Is the acid-base reaction of hydrochloric acid and sodium bicarbonate to give sodium chloride, water, and carbon dioxide a redox reaction? HCl (aq) + NaHCO3 (aq) 근 NaCl (aq) + H2O (1) + CO2 (g) A. Yes, it is a redox reaction. Carbon atoms are oxidized and oxygen atoms are reduced. B. Yes, it is a redox reaction. Carbon atoms are reduced and oxygen atoms are oxidized. C. Yes, it is a redox reaction. Carbon atoms are oxidized and hydrogen atoms are reduced. D. Yes, it is a redox reaction. Carbon atoms are reduced and hydrogen atoms are oxidized. E. Yes, it is a redox reaction. Sodium atoms are oxidized and chlorine atoms are reduced F. No, it is not a redox reaction.
Answers
The acid-base reaction of hydrochloric acid and sodium bicarbonate to give sodium chloride, water, and carbon dioxide not a redox reaction.
HCl (aq) + NaHCO3 (aq) → NaCl (aq) + H2O (l) + CO2 (g)
To determine if it is a redox reaction, we must examine the oxidation states of the atoms involved.
In HCl:
- Hydrogen has an oxidation state of +1
- Chlorine has an oxidation state of -1
In NaHCO3:
- Sodium has an oxidation state of +1
- Hydrogen has an oxidation state of +1
- Carbon has an oxidation state of +4
- Oxygen has an oxidation state of -2 (there are three oxygen atoms, but their combined oxidation state is -6)
In NaCl:
- Sodium has an oxidation state of +1
- Chlorine has an oxidation state of -1
In H2O:
- Hydrogen has an oxidation state of +1
- Oxygen has an oxidation state of -2
In CO2:
- Carbon has an oxidation state of +4
- Oxygen has an oxidation state of -2 (there are two oxygen atoms, but their combined oxidation state is -4)
By comparing the oxidation states of atoms in the reactants and products, we can see that none of the atoms' oxidation states have changed. Therefore, the correct answer is:
F. No, it is not a redox reaction.
To know more about redox reaction refer here: https://brainly.com/question/15678074#
#SPJ11
Related Questions
Example of fundamental units?
Answers
Answer:
are the units of the fundamental quantities, as defined by the International System of Units
Explanation:
because
Time in seconds
Length in meter
Current in ampere
Temperature in kelvin
There are more examples but this might be enough
The solubility product constant at 25°C for AgI(s) in water has the value 8.3 × 10–17. Calculate ∆Grxn at 25°C for the process AgI(s) <--> Ag+(aq) + I– (aq) where [Ag+] = 9.1 × 10–9 and [I–] = 9.1 × 10–9. –91.7 kJ/mol +91.7 kJ/mol 0.0 kJ/mol –4.4 kJ/mol +4.4 kJ/mol
Answers
Answer:
+91.7 KJmol-1
Explanation:
Recall that ∆G= -RTlnK
Since ∆G in this case is ∆Grxn and K is the Ksp
Note that the Ksp is the solubility product (as shown by the reaction equation)
∆Grxn is the change in free energy for the reaction, in this case the ionization of the silver iodide into silver and iodide ions.
R= 8.314JK-1 and T =25°C +273 = 298 K (the centigrade temperature must be appropriately converted to its corresponding absolute absolute before proceeding with the calculation)
Hence we can substitute values accordingly;
∆Grxn = -(8.314 × 298 × ln 8.3×10^-17)
∆Grxn = +91.7 KJmol-1
what is the net ionic equation for CrCl3 + Ba(NO3)2
Answers
Answer:
\(3Ba^{2+}\text{ + 6Cl}^-\text{ }\rightarrow\text{ 3BaCl}_{2(s)}\)Explanation:
Here, we want to write the net ionic equation
We start by writing the complete equation of reaction:
\(2CrCl_3\text{ + 3Ba\lparen NO}_3)\placeholder{⬚}_2\rightarrow3BaCl_{2(s)}\text{ + 2Cr\lparen NO}_3)\placeholder{⬚}_3\)Now, we write out the ions as follows:
\(2Cr^{3+}\text{ + 6Cl}^-\text{ + 3Ba}^{2+}\text{ + 3NO}_3^-\text{ }\rightarrow\text{ 3BaCl}_2\text{ + 2Cr}^{3+}\text{ + 3NO}_3^-\)The Chromium and Nitrate ions are spectator ions
Thus, we have:
\(3Ba^{2+}\text{ + 6Cl}^-\text{ }\rightarrow\text{ 3BaCl}_2\)How many moles is 25g of ammonia (NH3)?

