy in the form of a photon of light, and fall back down to their original energy level.
Explanation:
When properly stimulated, electrons in these materials move from a lower level of energy up to a higher level of energy and occupy a different orbital.
in which sell duplet rule is applied, why?
Answers
Answer:
its in explanationExplanation:
the tendency for items have 2 electrons in their outermost shell by interacting with others items through electron sharing or transfer is known as duplet rule. if an atom has 2 electrons in it's outermost shell then it is called duplet rule and if a 8 electrons in it's outermost shell than it is called octet rule .
Which of the following bond has the highes yield?
Baa2
BBB
Baa3
Baa1
Answers
Among the bond ratings provided, Baa1 has the highest yield. The bond ratings provided are based on the creditworthiness and risk associated with the issuer.
Generally, higher-yielding bonds indicate higher risk, which is reflected in lower credit ratings. Baa2, Baa3, and BBB all have lower credit ratings compared to Baa1, indicating a higher level of risk and, therefore, potentially higher yields. Key Learnings. Junk bonds, often known as high-yield bonds, are corporate financial securities that provide interest rates above those of investment-grade bonds. Low credit ratings, such as below BBB- from Standard & Poor's and Fitch or below Baa3 from Moody's, are typical of high-yield bonds.
To know more about highest yield
https://brainly.com/question/30902634
#SPJ11
when 1-methylcyclopentene is reacted with h2 with a pt catalyst, what will be the name of the resulting compound?
Answers
When 1-methylcyclopentene is reacted with H₂ in the presence of a platinum (Pt) catalyst, the resulting compound will be 1-methylcyclopentane.
The reaction between 1-methylcyclopentene and H₂ with a Pt catalyst is an example of a hydrogenation reaction. Hydrogenation involves the addition of hydrogen (H₂) across a carbon-carbon double bond, resulting in the conversion of an alkene into an alkane.
In the case of 1-methylcyclopentene, it is an unsaturated hydrocarbon with a double bond between two carbon atoms. The molecule can be represented as follows:
CH₃─CH=CH─CH₂─CH₂
The reaction involves the addition of two hydrogen atoms across the double bond, converting the alkene (cyclopentene) into an alkane (cyclopentane) by a process called hydrogenation.
Learn more about 1-methylcyclopentene from the link given below.
https://brainly.com/question/28258246
#SPJ4
What is the molarity of a solution containing 5. 0 moles of solute in 469 mL of a soltution
Answers
molarity of a solution containing 5. 0 moles of solute in 469 mL of solution is 10.66 mol/L
We know that the Molarity of a given solution is -
Molarity = n/V-------------------(i)
where
n= number of moles
V = Volume of solution in liters
now as per the question-:
number of moles n = 5 moles
volume of solution V= 469 ml or 0.469 ml { convert volume given in ml to L }
putting the values in equation (i)
\(Molarity =\frac{5}{0.469}\)Molarity = 10.66 mol/L
Therefore Molarity of the given solution is 10.66 mol/L
For more information on molarity
https://brainly.com/question/30404105
Can someone help me please?
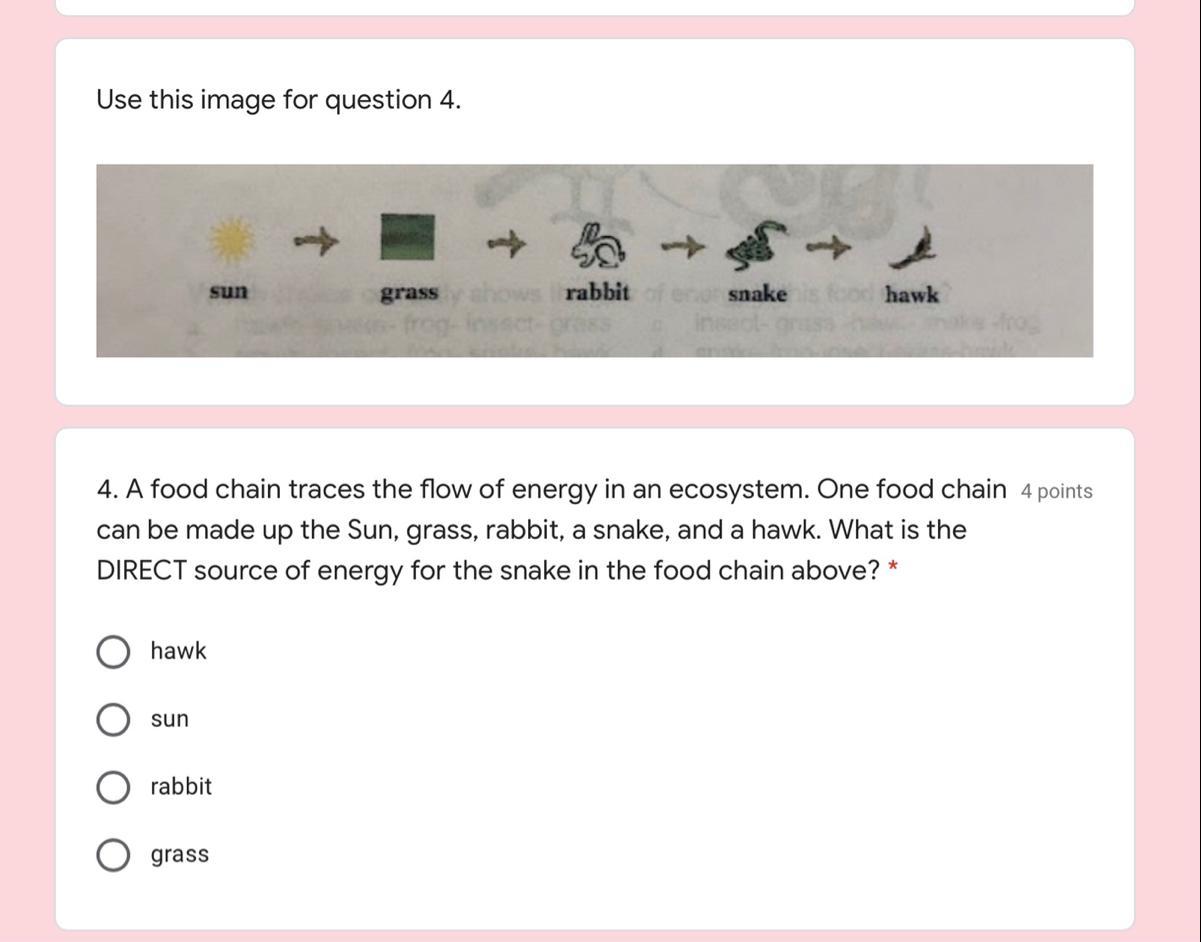
Answers
Answer:
SUN
Explanation:
Hope this helps! Plz mark as brainliest!
And hope it helps