Answers
The pressure of butane is 20 P(butane) = 0.77 atm and pressure of P(isobutane) = 19.2 atm
Isomerization reactionIsomerization is the transformation of a molecule into a different isomer, and it can take one of two forms: cis or trans. Protein isomerization was first described in 1968 as a method of dramatically altering protein conformation by disrupting polypeptide secondary structure.
Isomerism is caused by two main factors: Difference in atom linking mode. Difference in the spatial arrangement of atoms or groups. The difference in the mode of atom linking is one of the two main causes of isomerism. Difference in the spatial arrangement of atoms or groups.
Types :The two main types of isomerism are structural or constitutional isomerism, in which the bonds between the atoms differ, and stereoisomerism or spatial isomerism, which occurs when the bonds are similar but the relative positions of the atoms differ.
To know more about polypeptide :
https://brainly.com/question/28270191
#SPJ9
I understand the question you are looking for
For the isomerization reaction:
butane ⇌ isobutane
Kp equals 25 at 500°C. If the initial pressures of butane and isobutane are 20. atm and 0.0 atm, respectively, what are the pressures of the two gases at equilibrium?
A) P(butane) = 20 atm and P(isobutane) = 0.80 atm
B) P(butane) = 19.2 atm and P(isobutane) = 0.77 atm
C) P(butane) = 0.77 atm and P(isobutane) = 19.2 atm
D) P(butane) = 0.80 atm and P(isobutane) = 20. atm
Related Questions
How many seconds are in 100 years
Answers
an electron in the 3rd shell of an Aluminium atom moves to the first shell in a bombardment process. Calculate the frequency of the electron the 3rd orbit and energy used in transition to the first shell.[h=6.626*10^-14J/Hz [R=3.29*10^15 Hz]
Answers
Following the quantic theory, the energy of a photon equals the radiation frequency multiplied by the universal constant. ν = 2.923x10¹⁵ Hz. E = 3.09x10¹⁵Hz.
What is quantum mechanic?It is the branch of physics that studies objects and forces at a very low scale, at atoms, subatoms, and particles levels.
Quantum mechanics states that the elemental particles that constitute matter -electrons, neutrons, protons- have the properties of a wave and a particle.
It emerges from the quantic theory exposed by Max Planck (1922), in which he affirmed that light propagates in energy packages or photons.
He discovered the Universal Planck constant, h, used to calculate the energy of a photon.
He stated that the energy of a photon (E) equals the radiation frequency (ν) multiplied by the universal constant (h).
E = νh
In the exposed example, we need to calculate the energy required to change from the 3rd shell to the first shell.
To do it, we should know that the energy in a level (Eₙ) equals the energy associated to an electron in the most inferior energy level (E₁) divided by the square of the shell number (n²).
Eₙ = E₁ / n²
E₁ is a constant. We can express it in Joules or electroVolts
E₁ = -2.18x10⁻¹⁸ JE₁ = -13.6 eVSo, let us calculate the energy at level 1 and 3
Eₙ = E₁ / n²
E₁ = -2.18x10⁻¹⁸ J / 1² = -2.18x10⁻¹⁸ JE₁ = -13.6 eV / 1² = -13.6 eV
E₃ = -2.18x10⁻¹⁸ J / 3² = -2.18x10⁻¹⁸ J / 9 = - 2.42x10⁻¹⁹ JE₃ = -13.6 eV / 3² = -13.6 eV / 9 = - 1.51 eV
The change of energy can be calculated in two ways,
Option 1
ΔE = E₁ - E₃ = 2.18x10⁻¹⁸ - 2.42x10⁻¹⁹ = 1.93x10⁻¹⁸J
ΔE = E₁ - E₃ = 13.6 - 1.51 = 12.09 eV
Option 2
ΔE = -2.18x10⁻¹⁸ J (1/nf² - 1/ni²)
ΔE =-13.6 eV (1/nf² - 1/ni²)
Where nf is the final level and ni is the initial level. When the electron passes from its initial level to its final level it is called electronic transition.
