in which species is resonance most useful in explaining the observed bond lengths?
Answers
Resonance is most useful in explaining the observed bond lengths in species with delocalized electrons, such as in aromatic compounds like benzene (C₆H₆).
Resonance helps in explaining the observed bond lengths in compounds that have delocalized electrons, like the molecules with conjugated π systems (aromatic compounds), as shown below.
-C=C-C=C-C=C-
In these molecules, the electrons are not localized to a single bond but are shared among multiple bonds, leading to a stabilization of the molecule and a longer overall bond length. Here, the electrons are shared between multiple bond locations, leading to bond lengths that are intermediate between single and double bonds.
To know more about Resonance, click below.
https://brainly.com/question/31490176
#SPJ11
Related Questions
What is the name of this hydrocarbon?
a) A skeletal model starts with C H 3 at the left, goes down to a valley, up to a peak, and then down again.
b) The valley and the peak have C H 3; there is also a C H 3 on a line straight down from the end of the centerline of carbons. 2,3-dimethylpentane 3,4-dimethylbutane 3,4-diethylpentane 2,3-diethylbutane
Answers
Answer:
A. 2,3-dimethylpentane
Explanation:
edge 2022
Hydrocarbons are the simplest compound containing carbon and hydrogen in their molecular structure. 2, 3 dimethyl pentane is correct.
What is hydrocarbon ?Hydrocarbons are the simplest compound containing carbon and hydrogen in their molecular structure. There are four types of hydrocarbon as follows
Alkanes, alkenes, alkynes and aromatic hydrocarbons. Alkane contai carbon carbon single bond in their structure .Alkene containing carbon carbon double bond in their structure while alkynes contain carbon carbon triple bond in their structure.
Aromatic hydrocarbon contain benzene ring in their structure. Benzene is the example of aromatic hydrocarbon. Aromatic hydrocarbons are used as paint removal and are also used as a solvents.
Thus, 2, 3 dimethyl pentane is correct . It is a hydrocarbon containing carbon and hydrogen in their structure.
To learn more about hydrocarbon follow the link below;
https://brainly.com/question/17578846
#SPJ5
12. Label Each part of the atom:

Answers
Answer:
Atom Labeling
Explanation:
Y- neutron
Z-protron
X - electron
W- nucleus
Which body system includes the heart?
Answers
according to kinetic molecular theory, which of the following would not be considered an ideal gas
Answers
Answer:
A gas at very low volumes, when gas particles are very close together
A gas at very low temperatures, when gas particles have very little kinetic energy
A gas with highly polar molecules that have very strong inter-molecular forces
Explanation:
The Kinetic Molecular Theory:
particles in a gas are in constant, random motioncombined volume of the particles is negligibleparticles exert no forces on one anotherany collisions between the particles are completely elasticaverage kinetic energy of the particles is proportional to the temperature in kelvinsRM / NV / NF / EC / ET
Although none of the assumptions provided in the molecular theory of gases are strictly correct, they are fair enough for modeling some systems. It is an idealized approach of real systems. The fundamental presumptions are nearly identical to those of an ideal gas.
The most logical of the hypotheses is that of elastic collisions. Since gas molecules are treated as perfectly hard spheres in Newton's equations and elastic collisions, there is no energy lost in compressing the gas molecules during a collision.
For bulk, light gases at moderate temperatures and low to moderate pressures, it is acceptable to assume that there is an attractive force between the gas and the container wall. Since the walls of the containers only account for a minor portion of collisions in macroscopic quantities, they can typically be disregarded. Only until the gas's total density exceeds the kinetic energy do forces between its particles start to become significant. For light gases like He and straightforward diatomic gases, the kinetic energy of the gas molecules far outweighs the intramolecular interactions at normal temperatures.
But in a complete way of the KM theory being described:
The microscopic characteristics of atoms (or molecules) and their interactions, which result in observable macroscopic qualities, are described by the kinetic molecular theory of matter (such as pressure, volume, temperature). The idea may be used to explain why matter exists in distinct phases (solid, liquid, and gas), as well as how matter can transform between these phases.
