isotopes differ in the number of what particle? a) beta particles b) protons c) electrons d) neutrons e) gamma particles
Answers
Isotopes differ in the number of neutrons.
Each element is distinguished by the number of protons, neutrons and electrons that it possesses. The atoms of each chemical element have a defining and same number of protons and electrons, but crucially – not neutrons, whose numbers can vary.
Atoms with the same number of protons but different numbers of neutrons are called isotopes.
They share almost the same chemical properties, but differ in mass and therefore in physical properties. There are stable isotopes, which do not emit radiation, and there are unstable isotopes, which do emit radiation. The latter are called radioisotopes.
The first 80 elements on the periodic table have stable isotopes.
Learn more about isotopes and neutrons : https://brainly.com/question/364529
#SPJ11
Related Questions
A student investigates the number of particles of water that exist in a closed test tube throughout the phase
change of liquid to gas.
How many particles will be in the test tube after the water vaporizes and turns into a gas?
Answers
The number of particles of water that exist in a closed test tube after the water vaporizes and turns into a gas will be the same as the number of particles before the phase change.
This is because during the phase change, the molecules of water simply change their state from liquid to gas.the phase change from liquid to gas does not involve any change in the number of molecules, only a change in the physical state of the molecules. The molecules do not disappear or gain additional molecules from outside the test tube. As such, the number of particles of water in the test tube after the phase change is the same as before the phase change.
learn more about phase change Refer:brainly.com/question/30270780
#SPJ1
What are three concepts that you may explore while learning in a physical science course?
Answers
1-ethylycloheptene was treated with mcpba, followed by sodium methoxide in methanol. what was the product?
Answers
The reaction of 1-ethylcycloheptene with MCPBA (meta-chloroperoxybenzoic acid) followed by sodium methoxide in methanol leads to the formation of an epoxide.
MCPBA is a peracid that is commonly used to convert alkenes into epoxides through an epoxidation reaction. It adds an oxygen atom to the double bond of the alkene, resulting in the formation of an oxirane ring.
In this case, when 1-ethylcycloheptene reacts with MCPBA, an epoxide is formed. The specific product will depend on the regiochemistry and stereochemistry of the starting compound. Without further information on the exact structure and conditions of the reaction, it is difficult to determine the exact product.
However, the general product can be represented as an epoxide derived from 1-ethylcycloheptene:
Epoxide
1−ethylcycloheptene+MCPBA+NaOMe/MeOH→Epoxide
The exact position and stereochemistry of the epoxide ring would be determined by the specific structure of 1-ethylcycloheptene and the reaction conditions used.
For more such questions on sodium methoxide
https://brainly.com/question/31237886
#SPJ4
Which of these factors will cause a solid solute to dissolve faster?
Answers
Answer:
Heat
Explanation:
Higher temperatures cause solid solutes to dissolve at a faster rate than normal
Answer:
Higher temperature
Explanation:
Which of these factors is involved in earthquake formation?
A. plates getting larger
B. rocks breaking
C. stress that decreases
D. faults that remain stationary
Answers
Answer:
B
Explanation:
Edge 2023
What are the materials that good conductors and insulators?
Answers
Conductors
Silver
Gold
Copper
Aluminum
Mercury
Steel
Iron
Seawater
Concrete
Mercury
___________________
insulators
Aluminum foil
Glass
Air
Plastic
Rubber
Porcelain
Wood
Assumc that in an n-type gallium arsenide semiconductor at T = 300 K, the electron concentration varies linearly from 1X1018 to 7X1017 cm-3 over a distance of 0.10 cm. Calculate the diffusion current density if the electron diffusion coefficient is Dn= 225 cm2/s.
Answers
In order to calculate the diffusion current density in the given n-type gallium arsenide semiconductor, we can use Fick's first law of diffusion, which states that the diffusion current density (Jn) is equal to the product of the electron charge (q), the electron diffusion coefficient (Dn), and the gradient of the electron concentration (dn/dx).
First, we need to calculate the gradient of the electron concentration. The gradient is defined as the change in concentration divided by the change in distance. In this case, the change in concentration is (7X10^17 - 1X10^18) cm^-3, and the change in distance is 0.10 cm.
Substituting these values into the gradient formula, we have:
Gradient of electron concentration (dn/dx) = (7X10^17 - 1X10^18) cm^-3 / 0.10 cm
Next, we can calculate the diffusion current density by multiplying the electron charge (q), the electron diffusion coefficient (Dn), and the gradient of the electron concentration (dn/dx). The electron charge (q) is a constant equal to 1.6X10^-19 C.