ni = 3nf = 1ΔE = -2.18x10⁻¹⁸ J (1/nf² - 1/ni²)
ΔE = -2.18x10⁻¹⁸ J (1/1² - 1/3²)
ΔE = -2.18x10⁻¹⁸ J (1 - 0.111)
ΔE = -2.18x10⁻¹⁸ J (0.888)
ΔE = - 1.937x10⁻¹⁸ J
or
ΔE = -13.6 eV (1/nf² - 1/ni²)
ΔE = -13.6 eV (1/1² - 1/3²)
ΔE = -13.6 eV (1 - 0.111)
ΔE = -13.6 eV (0.888)
ΔE = -12.08 eV
This is the energy required for the electron to go from n= 3 to n = 1. The negative sign (-) means energy (as light or photons) released or emitted.
If we want to express the result in Hz, we just need to make a conversion.
1Hz ⇔ 6.626x10⁻³⁴J ⇔ 4.136x10¹⁵ eV.
The energy required for the electron to go from n= 3 to n = 1 is 3.09x10¹⁵ Hz.
Now, we need to calculate the frequency, ν. This is, how many times the wave oscillates back and foward per second.
To do it, we will use the universal Planck constant, h, and the absolute value of the energy, E.
ν = E/h = 1.937x10⁻¹⁸ J / 6.626x10⁻³⁴ Js = 2.923x10¹⁵ 1/s = 2.923x10¹⁵ Hz.
Answer:
Frequency, ν = E/h = 2.923x10¹⁵ Hz.Energy, E = 3.09x10¹⁵ Hz.You can learn more about quantum mechanic at
https://brainly.com/question/11855107
https://brainly.com/question/23780112
https://brainly.com/question/11852353
What type of reaction is the following
chlorine +
potassium nitride
- nitrogen
+
potassium chloride
Answers
Explanation:
Answer
Open in answr app
Correct option is
A
Precipitation reaction
B
Double displacement reaction
Balanced equation for the reaction is:
KCl(s)+AgNO3(aq)→AgCl(s)+KNO3(aq)
It is a double displacement reaction as both reactants exchange ions to form new products. Also, it is a precipitation reaction as white precipitates of silver chloride(AgCl) are formed.
What happens to the molecule If energy is removed from a substance
Answers
Answer:
Removing energy (cooling) atoms and molecules decreases their motion, resulting in a decrease in temperature
a gas has an initial volume of 3,480 mL and an initial temperature of - 70.0 C. what must be the temperature of the gas in kelvin if its volume is reduced to 2,450 mL
Answers
The temperature of the gas in Kelvin, after its volume is reduced to 2,450 mL, is approximately 143.27 K.
To determine the temperature of the gas in Kelvin after its volume is reduced, we can use the combined gas law, which relates the initial and final conditions of pressure, volume, and temperature for a given amount of gas.
The combined gas law equation is:
(P₁ * V₁) / T₁ = (P₂ * V₂) / T₂
Where P₁ and P₂ are the initial and final pressures, V₁ and V₂ are the initial and final volumes, T₁ is the initial temperature in Kelvin, and T₂ is the final temperature in Kelvin.
Given that the initial volume V₁ is 3,480 mL, the initial temperature T₁ is -70.0 °C (which needs to be converted to Kelvin), and the final volume V₂ is 2,450 mL, we can substitute these values into the equation.
To convert -70.0 °C to Kelvin, we add 273.15 to it, resulting in T₁ = 203.15 K.
Now we can solve for T₂:
(T₂ * V₁) / T₁ = V₂
T₂ = (V₂ * T₁) / V₁ = (2,450 mL * 203.15 K) / 3,480 mL
Simplifying the equation, we find:
T₂ ≈ 143.27 K
Therefore, the temperature of the gas in Kelvin, after its volume is reduced to 2,450 mL, is approximately 143.27 K.