The three states of matter are: As we transition from the solid to the gaseous phase, you'll notice that the distance between atoms or molecules widens.
According to the kinetic molecular theory of matter,
Particles that make up matter are continually moving.Every particle has energy, however the amount of energy changes with the temperature of the sample of matter. Thus, whether the material is in a solid, liquid, or gaseous form is determined. The least energetic molecules are those in the solid phase, whereas the most energetic particles are those in the gas phase.The average kinetic energy of the particles in a material may be calculated from its temperature.When the particles' energies are altered, the phase of the particles may vary.Matter atoms are separated by gaps. As a sample of matter transitions from the solid to the liquid and gas phases, the average amount of vacant space between molecules increases.Atoms and molecules interact by attraction forces, which intensify as the particles draw closer to one another. Intermolecular forces are the name for these pulling forces.How does kinetic molecular theory affect gases?According to the Kinetic Molecular Theory, gas particles collide in an elastic manner and are always in motion. Only absolute temperature directly affects a group of gas particle's average kinetic energy.
Part I of How the Kinetic-Molecular Theory Explains Gas Behavior.
If the volume is kept constant, the faster gas molecules collide with the container walls more frequently and more violently, raising the pressure according to Charles' law.
Answer:
See below
Explanation:
Gas at LOW PRESSURE or high temperature with little or no intermolecular forces would be closest to ideal gas behavour.
Why is the? The second ionization energy of sodium is about three times greater than the second ionization energy of magnesium
Answers
Answer:
~Na+1 is already in the preferred form. Because of this, the second ionization energy of sodium is higher than normal. Mg+1 loses an electron to form s2 p6 .
When a new substance is formed, what kind of change has taken place? *
Physical Change
Chemical Change
O Phase Change
O None of the above
Answers
Answer:
Chemical Change
Explanation:
Physical change and phase change are both changes in the matter's state. Such as solid, gas and liquid. Not what it's made of. So when a new substance is formed, it' a chemical change.
Bubbling during a chemical reaction is an indicator that a ______
I has been produce
Answers
Answer: gas
Explanation:
state three properties of light
Answers
Answer:
The primary properties of visible light are intensity, propagation-direction, frequency or wavelength spectrum and polarization.
Explanation:
PLS HELP ASAP I NEED HELP IMAGE BELOW

Answers
Answer:
1, 4, 5 are the correct answers
Explanation:
Hope this helps! :D
Which one of the following substances is an element?
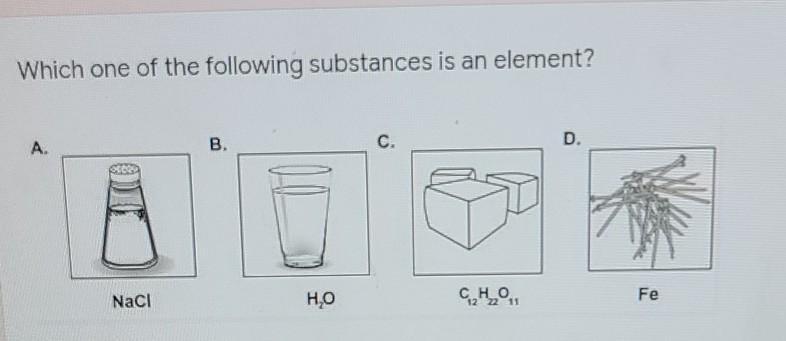
Answers
Answer: Fe
Explanation: Fe (Iron) is the only element since it involves only 1 atom. The other options are compounds, since there are more than 2 atoms bonded together.
Question 11
Which formula represents a hydrocarbon?
C₂H6
C₂H5OH
C₂H5Cl
C₂H6O
Answers
Answer:
C₂H6
Explanation:
Among the given options, the formula A) C₂H6 represents a hydrocarbon (specifically, ethane). Option A
A hydrocarbon is a compound that consists of only carbon and hydrogen atoms. It is important to identify the formula that represents a hydrocarbon among the given options:
A) C₂H6: This formula represents ethane, which is a hydrocarbon. Ethane consists of two carbon atoms bonded together with single bonds and six hydrogen atoms.