Diffusion current density (Jn) = q * Dn * (dn/dx)
Substituting the given values, we have:
Diffusion current density (Jn) = (1.6X10^-19 C) * (225 cm^2/s) * [(7X10^17 - 1X10^18) cm^-3 / 0.10 cm]
Simplifying the expression, we can calculate the diffusion current density. Please note that the result will depend on the values of the given concentrations and distance.
Remember to substitute the given values and perform the necessary calculations to find the diffusion current density.
To know more about diffusion visit:
https://brainly.com/question/13513898
#SPJ11
list 5 things which we can make using each of the following materials
Answers
Answer:
Glass
1.microscopes
2.mirrors
3.glass shelves
4.wrist watch glass
5.magnifying glass
Metal
1.keys
2.automobile body parts
3.coins
4.window frames
5.screws and nails
Plastic
1.bottles
2.boxes
3.balls
4.carry bags
5.buckets and mugs
Wood
1.chairs
2.tables
3.shoe stand
4.dressing table
5.shelves
Please Help Quick ASAP Hurry Chemistry Unit 4 Review Sheet
SO2(g) + O2(g) → SO3(g)
4. If 65.8 grams of SO3 are produced in the unbalanced reaction below, how many grams of oxygen reacted?
SO2(g) + O2(g) → SO3(g)
Work:
Ans:_________
Answers
The mass of oxygen that reacted would be 26.32 grams.
Stoichiometric problemThe balanced equation of the reaction would be as follows:
\(SO_2(g) + 2O_2(g) --- > 2SO_3(g)\)
Thus, the mole ratio of the \(SO_3\) that is produced and the \(O_2\) that reacts is 1:1.
Recall that, mole is the ratio of mass and molar mass. That is:
Mole = mass/molar mass
Mole of 65.8 grams of \(SO_3\) = 65.8/80 = 0.8225 mol
From the mole ratio, the equivalent mol of \(O_2\) that reacts would also be 0.8225 mol.
Mass of 0.8225 mol \(O_2\) = 0.8225 x 32
= 26.32 grams
In other words, the mass of oxygen that reacted to produce 65.8 grams of \(SO_3\) according to the reaction is 26.32 grams.
More on stoichiometric problems can be found here: https://brainly.com/question/28297916
#SPJ1
Use the reaction below to balance the nuclear reaction equation.
Superscript 22 subscript 11 upper N right arrow superscript a subscript z variable X plus superscript 0 subscript plus 1 e.
The isotope produced from the beta plus decay of sodium-22 is
.
Answers
Answer:
neon-22
Explanation:
i got it right..have a great day.Jesus loves you<3
The isotope produced from the beta plus decay of sodium-22 is neon 22.
What are isotope ?Isotopes are two or more types of atoms that have the same atomic number and position in the periodic table but differ in nucleon numbers because their nuclei have different numbers of neutrons.
Carbon 12 and Carbon 14 are both carbon isotopes, one with 6 neutrons and the other with 8 neutrons (both with 6 protons). Carbon-12 is an isotope that is stable, whereas carbon-14 is a radioactive isotope (radioisotope). Uranium-235 and uranium-238 are naturally occurring elements in the Earth's crust. Both have extremely long half-lives.
Different elements exist when two atoms have different numbers of protons. If two atoms have the same number of protons but different numbers of neutrons, they are referred to as isotopes. We use two terms to identify nuclides.
Thus,The isotope produced from the beta plus decay of sodium-22 is neon 22.
To learn more about an isotope, follow the link;
https://brainly.com/question/21536220
#SPJ2
10. If 3.5 kJ of energy are added to a 28.2 g sample of iron at 20°C, what
is the final temperature of the iron in kelvins? The specific heat of iron
is 0.449 J(g•K).
Answers
Answer:
569K
Explanation:
Q = 3.5kJ = 3500J
mass = 28.2g
∅1 = 20°C = 20 + 273 = 293K
∅2 = x
c = 0.449
Q = mc∆∅
3500 = 28.2×0.449×∆∅
3500 = 12.6618×∆∅
∆∅ = 3500/12.6618
∆∅ = 276.4220
∅2 - ∅1 = 276.4220
∅2 = 276.4220 + ∅1
∅2 = 276.4220 + 293
∅2 = 569.4220K
∅2 = 569K
When the temperature is increased, there is the increase in thermal energy of the system. The final temperature of the iron in kelvins is 570 K.