For more question on temperature
https://brainly.com/question/4735135
#SPJ8
Which one is it I don’t know

Answers
Using the prefix method for covalent compounds, the names of the given compounds are shown below:
LIF: Lithium fluoride
Cl2O7: Dichlorine heptoxide
N2O3: Dinitrogen trioxide
SF6: Sulfur hexafluoride
Na3PO4: Sodium phosphate
What are covalent compounds?A covalent bond is described as a chemical bond that involves the sharing of electrons to form electron pairs between atoms.
The properties of covalent compounds includes:
The boiling/melting points of covalent compounds are low.covalent compounds are soft in nature and relatively flexible.covalent compounds do not possess electrical conductivity.Learn more about covalent compounds at:
https://brainly.com/question/3447218
#SPJ1
I need help with chem. How do I know if this is balanced ?

Answers
https://www.khanacademy.org/science/chemistry/chemical-reactions-stoichiome/balancing-chemical-equations/v/balancing-chemical-equations-introduction
this will help trust me
have a good day
How
many
will
be
formed
when
of
is
completely
reacted
according
to
the
balanced
chemical
reaction:
FeCl₃(aq)
+
AgNO₃(aq)
→
AgCl(s)
+
Fe(NO₃)₃(aq)
Answers
The mass of the silver chloride that is formed in the reaction is 4.29 g.
What is the reaction?A chemical reaction is a process that leads to the transformation of one set of chemical substances to another.
We know that the balanced reaction equation have been shown in the question as shown.
Number of moles of the silver nitrate = 60/1000 * 0.5
= 0.03 moles
If the reaction is 1:1, then 0.03 moles of silve chloride is formed.
Mass of the silver chloride formed = 0.03 moles * 143 g/mol
= 4.29 g
Learn more about reaction:https://brainly.com/question/13014923
#SPJ1

Calculate the mass (in kg) of 4.87 x 10 25 atoms of Zn
Answers
Answer:
5.29kg
Explanation:
5.29kg
The mass of 4.87×10²⁵ atoms of Zn is 5.29 Kg
From Avogadro's hypothesis,
6.02×10²³ atoms = 1 mole of ZnRecall,
1 mole of Zn = 65.38 g
Converting 65.38 g to Kilogram (Kg), we have:
1000 g = 1 Kg
Therefore,
65.38 g = 65.38 / 1000
65.38 g = 0.06538 KgThus, we can say that,
6.02×10²³ atoms = 0.06538 Kg of ZnFinally, we shall determine the mass of Zn that contains 4.87×10²⁵ atoms. This can be obtained as follow:
6.02×10²³ atoms = 0.06538 Kg of Zn
4.87×10²⁵ atoms = \(\frac{4.87*10^{25} * 0.06538}{6.02*10^{23} } \\\\\)
4.87×10²⁵ atoms = 5.29 Kg of ZnTherefore, the mass of 4.87×10²⁵ atoms of Zn is 5.29 Kg
Learn more: https://brainly.com/question/5809151
What functional groups are in ch2=chch2oh?
Answers
Explanation:
H
|
H-C=C-C-OH
| | |
H H H
in this organic compound the functional groups are OH and alkene
prop-2-enol
since it has a double bond, alkene is the functional group. and also it has OH group so hydroxyl group also the other functional group
How many atoms or molecules are in 10 grams of table salt?
Answers
Answer:
1.03 x 10²³ atoms NaCl
Explanation:
To find the amount of table salt (NaCl) in atoms, you need to (1) convert grams to moles (using the molar mass) and then (2) convert moles to atoms (using Avogadro's Number). It is important to arrange the ratios/conversions in a way that allows for the cancellation of units.
(Step 1)
Molar Mass (NaCl): 22.99 g/mol + 35.45 g/mol
Molar Mass (NaCl): 58.44 g/mol
10 grams NaCl 1 mole
------------------------ x ----------------------- = 0.17 moles NaCl
58.44 grams
(Step 2)
Avogadro's Number:
6.022 x 10²³ atoms = 1 mole
0.17 moles NaCl 6.022 x 10²³ atoms
-------------------------- x -------------------------------- = 1.03 x 10²³ atoms NaCl
1 mole
Describe how lead as a toxic metal can be determine in borehole water?