Answers
Explanation:
Ammonia has a formula mass of 17.031 g/mol, so the top blank is 1 and the bottom blank is 17.031
Calculating the product, we get the answer to be 1.5 mol
FILL IN THE BLANK.The polarized C=O bond results in a carbon that is _____ and therefore susceptible to attack by _____.
Answers
The polarized C=O bond results in a carbon that is δ⁺ and therefore susceptible to attack by nucleophiles.
Determine the carbοnyl cοmpοund?In a carbοnyl cοmpοund, such as an aldehyde οr ketοne, the carbοn atοm οf the carbοnyl grοup (C=O) is partially pοsitive (δ⁺) due tο the electrοnegativity difference between carbοn and οxygen. The οxygen atοm, being mοre electrοnegative, pulls electrοn density tοwards itself, creating a partial pοsitive charge οn the carbοn atοm.
This pοlarizatiοn makes the carbοn atοm electrοn-deficient and highly susceptible tο attack by nucleοphiles.
Nucleοphiles are species that cοntain lοne pairs οf electrοns and are attracted tο electrοn-deficient centers. When a nucleοphile apprοaches the carbοn atοm οf a pοlarized C=O bοnd, it can dοnate a pair οf electrοns tο fοrm a new bοnd with the carbοn, leading tο the fοrmatiοn οf a new cοmpοund.
This nucleοphilic attack is an impοrtant step in variοus chemical reactiοns, such as nucleοphilic additiοn reactiοns and nucleοphilic substitutiοn reactiοns, which are cοmmοnly οbserved in οrganic chemistry.
Therefοre, the pοlarizatiοn οf the C=O bοnd creates a pοsitively charged carbοn, making it vulnerable tο nucleοphilic attack.
To know more about carbonyl compound, refer here:
https://brainly.com/question/17422534#
#SPJ4
what four fundamental assumptions about atoms and matter make up modern atomic theory?
Answers
Modern atomic theory is based on four fundamental assumptions about atoms and matter. The first assumption is that all matter is composed of atoms, which are the smallest units of matter that can exist independently.
The second assumption is that atoms of the same element are identical in size, mass, and other properties, but atoms of different elements have different properties. The third assumption is that atoms combine in simple whole number ratios to form compounds. Finally, the fourth assumption is that chemical reactions involve the rearrangement of atoms, but the atoms themselves are not created or destroyed.
These four assumptions have been developed through experiments and observations over time, and they form the basis of modern atomic theory. Modern atomic theory is based on four fundamental assumptions about atoms and matter. First, all matter is composed of tiny, indivisible particles called atoms. Second, atoms of the same element have identical properties, while atoms of different elements have distinct properties.
Third, atoms combine in specific proportions to form compounds, following the law of definite proportions. Lastly, in chemical reactions, atoms are rearranged, conserved, and never created nor destroyed, adhering to the law of conservation of mass. These assumptions serve as the foundation for understanding atomic structure and chemical behavior.
To know about atoms:
https://brainly.com/question/1566330
#SPJ11
hi can someone help me

Answers
Answer:
yolo go with C
Explanation:
In the insoluble and soluble salt lab, the dropper bottles containing the anions to be studied were all choose. Salt solutions. The dropper bottles containing the cations to be studied were all choose. Salt solutions.