What is energy?The energy is the ability to do work.
Given is the energy Q = 3.5 kJ = 3500 J, mass of sample m = 28.2 g, specific heat of iron Cp = 0.449 J(g•K).
The initial temperature in kelvins is T1 = 20°C = 20 + 273 = 293K
The heat is related to the temperature difference as
Q = m c ∆T
Substitute the values into the expression,
3500 = 28.2 × 0.449 × ∆T
3500 = 12.6618 × (T2 -T1)
T2 - T1 = 276.4220
T2 = 276.4220 K +293 K
T2 = 569.4220K
The temperature of the iron is approximately 570 K.
Thus, the final temperature of the iron in kelvins is 570K.
Learn more about energy.
https://brainly.com/question/1932868
#SPJ2
Give one example of each of the following, that happens to us in our everyday life: Explain a bit about the science behind it, so for example, for melting you can say ice cream melting in your hand, which turns from a solid to a liquid, which is melting. If you are unsure please do not answer, though if you are confident please be free to do so! Have a wonderful day or night!
a) Melting:
b) Freezing:
c) Condensation:
d) Evaporation:
e) Sublimation.
Answers
a) Melting: An example of melting that occurs in our everyday life is when we heat butter on a stovetop.
b) Freezing: Freezing is the process in which a liquid transforms into a solid upon cooling.
c) Condensation: One example of condensation that we encounter regularly is when water droplets form on the surface of a cold drink on a hot day.
d) Evaporation: Evaporation is the process by which a liquid transforms into a gas or vapor.
e) Sublimation: Sublimation refers to the transformation of a substance directly from a solid to a gas without passing through the liquid state.
a) Melting: Butter is a solid at room temperature, but when heat is applied, it melts into a liquid. This change is a result of the increase in temperature, which provides enough energy to overcome the intermolecular forces holding the butter molecules together.
b) Freezing:Eventually, the temperature reaches the freezing point of water (0°C or 32°F), at which the water molecules slow down and arrange themselves into a regular, crystalline structure. This transformation from a liquid to a solid state is accompanied by the release of heat energy.
c) Condensation: As the temperature decreases, the air's capacity to hold moisture decreases, causing the water vapor in the air to condense into liquid water droplets. This process occurs due to the transfer of heat energy from the warm air to the cold surface, leading to the saturation of the air and the conversion of water vapor into liquid form.
d) Evaporation: As the sun's heat energy is absorbed by the water molecules on the clothes' surface, their kinetic energy increases, causing them to break free from the liquid phase and escape into the surrounding air as water vapor. This process occurs because the molecules at the liquid surface with sufficient energy can overcome the attractive forces within the liquid and enter the gas phase.
e) Sublimation: Sublimation refers to the transformation of a substance directly from a solid to a gas without passing through the liquid state. An example of sublimation is the process of dry ice (solid carbon dioxide) converting into carbon dioxide gas.
For more such questions on Freezing visit:
https://brainly.com/question/40140
#SPJ8
The molar mass of calcium hydroxide, Ca(OH)2, is 74.10 g/mole 58.09 g/mole 57.09 g/mole 54.02 g/mole r 114.2 g/mole How many moles of K SO4 are in 15.0 g of KSO.? 0.119moles 0.0861 moles 0.111 moles 2.61 x 103 moles 0.172 moles How many grams of glucose (CeH120s) are in 3.55 moles of glucose? 180. g 426 g 103 g 640. g 507 g
Answers
The molar mass of calcium hydroxide, Ca(OH)₂ is 74.10 g/mol. The moles of the K₂SO₄ is 0.086 mol . The grams of the glucose is 639 g.
The molar mass of the Calcium oxide , Ca(OH)₂ is :
Ca(OH)₂ = 40 + 2( 15.99 + 1 )
= 74.10 g/mol
The moles of the K₂SO₄ in the 15 g of the K₂SO₄ :
Moles = mass / molar mass
Moles = 15 / 174.25
Moles = 0.086 mol
The moles of the glucose, C₆H₁₂O₆ = 3.55 mol
The mass of the glucose = moles × molar mass
= 3.55 × 180
= 639 g
To learn more about moles here
https://brainly.com/question/26416088
#SPJ4
_____________ is the method of determining the concentration of a solution of unknown concentration by adding a controlled and measured amount of a solution of a known concentration until a desired chemical reaction occurs.