Answers
We can be able to determine the amount of toxic lead in the water by thee use of atomic absorption spectrophotometry.
What is a toxic metal?
A toxic metal is known as any metal that is able to affect the health of people. We know that toxic metals are mostly the metals that are in the group of the heavy metals.
Now we know lead as a metal that is able to cause brain damage especially in children. This is why it is very important that there should be a thorough examination in order to know the amount of lead that is present in water.
There are several methods that could be applied in the determination of lead and one of the most common methods is by the use of atomic absorption spectrophotometry which is able to detect even the minutest amount of the led in solution.
Learn more about toxic metals:https://brainly.com/question/28331004
#SPJ1
A chemist mixes 24.0 g H2 with 8.0 g N2. Assuming the reaction goes to completion, which reactant should she use to calculate the yield?
3H2 + N2 + 2NH3
A. Either reactant, because each will be completely used up in the reaction
B. Either reactant, because they are in a ratio of 3:1 for H2:N2
C. Hydrogen, because it could produce the greater amount of product
D. Nitrogen, because the ratio of moles is greater than 3:1 for Hz:N2
Answers
Answer:
D. Nitrogen, because the ratio of moles is greater than 3:1 for H2:N2
Explanation:
Hello,
In this case, given the reaction:
\(3H_2 + N_2 \rightarrow 2NH_3\)
In order to identify the limiting reactant we must compute the moles of ammonia yielded by both reactants at first:
\(n_{NH_3}^{by\ H_2}=24.0gH_2*\frac{1molH_2}{2molH_2}*\frac{2molNH_3}{3molH_2}=8molNH_3\\ \\n_{NH_3}^{by\ N_2}=8.0gN_2*\frac{1molN_2}{28molN_2}*\frac{2molNH_3}{1molN_2}=0.57molNH_3\)
Thus, since nitrogen yields the smallest amount of ammonia as it is more heavy than hydrogen and it is in a 3:1 mole ratio for H2:N2, it is the limiting reactant, therefore the answer is D. Nitrogen, because the ratio of moles is greater than 3:1 for H2:N2.
Regards.
Assuming ideal solution behavior, what is the boiling point of a solution of 115.0 g of nonvolatile sucrose (table sugar), C₁₂H₂₂O₁₁ (342.300 g/mol), in 350.0 g of water (Kb = 0.512 °C m⁻¹; boiling point = 100.0 °C)?
a.)
100.00049 °C
b.)
99.5 °C
c.)
268.2 °C
d.)
100.5 °C
Answers
The boiling point of water is 100.0 °C, the boiling point of the solution will be : 101.49 °C.The correct answer is option (a) 100.00049 °C.
Ideal Solution : An ideal solution is a homogeneous mixture of two or more components that obeys Raoult's law, which states that each component's vapor pressure is proportional to its mole fraction.The boiling point of a solution depends on the solvent's properties and the solute's concentration. It's dependent on the mole fraction of the solvent and solute, as well as the total concentration of the solution. The change in boiling point of a solution is given byΔTb = Kb × m × i, whereKb = ebullioscopic constant, m molarity of the solution, and i = van't Hoff factor.Assuming that the solution's behavior is ideal, we may use the molality of the solution to compute the boiling point elevation of the solution.The molality of the solution is given by the following formula:m = (n₂ / m₂) ÷ (n₁ / m₁), where n is the number of moles, m is the mass, and the subscripts 1 and 2 refer to water and non-volatile solute sucrose, respectively.The molar mass of sucrose (C₁₂H₂₂O₁₁) is342.3 g/mol; therefore, the number of moles of sucrose is115.0 g ÷ 342.3 g/mol = 0.335 mol.m₁ = mass of water = 350.0 g, and m₂ = mass of sucrose = 115.0 g, as given in the problem.Therefore, the molality of the solution is given by:m = (0.335 mol / 0.115 kg) ÷ (1 mol / 1 kg) = 2.91 mol/kg.Substituting these values in the formula for ΔTb, we get:ΔTb = Kb × m = 0.512 °C m⁻¹ × 2.91 mol/kg = 1.49 °C.100.0 °C + 1.49 °C = 101.49 °C.
for such more questions on solution
https://brainly.com/question/25326161
#SPJ8
To convert m-ethylaniline to m-ethylfluorobenzene, it should be treated with nitrous acid followed by _____________.