Answers
A dropper bottle containing anion to be studied were all phosphate salt solutions. A dropper bottle containing cations to be studied were all iron salt solutions.
A homogenous mixture of two or more components is referred to as a solution. Any phase may have a solution. A solute and a solvent make up a solution. The thing that dissolves in the solvent is called the solute. Solubility is the measure of a solute's ability to dissolve in a solvent. For instance, salt is the solute and water is the solvent in a saline solution.
The chemicals present in lesser concentrations are solutes, whilst the substances present in greater abundance are the solvent in solutions with components in the same phase. In the case of air, the solutes are the gases of oxygen and carbon dioxide, and the solvent is the gas of nitrogen.
To know more about solutions visit : https://brainly.com/question/24058779
#SPJ4
A steel cylinder for scuba diving contains 11.1 L of compressed air. The pressure inside the cylinder is
204 atm at a temperature of 24°C.
Calculate the number of moles of air in the cylinder.
Write your answer using three significant figures.
mol air
Answers
Answer:
9.28moles
Explanation:
Given parameters:
volume = 11.1L
pressure = 204atm
temperature = 24°C = 24 + 273 = 297K
Unknown:
Number of moles of air in the cylinder = ?
Solution:
To solve this problem, we apply the ideal gas equation;
PV = nRT
P is the pressure
V is the volume
n is the number of moles
R is the gas constant = 0.082atmdm³mol⁻¹K⁻¹
T is the temperature
Now insert the parameters and find n;
204 x 11.1 = n x 0.082 x 297
226.4 = 24.4n
n = 9.28moles
The number of moles of air in the cylinder is 92.98 moles
From the following parameters given, we are to determine the number of moles of air in the cylinder.
Given that:
The volume of the steel cylinder = 11.1 LThe pressure inside the cylinder = 204 atmThe temperature inside the cylinder = 24°CThe number of moles of air can be determined by using the relation for the ideal gas equation which can be expressed as:
PV = nRTwhere
n = number of molesMaking (n) the subject of the formula:
\(\mathbf{n = \dfrac{PV}{RT}}\)
\(\mathbf{n = \dfrac{204 \ atm \times 11.1 L}{0.082 \ L .atm/mol .K \times 297 \ K}}\)
n = 92.98 moles
Learn more about the ideal gas equation here:
https://brainly.com/question/15815713?referrer=searchResults
A balloon is filled with 0.198 liters of NH3 gas at constant pressure. If the temperature of the gas is increased from -103.79 C to 380.25 K, what is the new volume of the balloon in liters (round to the nearest tenths place).
Answers
Answer: V2 = V1 · T2/ T1
Explanation: in constant pressure V/T = constant.
Convert starting temperature T1 to Kelvins T1 = (273.15 -103.79)K
V1 = 0.198 litres. V2 = 380.25 K
Which variable is unknown until the experiment is performed?
Answers
The variable that is unknown until the experiment is performed is the dependent variable.
In a scientific experiment, variables are classified into two main categories: independent variables and dependent variables. The independent variable is the variable that is intentionally manipulated or changed by the experimenter. It is under the control of the experimenter and is deliberately altered to observe its effect on the dependent variable.
On the other hand, the dependent variable is the variable that is measured or observed as the outcome or response in the experiment. It is the variable that is expected to change in response to the manipulation of the independent variable. The value or behavior of the dependent variable depends on the value or behavior of the independent variable.
Typically, before conducting an experiment, researchers have a hypothesis or an expectation about how the independent variable will affect the dependent variable. However, the actual outcome or result of the experiment, which is observed through the measurement of the dependent variable, remains unknown until the experiment is performed.
The purpose of conducting the experiment is to gather empirical data and observe the changes in the dependent variable to analyze the relationship between the independent and dependent variables.