Answers
Titration is the method of determining the concentration of a solution of unknown concentration by adding a controlled and measured amount of a solution of a known concentration until a desired chemical reaction occurs.
In titration, the solution of known concentration is called the titrant, and the solution of unknown concentration is called the analyte. The reaction between the two solutions is carefully monitored by observing a change in physical properties, such as color, or by measuring a change in the conductivity or pH of the solution. The volume of titrant needed to cause the reaction is used to calculate the concentration of the analyte. Titration is widely used in analytical chemistry, quality control, and industrial processes to determine the precise concentration of a variety of substances in solution.
Learn more about Titration:
brainly.com/question/2728613
#SPJ4
1: At which temperature would a reaction withΔH = -102 kJ/mol, ΔS = -0.188 kJ/(mol×K) be spontaneous? 2: At which temperature would a reaction withΔH = 132 kJ/mol, ΔS = 0.200 kJ/(mol×K) be spontaneous?
Answers
Answer:
1: At temperatures below 542.55 K
2: At temperatures above 660 K
Explanation:
Hello there!
In this case, according to the thermodynamic definition of the Gibbs free energy, it is possible to write the following expression:
\(\Delta G=\Delta H-T\Delta S\)
Whereas ΔG=0 for the spontaneous transition. In such a way, we proceed as follows:
1:
\(0=\Delta H-T\Delta S\\\\T=\frac{-102kJ/mol}{-0.188kJ/mol-K} \\\\T=542.55K\)
It means that at temperatures lower than 542.55 K the reaction will be spontaneous.
2:
\(0=\Delta H-T\Delta S\\\\T=\frac{132kJ/mol}{0.200kJ/mol-K} \\\\T=660K\)
It means that at temperatures higher than 660 K the reaction will be spontaneous.
Best regards!
*The diagram has to do with the question*
Pls help

Answers
Wgsystststsgdhdb
Oil refineries use state of the art distillation processes to create synthetic oils for use. Which description best explains this process?
A) Crude oil is cooled to separate into synthetic oils.
B) Crude oil is heated at different temperatures to create synthetic oils.
C) Crude oil is separated by allowing the crude oil impurities to evaporate.
D Crude oil is separated into different synthetic oils using high tech filters.
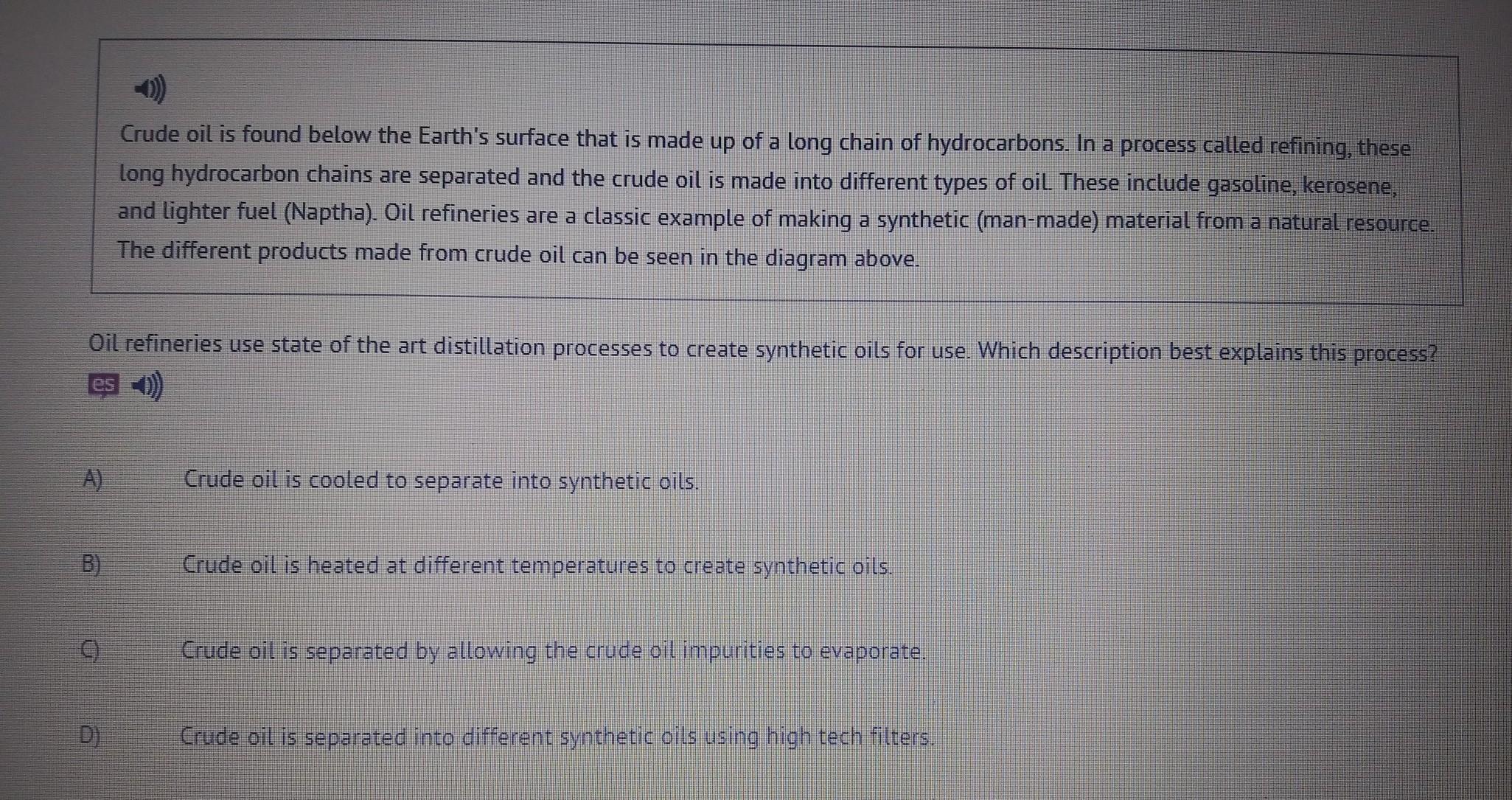
Answers
Answer:
A
Explanation:
all of the following may change during a chemical reaction except a) the total number of atoms in the system. b) the temperature of the system. c) the color of the system. d) the total number of molecules in the system. e) the physical state of the system.
Answers
All the following may change during a chemical reaction except (a) the total number of atoms in the system.
Chemical reactions follow the law of conservation of mass which states that mass is neither destroyed nor created during a chemical reaction. This means that the total number of atoms during the reaction remains constant.
In physics and chemistry, the Law of Conservation of Mass, or the Principle of Conservation of Mass, states that in a system that is closed to all transfers of matter and energy, the mass of the system must remain constant over time. increase. The system cannot be changed. In other words, you cannot add or remove quantities. Mass is therefore conserved over time. [1]
This law implies that mass can be rearranged in space and change the shape of the entity associated with it, but it cannot be created or destroyed.
Learn more about law of conservation of mass here :
https://brainly.com/question/15289631
#SPJ4
what is the molecular shape of the osbcl− 2 molecule? sb is the central atom. select all that apply
Answers
The molecular shape of the "OsbCl2" molecule can be determined by using the VSEPR (Valence Shell Electron Pair Repulsion) theory. This theory predicts the molecular shape based on the repulsion between the electron pairs surrounding the central atom (in this case, the "sb" atom).
Based on the VSEPR theory, the molecular shape of OsbCl2 is:Square Planar In a square planar molecular geometry, the central atom is surrounded by four electron pairs (or regions of high electron density) arranged in a square shape. These electron pairs are in a plane, and the bond angles are approximately 90°.The molecular shape of OsbCl2 is Square Planar, which means the central atom (sb) is surrounded by four electron pairs arranged in a square shape and the bond angles are approximately 90°
Learn more about molecular shape here:
https://brainly.com/question/12449743
#SPJ4
can there be an ionic bonding between 2 metals or 2 non-metals?
Answers
Answer:
No
Explanation:
Ionic bonding is where an one atom wants to gain e- while the other wants to lose e-
Such as NaCl.
What is the pH and pOH of a solution prepared by dissolving 1.40 g of Ca(OH)2 in water to make 865 mL of solution?