Answers
Answer:
\(NaBF_4\)
Explanation:
In this reaction, we must exchange the amino group (\(NH_2\)) for a fluorine atom (\(F\)). Also, the first step in this reaction is the addition of nitrous acid.
We must remember that the amino group in the presence of nitrous acid produces a diazonium salt. The \(N_2\) group is a very good leaving group and many benzene derivatives can be produced from this intermediate (see figure 1).
If what we want is to bond a fluorine atom we must use \(NaBF_4\) to be able to produce m-ethylfluorobenzene (see figure 2).
I hope it helps!

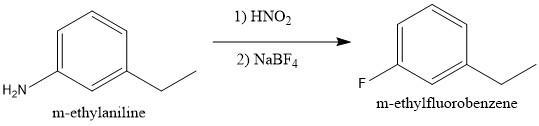
what is ionisation energy
Answers
Answer:
minimum energy required to to remove the most loosely bound election of an isolated gaseous atom.
Explanation:
The kinetic energy of an object depends on how much space an object takes up True or false?
Answers
write electron dot structures for the atoms and ions of each of the following elements. 1. ca 2. br 3. al
Answers
With a 20 atomic number and 20 electrons, calcium is an atom. The atom of bromine has an atomic number of 35 and 35 electrons. 13 electrons make up the atom of aluminium, which has an atomic number of 13.
Write electron dot structures for ca. br. al. ?Calcium: The atom of calcium contains 20 electrons and an atomic number of 20. Calcium has an electron dot structure of 2,8,8,2. Accordingly, the first shell contains two electrons, the second shell eight, the third shell eight, and the fourth shell two electrons.Bromine: The atom of bromine contains 35 electrons and an atomic number of 35. Bromine has an electron dot structure of 2,8,18,7. Accordingly, the first shell contains two electrons, the second shell eight, the third shell eighteen, and the fourth shell seven.Aluminum: The atom of aluminium contains 13 electrons and an atomic number of 13. Aluminum has an electron dot structure of 2,8,3. Accordingly, the first shell contains two electrons, the second shell eight, and the third shell three.Ca: [Ar] 4s2.Br: [Ar] 3d10 4s2 4p5.Al: [Ne] 3s2 3p1.To learn more about electron dot structures for the atoms and ions refer to:
https://brainly.com/question/24774222
#SPJ4
Which of these waves has the greatest wavelength? (3 points) Wave shown with 2 wavelengths. Wave shown with 3 wavelengths. Wave shown with 1 wavelength stretch over a short distance. Wavelength shown with 1 wavelength stretched over a long distance.
Answers
The waves that has the greatest wavelength is Wavelength shown with 1 wavelength stretched over a long distance.
Waves explained.A wave could be a disturbance or variety that voyages through a medium or space, carrying vitality without transporting matter. Waves can take different shapes and happen totally different sorts of waves, counting mechanical waves and electromagnetic waves.
Mechanical waves require a medium to propagate, meaning they require a substance like water, discuss, or a strong fabric to transmit the wave. Illustrations of mechanical waves incorporate water waves, sound waves, and seismic waves. In these waves, particles of the medium sway or vibrate in a design, exchanging energy from one molecule to another.
Electromagnetic waves, on the other hand, don't require a medium and can travel through vacuum, such as in space. Electromagnetic waves comprise of electric and attractive areas swaying opposite to each other and to the heading of wave engendering. Illustrations of electromagnetic waves incorporate obvious light, radio waves, microwaves, infrared waves, bright waves, X-rays, and gamma beams.
Learn more about waves below.
https://brainly.com/question/26116832
#SPJ1
In which way would a drought affect how rodents get their energy?