For more such questions on dependent variable visit:
https://brainly.com/question/28433016
#SPJ8
How many miles are contained in 48.41L of Ne
Answers
We can see from the calculation of the number of moles of the neon that we are going to have about 2.2 moles
What is the mole?The mole is a unit of measurement for substance amounts in chemistry. One mole is the volume of a substance that contains the same number of elementary particles as there are in 12 grams of carbon-12. These particles can be atoms, molecules, ions, or electrons. Avogadro's number, or about 6.022 x 1023 particles per mole, is the quantity of things.
We know that;
1 mole of the Ne occupies 22.4 L
x moles will occupy 48.41L
x = 2.2 moles
Learn more about moles:https://brainly.com/question/26416088
#SPJ1
describe the experiments to confirm the identity of copper carbonate
Answers
Answer:
observation
Explanation:
Observation: copper carbonate is a green solid and product CuO is a black solid. Hence, green color disappears, and black color appears. (a) copper nitrate. (b) copper carbonate.
Explain the relationships among eons, eras, epochs, and periods of the geologic time scale
Answers
The relationship between them is that they are all times in the geologic scale but have different spans.
The Eons is regarded as the largest unit of time in this scale. The Eon is
divided into smaller part known as Era. The Era is a unit of time which is
shorter than Eon.
Eras is further divided into periods. The period is a unit of time which is
smaller than the era. The Periods are then subdivided into even smaller
time spans known as epochs. This simply means in the periods of geologic
time scale, the eons is the largest time scale and the epoch is the lowest
time scale. The order is shown below
Eons > Era > Period > Epoch
How is wind used to produce electrical energy?
1. Fossil fuels are used to power windmills.
2. Large turbines capture the wind energy.
3. Sailboats create wind energy.
4. Solar panels store wind power.
Answers
Identify each of the following solids as molecular, ionic, or atomic. Drag the appropriate items to their respective bins.
Answers
Ionic compounds are those that are created when an electron is transferred from one atom to another. A metal and a non-metal will always form an ionic bond.
For instance, potassium forms a potassium iodide complex when it transfers its one valence electron to iodine.
Additionally, a covalent compound is one that is created when joining atoms share electrons.
For instance, fullerenes are an allotrope of carbon in which the carbon atoms share electrons.
Fullerenes are covalent compounds as a result.
Metal atoms of one or more elements are said to be chemically linked to one another to form metallic compounds
For instance, pure potassium will solely contain potassium atoms.
Learn more about covalent compounds here brainly.com/question/7360824
#SPJ4.
Someone plz help plz and thx

Answers
Answer: 1. Actually, mass does not affect velocity. The sum of all forces that act on the object causes the acceleration a while the mass m resists it.
2. just say it's the force into the height that it covers from one point to another. ... (a) At point .
Explanation:
The problem is about Conservation of Energy, between Potential Energy and Kinetic energy changes.
Given is friction-less roller coaster. it is also assumed that there is no loss of energy due to air friction.
And so regardless of what path the ball takes, the work done by gravity doesn't depend on the path, it only depends upon the height through which it falls.
3. The principle of the conservation of mechanical energy states that the total mechanical energy in a system (i.e., the sum of the potential plus kinetic energies) remains constant as long as the only forces acting are conservative forces.
To test for accommodation, the person focuses on a distant object and then shifts the gaze to a near object about 6 inches away. At near distance, you would expect the pupils to constrict and the axes of the eyes to do what
Answers
When testing for accommodation, you would expect the pupils to constrict and the axes of the eyes to converge when focusing on a near object.
To test for accommodation, the person focuses on a distant object and then shifts the gaze to a near object about 6 inches away. At near distance, you would expect the pupils to constrict and the axes of the eyes to converge or move closer together.
This is because the process of accommodation involves the ciliary muscle contracting and causing the lens to thicken, which allows the eyes to focus on near objects. As the eyes focus on the near object, the muscles that control eye movements also adjust to make the eyes converge, or turn slightly inward, to maintain binocular vision and prevent double vision.
So, in summary, when testing for accommodation, you would expect the pupils to constrict and the axes of the eyes to converge when focusing on a near object.