B) C₂H5OH: This formula represents ethanol, which is not a hydrocarbon. Ethanol contains a hydroxyl group (-OH), indicating the presence of oxygen in addition to carbon and hydrogen atoms. It is an alcohol, not a hydrocarbon.
C) C₂H5Cl: This formula represents ethyl chloride, which is not a hydrocarbon. Ethyl chloride contains a chlorine atom (Cl) in addition to carbon and hydrogen atoms. It is a haloalkane, not a hydrocarbon.
D) C₂H6O: This formula represents ethanol, which, as mentioned before, is not a hydrocarbon. Ethanol contains an oxygen atom (O) in addition to carbon and hydrogen atoms. It is an alcohol, not a hydrocarbon.
Among the given options, the formula A) C₂H6 represents a hydrocarbon (specifically, ethane). It consists only of carbon and hydrogen atoms, making it a suitable representation of a hydrocarbon.
In summary, the formula C₂H6 (option A) represents a hydrocarbon, while the other options contain additional elements (oxygen or chlorine) that make them non-hydrocarbon compounds. Option A
For more such questions on hydrocarbon visit:
https://brainly.com/question/21281906
#SPJ8
PLEASE HELP WILL GIVE BRAILIST!!!
b) Type one to two paragraphs that describe your models and relate them to the law of
conservation of mass. Your document should:
i. identify the names of the reactants and products in the reaction.
ii. identify the number of molecules that make up the reactants and products.
iii. identify the type and number of atoms in each molecule of the reactants and products.
iv. explain what happens during the chemical reaction.
v. explain how mass is conserved during the chemical reaction
Answers
Answer:
The first one is on google
Explanation:
Gravitational influence of the sun on the different planets. Which is it the least on? Which is it the greatest on?
help please
Answers
Gravitational influence of the sun on the different planets least is mercury 0.38 g and greatest is neptune 1.14 g
Gravity, also called gravitation, in mechanics, the universal force of attraction acting between all matter and it is by far the weakest known force in nature and thus plays no role in determining the internal properties of everyday matter and the sun is the body that has the greatest gravitational influence on all the other components of the system that revolve around it and least is mercury 0.38 g and greatest is neptune 1.14 g
Know more about Gravitation
https://brainly.com/question/852528
#SPJ1
(PLEASE HELP, i will give brainlist, and the question is 15 points)
What is the volume of a 4.25 liter of Neon at STP whose pressure is now 50 kPa
1) 2.52 L
2) 16.78 L
3) 8.6 L
Answers
when mechanical energy is lost due to friction, it becomes _____ energy.
Answers
When mechanical energy is lost due to friction, it becomes thermal energy.
Mechanical energy is the sum of kinetic energy and potential energy in an object that is used to accomplish a job or to move an object. Mechanical energy can be transformed between kinetic and potential energy, and it can be transformed between various forms of energy such as heat, light, and sound.
Friction is a force that opposes movement between two surfaces that are in touch with one another. When two surfaces are rubbed together, the force of friction opposes the motion of the objects.When mechanical energy is lost due to friction, it becomes thermal energy. As a result of friction, the mechanical energy of an object transforms into heat, which is referred to as thermal energy.
The heat produced by friction is due to the motion of molecules in the surface. The conversion of mechanical energy into thermal energy is a natural process that occurs all the time as a result of friction. Therefore, it is important to reduce friction in order to conserve mechanical energy, and hence reduce wastage.
Learn more about friction from the given link
https://brainly.com/question/24338873
#SPJ11
help me name the molecule

Answers
Answer:
ether
Explanation:
ether is -OR
- Hope that helps! Please let me know if you need further explanation.