Answers
Number of moles of Ca(OH)2 = 1.40 g / 74.10 g/mol = 0.0189 mol
Volume of solution = 865 mL = 0.865 L
Concentration of OH- = 2 x 0.0189 mol / 0.865 L = 0.0437 M
From this, we can calculate the pOH:
pOH = -log[OH-] = -log(0.0437) = 1.36
Using the fact that pH + pOH = 14, we can calculate the pH:
pH = 14 - pOH = 14 - 1.36 = 12.64
So, the pH of the solution is 12.64 and the pOH is 1.36.
(If this doesn’t seem right to you make sure you comment!)
the root-mean-square speed of nitrogen molecules in m/s, at 125 oc is closest to...
Answers
The root-mean-square (rms) speed of nitrogen molecules can be calculated using the formula vrms = √(3kT/m), where k is Boltzmann's constant, T is the temperature in Kelvin, and m is the mass of one nitrogen molecule. At 125°C, which is 398 K, the vrms of nitrogen molecules is closest to 585 m/s.
To arrive at this answer, we need to convert the temperature to Kelvin (398 K) and the mass of a nitrogen molecule is 28 atomic mass units. Using these values and the formula, we can calculate the vrms of nitrogen molecules to be 585 m/s.
The root-mean-square speed (RMS speed) of nitrogen molecules at 125°C can be calculated using the formula:
RMS speed = √(3RT/M)
where R is the ideal gas constant (8.314 J/(mol·K)), T is the temperature in Kelvin (125°C + 273.15 = 398.15 K), and M is the molar mass of nitrogen (28.02 g/mol x 0.001 kg/g = 0.02802 kg/mol).
Plugging these values into the formula:
RMS speed = √(3 × 8.314 × 398.15 / 0.02802)
RMS speed ≈ 515 m/s
So, the root-mean-square speed of nitrogen molecules at 125°C is closest to 515 m/s.
To know about RMS visit:
https://brainly.com/question/29662026
#SPJ11
If a sample of nitrogen gas is compressed to fit in a smaller container, what happens to the
pressure, assuming the temperature remains constant?
Answers
Answer:
The pressure increases
Explanation:
In this action it is best to apply Boyle's law to resolve the problem. This is an interplay between Volume, Pressure and Temperature.
According to Boyle's Law "the volume of a fixed mass of a gas varies inversely as the pressure changes, if the temperature is constant". Since the volume is being reduced to cram into the container, the pressure of the gases increases. In the smaller container, pressure rises and the volume reduces if temperature remains constant.Balance the chemical equations

Answers
Answer:
Explanation:
1). Ca(NO₃)₂ + KI → CaI + K(NO₃)₂
This equation is incorrect.
When Ca⁺⁺ reacts with I⁻, final product is CaI₂
And when K⁺ react with NO₃⁻, final product is KNO₃
Hence the equation will be,
Ca(NO₃)₂ + KI → CaI₂ + KNO₃
Now we have to balance this equation.
Ca(NO₃)₂ + 2KI → CaI₂ + KNO₃
↓
Ca(NO₃)₂ + 2KI → CaI₂ + 2KNO₃
2). Ca(NO₃)₂ + KOH → CaOH + K(NO₃)₂
This equation is incorrect,
Since the reaction of Ca⁺⁺ with OH⁻ gives the final product Ca(OH)₂
And final product of K⁺ and NO₃⁻ is KNO₃
Therefore, the equation will be,
Ca(NO₃)₂ + KOH → Ca(OH)₂ + KNO₃
Now we will balance this equation by changing the coefficients of the molecules until the number of atoms on both the sides become equal.
Ca(NO₃)₂ + KOH → Ca(OH)₂ + 2KNO₃
↓
Ca(NO₃)₂ + 2KOH → Ca(OH)₂ + 2KNO₃
3). Ca(NO₃)₂ + Na₂C₂O₄ → CaC₂O₄ + 2Na(NO₃)₂
This equation is incorrect,
Since the reaction of Na⁺ and NO₃⁻ gives the final product NaNO₃.
Therefore, the correct equation will be,
Ca(NO₃)₂ + Na₂C₂O₄ → CaC₂O₄ + 2NaNO₃
This equation is in the balanced form.
How do you find the number of neutrons in a atom?
Answers
Answer:
To find the number of neutrons in an atom simply calculate the difference between the atomic mass of the atom and the number of protons in the atom. You can find the number of protons in an atom by looking at it's atomic number. The reason this works is because atomic mass is the amount of protons and neutrons. Subtracting the protons from that amount leaves us with the neutrons.