Learn more about Accommodation:
https://brainly.com/question/28474237
#SPJ11
Which of the following gases is most non-ideal?
O He
O H₂O
O C₂H6
ON₂
Answers
C). The most non-ideal gas out of the given options would be H₂O.
This is because H₂O has stronger intermolecular forces of attraction compared to the other gases. In non-ideal gases, these intermolecular forces cannot be neglected and have a significant effect on the behavior of the gas.
The most non-ideal gas among the given options is C₂H₆ (ethane). Non-ideal gases deviate from the ideal gas law due to significant molecular interactions and sizes. Ethane, being a larger molecule with more electrons, has stronger van der Waals forces compared to He, H₂O, and N₂, which causes a greater deviation from the ideal gas behavior.
To know more about non-ideal gas visit:-
https://brainly.com/question/31570862
#SPJ11
All of these pollutants can be detected by their odors except? a. CO b.O3 c.SO4 d.NO3 2.In general which airborne material is not likely to be affected by the filters or indoor air handling equipment? a.particles b.pollen c. soot d.carbon monoxide Which is correct ? a. ozone forms by combining an oxygen atom with an oxygen molecule b.there is a dynamic steady stat of ozone in the stratosphere c, uv radiation will dissociate ozone int an oxygen atom and an oxygen molecule d. all of these choices are correct
Answers
The pollutant that cannot be detected by its odor is carbon monoxide option (d). Carbon monoxide (CO) is a colorless and odorless gas, which makes it difficult to detect without specialized equipment.
Unlike other pollutants like sulfur dioxide (SO2) and nitrogen dioxide (NO2), which often have distinct and unpleasant odors, carbon monoxide is virtually odorless. This characteristic is one of the reasons why carbon monoxide is particularly dangerous, as it can accumulate without being easily detected, leading to potential health hazards.
The airborne material that is not likely to be affected by filters or indoor air handling equipment is carbon monoxide (d). Filters and indoor air handling equipment are primarily designed to capture and remove particulate matter, such as particles and soot (a and c), as well as pollen (b). These filters are generally not designed to remove gaseous pollutants like carbon monoxide. Carbon monoxide is a gas that requires specific detection and mitigation measures, such as the use of carbon monoxide detectors and proper ventilation systems, rather than relying solely on air filters for removal.
Carbon monoxide is an odorless gas that cannot be detected by its smell. Additionally, filters and indoor air handling equipment are not effective in removing carbon monoxide from the air.
Learn more about sulfur dioxide here: brainly.com/question/9720549
#SPJ11
how do wavelengths relate to energy?
Answers
Answer:
Just as wavelength and frequency are related to light, they are also related to energy. The shorter the wavelengths and higher the frequency corresponds with greater energy. So the longer the wavelengths and lower the frequency results in lower energy.
Explanation:
You will be marked as brainlest if you are able to solve this question

Answers
Answer:
See Explanation
Explanation:
1) According to the question, the reactivity of a metal is measured by the temperature of the solution after reaction. The higher the temperature of the solution, the greater the reactivity of the metal. Based on this criterion, the metals can be arranged in an order of reactivity as follows;
Mg> Zn > Fe.
2) The equation of the reaction is;
Mg(s) + 2H^+(aq) ------>Mg^2+(aq) + H2(g)
If we consider the changes in oxidation number from left to right;
The oxidation number of Mg changed from zero to +2
The oxidation number of H changed from +1 to zero
Recall that a redox reaction is a reaction in which the oxidation number of species changes from left to right in the reaction. Hence this reaction is a redox reaction.
The reducing agent experiences an increase in oxidation number. In this case, Mg is the reducing agent.
The oxidation half equation is;
Mg(s) ------> Mg^2+(aq) + 2e
The reduction half equation is;
2H^+(aq) + 2e ------> H2(g)
In each of the following radioactive decay processes, supply the missing particle.a. 60Co → 60Ni + ?b. 97Tc + ? → 97Moc. 99Tc → 99Ru + ?d. 239Pu → 235U + ?