2. How many moles are in 7.30 X 10^23 molecules of NaCl?
Answers
Answer:
\( \huge{ \boxed{1.213 \: \text{moles}}}\)
Explanation:
To find the number of moles in a substance given it's number of entities we use the formula
\( \bold{n = \frac{N}{L}} \\ \)
where
n is the number of molesN is the number of entitiesL is the Avogadro's constant which is 6.02 × 10²³ entitiesFrom the question
N = 7.30 × 10²³ NaCl molecules
\(n = \frac{7.30 \times {10}^{23} }{6.02 \times {10}^{23} } = \frac{7.30}{6.02} \\ = 1.2126\)
We have the final answer as
1.213 molesFor every 6 mols of H2 how many mols of h20 will be produced?
For every 2 mols of H2 how many mols of h20 will be produced?
For every 5.67 mols of H2 how many mols of h20 will be produced?
Answers
The moles would be 12 moles, 4 moles and 11.34 moles
How to solve for the molesWhen hydrogen gas (H2) reacts with oxygen gas (O2) to produce water (H2O), the balanced chemical equation is:
2H2 + O2 -> 2H2O
So,
For every 6 moles of H2, 12 moles of H2O will be produced
For every 2 moles of H2, 4 moles of H2O will be produced
For every 5.67 moles of H2, 11.34 moles of H2O will be produced.
Read more on the moles here:https://brainly.com/question/15356425
#SPJ1
How many degrees would the temperature of a 45.0 g piece of iron increase if 7,680 J of energy are applied to it? (The specific heat of iron is 0.449 J/g°C)
Question 10 options:
76.6°C
155,000°C
380°C
0.0130°C
Answers
Answer:
380
Explanation:
Q=mco
Q is the energy
M is mass
C is specific heat
O is increased in temperature
the ksp of agi is 1.5 × 10–16. calculate the molar solubility of silver iodide. give the answer in 2 sig. figs. question blank 1 of 2 type your answer... x 10^ question blank 2 of 2
Answers
The molar solubility of silver iodide can be calculated using the molar solubility of silver iodide is 1.2 × 10–8 M, rounded to 2 significant figures..
The solubility product constant (Ksp) is a measure of the degree to which a sparingly soluble salt dissociates into its constituent ions in solution. The Ksp expression is written as the product of the concentrations of the ions raised to their stoichiometric coefficients in the balanced chemical equation. By assuming that the substance dissociates completely, we can use the Ksp expression to calculate the molar solubility of the salt. In this case, the molar solubility of silver iodide is calculated to be 1.2 × 10–8 M, which indicates that only a very small amount of AgI dissolves in water.
learn more about solubility here:
https://brainly.com/question/28170449
#SPJ11
sulfur and oxygen can react to form both sulfur dioxide and sulfur trioxide in sulfur dioxide there are 32.06 grams of sulfur and 32 grams of oxygen in sulfur dioxide there are 32.06 grams of sulfur are combined with 48 grams of oxygen
a. what is the ratio of the weights of oxygen that combine with 32.06 g of sulfur ?
b. How do these data illustrate the law of multiple proportions?
Answers
Answer:
Explanation:
In sulfur dioxide there are 32.06 grams of sulfur and 32 grams of oxygen .
In sulfur trioxide there are 32.06 grams of sulfur are combined with 48 grams of oxygen.
The ratio of oxygen which reacts with 32.06 gram of sulfur is 32: 48 .
This ratio is equal to 2 : 3.
This is in accordance with law of multiple proportion because , the ratio of mass of oxygen which reacts with constant mass of sulfur is integral ratio . Hence they are in accordance with law of multiple proportions.
Answer:
sulfur and oxygen can react to form both sulfur dioxide and sulfur trioxide in sulfur dioxide there are 32.06 grams of sulfur and 32 grams of oxygen in sulfur dioxide there are 32.06 grams of sulfur are combined with 48 grams of oxygen
a. what is the ratio of the weights of oxygen that combine with 32.06 g of sulfur?
b. How do these data illustrate the law of multiple proportions?
Explanation:
In sulfur dioxide (\(SO_2\))
32.06 g of sulfur reacts with 32.0 g of oxygen.
In sulfur trioxide (\((SO_3)\) 32.0 g of sulfur reacts with 48.0 g of oxygen.