Example: Let's look at Arsenic (my favorite element lol) which has an atomic number of 33 and atomic mass of about 75 (always round to nearest whole number) which means that the number of neutrons is 75 - 33 = 34.
A sample of no2 occupies 16,500 ml at stp. How many no2 molecules are in the sample?.
Answers

According to Avogadro's law equal volume of all gases at the same temperature and pressure contain equal number of molecules. At STP one mole of any gas behaving ideally occupies a volume of 22.414 L.
What is molar volume?The volume occupied by one mole of a substance at a given temperature and pressure is defined as the molar volume at that temperature and pressure. The value of molar volume of an ideal gas at STP is important in stoichiometric calculations.
At STP, the number of molecules of a gas is given as:
Number of molecules of a gas (N) = V₀ in L / 22.414 × 6.022 × 10²³
Here V₀ in L = 16500 = 16.5 L
N = 16.5 / 22.414 × 6.022 × 10²³
N = 4.433 × 10²³
Thus the number of molecules present is 4.432 × 10²³.
To know more about number of molecules, visit;
https://brainly.com/question/19109598
#SPJ2
What is the molar mass of Al(NO3)3 · 3H2O?
Answers
Answer:
Go to your periodic table and look for each element. Find the mass for Aluminum, Nitrogen, Oxygen, and Hydrogen.
After you find those, for each compound you must add them together and find the least amount of sig figs after the decimal point.
For the first compound, Al(NO3)3, you will have a total of
1 Aluminum atom
3 Nitrogen atoms
9 Oxygen atoms
Aluminum has 26.982 g/mol
Nitrogen has 14.007 g/mol
Oxygen has 15.999 g/mol
Now multiply those numbers by the amount of atoms of each element.
(1)26.982 g/mol = 26.982 g/mol
(3)14.007 g/mol = 42.021 g/mol
(9)15.999 g/mol = 143.991 g/mol
now add all of those numbers together, and you see that their least significant figure after the decimal is 3, so round to 3 digits after the decimal point.
26.983 + 42.021 + 143.991 = 212.994
now do the same for the other compound
to start you off.. you have
6 Hydrogen atoms
3 Oxygen atoms
Hydrogen has 1.009 g/mol
Oxygen has 15.999 g/mol
the least significant figure after the decimal point is 3, so round you 3 digits after the decimal point.
after you finish getting your totals, you. until them and find the greatest sig fig over all. comment on this if you need further instruction :)
approximately how many joules of heat required to raise the temperature of 20 g of water from thirty degrees celsius to 40 degrees celsius
Answers
To raise the temperature of 20 grams of water from 30 degrees Celsius to 40 degrees Celsius requires approximately 8.4 joules of heat.
This can be calculated using the equation Q = mcΔT, where m is the mass of the water (20 grams), c is the specific heat capacity of water (4.184 J/g°C), and ΔT is the change in temperature (10°C). Therefore, the required heat is 8.4 J.
The equation can be used to calculate the amount of energy required to raise the temperature of a given mass of a substance from one temperature to another. For example, if we wanted to calculate the amount of energy required to raise the temperature of 20 grams of water from 30 degrees Celsius to 40 degrees Celsius, we would use the following equation: Q = (20 grams)(4.184 J/g°C)(10°C) = 8.4 J.
This means that 8.4 joules of energy are required to raise the temperature of 20 grams of water from 30°C to 40°C.
Learn more about temperature:
https://brainly.com/question/12791197
#SPJ4
5. Which of the following are all physical properties of matter?
a. density, color, hardness
b. density, reactions, hardness
c chemistry, freezing point, color
d. lightness, electrons, boiling point
Answers
Hw does water vapor response, if temperature rises 1K?
Answers
When the temperature rises by 1 Kelvin (1K), the response of water vapor is typically an increase in its concentration in the atmosphere. This is because warmer air can hold more water vapor due to its increased capacity to hold moisture.
As the temperature increases, the kinetic energy of water molecules also increases, leading to greater evaporation from liquid water sources such as oceans, lakes, and rivers. This increased evaporation results in an increased amount of water vapor in the air. Water vapor is a greenhouse gas, meaning it has the ability to absorb and emit infrared radiation. As the concentration of water vapor increases in response to rising temperature, it enhances the greenhouse effect, trapping more heat in the atmosphere. This positive feedback loop can further amplify the temperature increase, leading to more evaporation and an even higher concentration of water vapor.
Learn more about temperature here: brainly.com/question/29524629
#SPJ11