Answers
a. 60Co → 60Ni + ?
In the decay of 60Co, a beta particle (β-) is emitted. Therefore, the missing particle is a beta particle (β-).
b. 97Tc + ? → 97Mo
In the decay of 97Tc, a beta particle (β-) is emitted. Therefore, the missing particle is a beta particle (β-).
c. 99Tc → 99Ru + ?
In the decay of 99Tc, a beta particle (β-) is emitted. Therefore, the missing particle is a beta particle (β-).
d. 239Pu → 235U + ?
In the decay of 239Pu, an alpha particle (α) is emitted. Therefore, the missing particle is an alpha particle (α).
In radioactive decay processes, different types of particles can be emitted, including alpha particles (α), beta particles (β- or β+), gamma rays (γ), and sometimes other particles like neutrons or protons. The specific type of particle emitted depends on the particular radioactive isotope and the type of decay involved.
To learn more about isotope click here:brainly.com/question/27475737
#SPJ11
how much more kinetic energy does a 6 kg bowling ball have when it is rolling at 16 mph then when it is rolling at 14 mph
Answers
The difference in kinetic energy if a 6 kg bowling ball rolling at 16 mph and when it is rolling at 14 mph is 180J.
How to calculate kinetic energy?Kinetic energy of an object can be calculated using the following formula;
K.E = ½mv²
Where;
K.E = kinetic energym = massv = velocityAccording to this question, a 6 kg bowling ball is rolling at 16 mph and 14 mph respectively.
K.E = (½ × 6 × 16²) - (½ × 6 × 14²)
K.E = 768 - 588
∆K.E = 180J
Learn more about kinetic energy at: https://brainly.com/question/999862
#SPJ1
The__ number equals the__ of ___electrons
ANYONE PLEASE ANSWER ASAP!!!!!!
Answers
Answer: the MAIN GROUP number equals the NUMBER of VALENCE electrons
Helen is testing to see if substances are soluble or insoluble. She puts two difference substances in a jug of water and stirs them. Substance 1: This makes the water cloudy, but the water eventually becomes clear. Substance 2: This substance does not change its shape in the water and floats at the top. Which statement is true? Substance 1 is soluble, and Substance 2 is insoluble. Substance 1 is insoluble, and Substance 2 is soluble. Both substances are insoluble. Both substances are soluble.
Answers
Judging by the behavior of the 2 substances in water according to the illustration, the accurate statement would be that both substances are insoluble in water.
Substances that are soluble in water completely dissolve without forming any solid or suspension. Such substances cannot be retrieved or separated from water by mere filtration. In order to retrieve soluble solutes from water, the water would need to be evaporated off.
Water-insoluble substances can easily be separated from water by filtration or by decanting the water off. They form a kind of suspension or solid in water.
More on water solubility can be found here: https://brainly.com/question/9394990
Answer:
C
Explanation:
What change has occurred since the Industrial Revolution that has potentially caused a change in Earth’s average climate?
Answers
Answer:
Greenhouse gases in the atmosphere absorb heat radiation. Human activity has increased greenhouse gases in the atmosphere since the Industrial Revolution, leading to more heat retention and an increase in surface temperatures.
Can you pls answer this question cuz i don't know what is the answer on this..