So, both th ecom[pounds are made from sulfur and oxygen,
But the amount of oxygen reacts with fixed amount of sulfur that is 32.06 g and it is in proportions that is:
32.0 g : 48.0 g
=2:3.
Hence, the ratio of oxygen combines with sulfur is in the ratio of 2:3.
b.
This data illustrates the law of multiple proportions.
Because the oxygen which is combining with fixed amount of sulfur is in proportions.
ASAPP!!! CHEM HW HERE

Answers
Argon’s period is 3
It is in group 18
It’s atomic number is also 18
For the explanation:
A period is the horizontal row across the periodic table
The atomic number is the number of protons in the nucleus
For the reaction below , label each reactant as an electron pair acceptor or electron pair donor and as a Lewis acid or a Lewis base. AlCl 3 +Cl^ -; AlCl 4 ^ -
Answers
Since the reaction shown in the question is an acid - base reaction in the Lewis sense; the Lewis acid here is AlCl3 while the Lewis base here is Cl^- .
What is a Lewis acid?A Lewis acid is a substance that accepts electron pair while a Lewis base donates an electron pair.
Now consider the given reaction; AlCl3 +Cl^- ------> AlCl 4 ^-. The Lewis acid here is AlCl3 while the Lewis base here is Cl^- .
Learn more about acid - base reaction: https://brainly.com/question/14356798
Hydrogenation is a chemical reaction between hydrogen gas and another substance. Hydrogenation of vegetable oils takes place in the presence of a metal catalyst. What kind of catalyst is this?
Answers
The catalyst used in the hydrogenation of vegetable oils is a transition metal, commonly nickel, which facilitates the addition of hydrogen atoms to the unsaturated carbon-carbon double bonds in the oil molecules, resulting in a solid product with improved stability and shelf life.
Hydrogenation is a chemical reaction where hydrogen gas (H2) is combined with another substance, often to convert unsaturated molecules into saturated ones. In the case of vegetable oils, hydrogenation is employed to turn liquid oils into solid fats, such as margarine or shortening. This process enhances the stability, shelf life, and melting point of the oils.
The catalyst used in the hydrogenation of vegetable oils is typically a metal, often a transition metal. Common catalysts include nickel, palladium, platinum, and sometimes even rhodium. These metals facilitate the addition of hydrogen atoms to the unsaturated carbon-carbon double bonds found in the vegetable oil molecules. Nickel, being relatively inexpensive and effective, is the most commonly used catalyst in this process.
During the hydrogenation reaction, the vegetable oil is heated and mixed with hydrogen gas. The metal catalyst is introduced, and its presence accelerates the reaction, allowing the hydrogen atoms to be added to the oil molecules efficiently. The result is a product with a higher percentage of saturated fatty acids, leading to its solid state at room temperature.
To know more about hydrogenation, refer to the link below:
https://brainly.com/question/13130218#
#SPJ11
The major product expected from the sequential reaction of cyclopentene with Br2/H2O, followed by sodium hydroxide is: a) I b) II c) III d) IV e) None of these choices.
Answers
The major product expected from the sequential organic reaction of cyclopentane with Br2/H2O, followed by sodium hydroxide , then the correct option is e) None of these choices.
The sequential organic reaction of cyclopentane with Br2/H2O, followed by sodium hydroxide, leads to the formation of a major product.
The reaction of cyclopentane with Br2/H2O results in bromination of the double bond, adding Br atoms to the cyclopentene molecule. This forms a cyclic bromonium ion intermediate.
Subsequent treatment with sodium hydroxide (NaOH) triggers an intramolecular nucleophilic attack by the hydroxide ion (OH-) on the bromonium ion, resulting in the formation of a cyclic alcohol.
Among the choices given, we can analyze the possible products based on the given information. Since we don't have a visual representation of the structures, it is not possible to directly determine the specific product. However, based on the reaction conditions, the expected product is likely to involve the formation of a cyclic alcohol.
Given the options provided (I, II, III, IV), the correct answer cannot be determined without additional information. Hence, the correct choice is e) None of these choices.
Learn more about organic reactions here:
brainly.com/question/9585105
#SPJ11
analysis definition?
Answers
A student designed an experiment to determine the effect of increasing the temperature of the solvent on the amount of
solute that could be dissolved. Which of the following would be the dependent variable?
A Temperature of the solute
Answers
Answer: A Temperature of the solute
Explanation:
Help what’s the answer?

Answers
The volume (in L) at STP occupied by 1.24 mole of hydrogen gas is 27.78 liters
How do i determine the volume at STP?From the question given above, the following data were obtained:
Number of mole of hydrogen gas = 1.24 moleVolume of hydrogen gas =?The volume of 1.24 mole of hydrogen gas can be obtained as illustrated below:
At standard temperature and pressure, STP,
1 mole of hydrogen gas = 22.4 Liters
Therefore,
1.24 mole of hydrogen gas = (1.24 mole × 22.4 Liters) / 1 mole
1 .24 mole of hydrogen gas = 27.78 liters
Thus, we can conclude from the above calculation that the volume of hydrogen gas is 27.78 liters
Learn more about volume:
https://brainly.com/question/225322
#SPJ1
MARKING BRAINLIEST!! - Determine the molar mass [MM] of a gas if 2 L of the gas weights 0.500 g at 298 K and 2.00 atm.
Answers
Answer:
3.125g/mol
Explanation:
To find the molar mass of the gas, we need to initially find the number of moles (n) contained in the gas. To find the number of moles, we use the general gas law whose equation is:
PV=nRT
Where; P= Pressure
V= Volume occupied by gas
n= number of moles
R= general gas constant
(0.0821 L atm mol/K)
T= absolute temperature
According to the question; P= 2.0atm, V= 2.0L, n= ?, T= 298K
To find n, we make it the subject of the formula:
n= PV/RT
n= 2.0 × 2.0 / 0.0821 × 298
n= 4/ 24.4658
n= 0.16mol
If number of moles (n) of the gas is 0.16mol and it weighs 0.500g, its molar mass can be found using:
number of moles (n) = mass (g) / molar mass
Making MM subject of the formula;
molar mass = mass / number of moles
MM= 0.500/0.16
MM= 3.125
Hence, the molar mass of the gas is 3.125g/mol.
One of the main components of an airbag is the gas that fills it. As part of the design process, you need to determine the exact amount of nitrogen that should be produced. Calculate the number of moles of nitrogen required to fill the airbag. Show your work. Assume that the nitrogen produced by the chemical reaction is at a temperature of 495°C and that nitrogen gas behaves like an ideal gas
Answers
The number of moles of nitrogen required to fill the airbag is approximately 0.0295 mol.
What is Temperature?
Temperature is a physical property that measures the degree of hotness or coldness of an object or system, as compared to a standard reference point. It is a measure of the average kinetic energy of the particles (atoms or molecules) in a substance or system. The SI unit for temperature is the kelvin (K), but it can also be measured in degrees Celsius (°C) or Fahrenheit (°F).
To calculate the number of moles of nitrogen required to fill the airbag, we need to know the volume of the airbag and the conditions under which the nitrogen will be produced.
Let's assume that the airbag has a volume of 50 L and that the temperature and pressure inside the airbag are both 25°C (298 K) and 1 atm, respectively. We also need to know the molar volume of an ideal gas at these conditions, which is approximately 24.5 L/mol.
Now, we can use the ideal gas law to calculate the number of moles of nitrogen required:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles, R is the gas constant (8.31 J/mol K), and T is the temperature.
First, we need to convert the temperature of the nitrogen from 495°C to Kelvin:
495°C + 273.15 = 768.15 K
Next, we can rearrange the ideal gas law to solve for n:
n = PV/RT
Substituting in the values we have:
n = (1 atm)(50 L)/(8.31 J/mol K)(768.15 K)
n = 0.0295 mol
Learn more about Temperature from the given link
https://brainly.com/question/25677592
#SPJ1