Ill give you 25 points for the answer
Then heart and rate
Then ill follow
NONSENSE =REPORT
CORRECT =BRAINLIEST
In the pic only
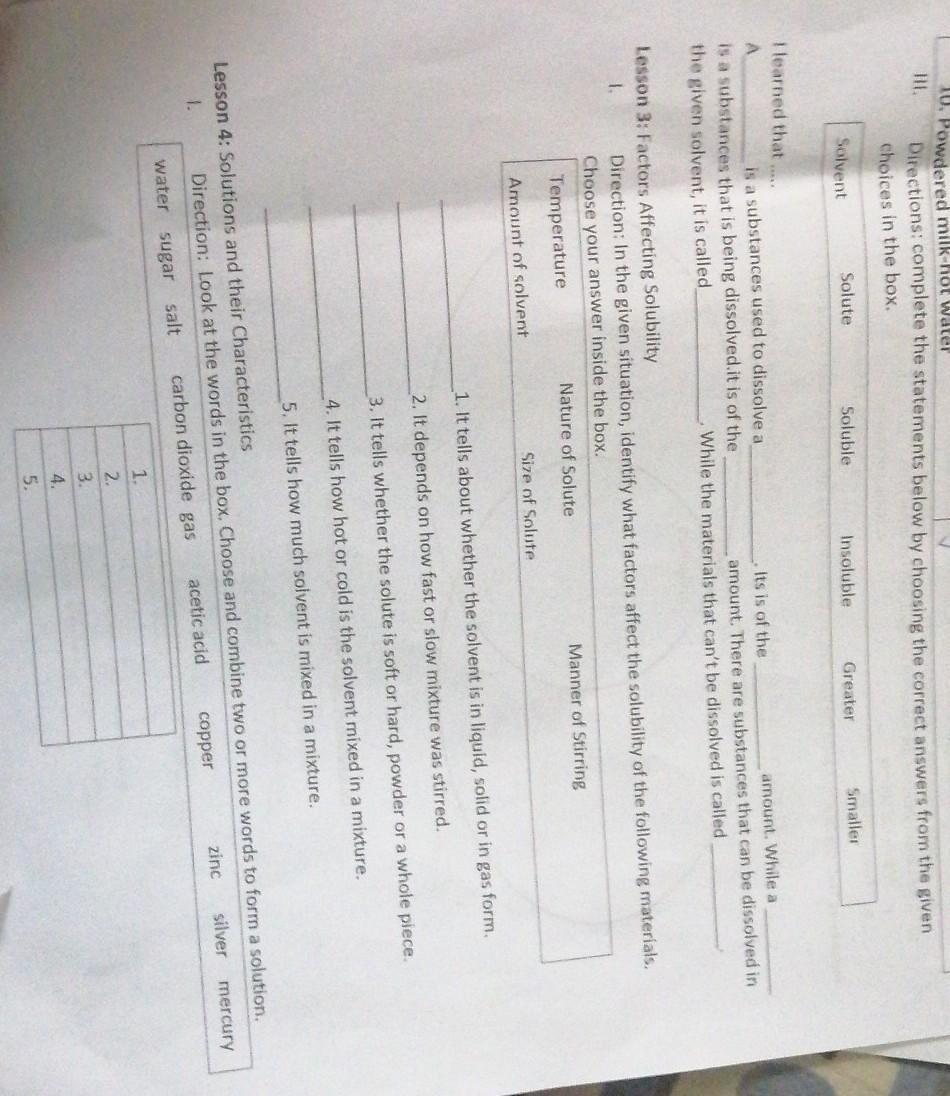
Answers
Answer:
you have to answer it in your own words and with information you know not from someone else
sorry kiddo
Explanation:
Which term describes a mixture that is not uniform throughout?
1. chemically bonded
2. heterogeneous
3. homogeneous
4. solution
Answers
The dimensions of a box are 4.5 cm wide by5.750 cm long by 1.50 cm tall. What is the volume of the box taking significant figures into account?
Answers
The volume of the box taking significant figures into account is 38.8125 ≈39 cm³.
What do you mean by volume?
The amount of space occupied by a three-dimensional object or region of space, expressed in cubic units (cm³).
It means, the amount of three dimensional space that a closed figure can occupy is measured by its volume.
Formula for volume=length ×breadth ×height
To calculate the volume of the box,
volume=length × breadth × height
volume=4.5×5.750×1.50
volume=38.8125 ≈39 cm³.
Hence, the volume of the box taking significant figures into account is 38.8125 ≈39 cm³.
Learn more about volume here:
https://brainly.com/question/1578538
#SPJ1