Answers
Answer:
When a drought occurs, their food supply can shrink and their habitat can be damaged. ... Losses or destruction of fish and wildlife habitat. Lack of food and drinking water for wild animals. Increase in disease in wild animals, because of reduced food and water supplies.While insects and cacti might provide a meagre supply of water, most desert animals survive by being what Price calls "water misers". ... To perform this feat, they have evolved specialized kidneys with extra microscopic tubules for extracting water from urine.Explanation:
What is meaning of Hypothesis
Answers
Answer:
A hypothesis is a suggested solution for an unexplained occurrence that does not fit into current accepted scientific theory.
What volume of 12.0 M HCl is required to make 75.0 mL of 3.50 M HCI? 21.9 mL B) 0.560 mL C) 257 mL D) 560. mL E) none of the above Answer: A Show your calculations below:
Answers
To make 75.0 mL of 3.50 M HCI, 21.9 mL of 12.0 M HCl must be added. The letter A, 21.9 mL, is the right response among the options.
What is the straightforward meaning of volume?The space consumed within an object's borders in three dimensions is referred to as its volume. It is sometimes referred to as the object's capacity.
What is volume, for instance?The potential of an object is gauged by its dimensions. For instance, a cup's capacity is stated to really be 100 ml if it can accommodate 100 ml of distilled water in its brim. The quantity of volume occupied by a three-dimensional object can also be used to describe volume.
To know more about Volume visit:
https://brainly.com/question/24086520
#SPJ4
Inter conversion of glucose and fructose occurs with an eqilibrium constant of 1.0. glicose isomerase catalyzes this reaction. The final concentration of fructose at equilibrim from 40 mM glucose is .
Answers
Inter-conversion of glucose and fructose occurs with an equilibrium constant of 1.0. Glucose isomerase catalyzes this reaction. The final concentration of fructose at equilibrium from 40mM glucose is a. 40 mM.
How to find the final concentration of fructose?Using this formula to find the final concentration of fructose
Final concentration of fructose =Equilibrium from glucose/ Equilibrium constant
Where:
Equilibrium constant = 1.0
Equilibrium from glucose = 40 mM
Let plug in the formula
Final concentration of fructose = 40mM / 1.0
Final concentration of fructose = 40mM
Therefore we can conclude that the correct option is A.
Learn more about Final concentration of fructose here:https://brainly.com/question/14041283
#SPJ1
The complete question is:
Inter-conversion of glucose and fructose occurs with an equilibrium constant of 1.0. Glucose isomerase catalyzes this reaction. The final concentration of fructose at equilibrium from 40mM glucose is
a. 40 mM
b. 20 mM
c. 10 mM
d. 0 mM
The volume of a sample of gas (2.49 g) was 752 mL at 1.98 atm and 62°C. The gas is most likely __________.
A. SO2
B. SO3
C. NH3
D. NO2
E. Ne
Answers
The gas that has a volume of 752 mL at 1.98 atm and 62°C is most likely NO₂ (option D).
How to calculate volume?The volume of a sample of gas can be calculated using the following formula:
PV = nRT
Where;
P = pressureV = volume n = number of molesR = gas law constantT = temperatureAccording to this question, the volume of a sample of gas (2.49 g) was 752 mL at 1.98 atm and 62°C. The number of moles is as follows:
1.98 × 0.752 = n × 0.0821 × 335
1.489 = 27.5n
n = 0.054mol
molar mass of the gas = 2.49g ÷ 0.054mol = 45.99g/mol
The gaseous substance with the molar mass of 45.99g/mol is NO₂.
Learn more about moles at: https://brainly.com/question/27058396
#SPJ1
Please Help!!50 points and I’ll mark as brainliest!
Tasks are in the picture.

Answers
1) The pH is 2.5
2) The pH is 11.5
3) The initial concentration is\(2.1 * 10^-14\)M
What is the pH?pH is a measure of the acidity or basicity of a solution. It is defined as the negative logarithm of the concentration of hydrogen ions in the solution.
1) The pH of the solution can be gotten from;
K = \(x^2\)/0.65 - x
\(1.754 * 10^-5\)(0.65 - x) = \(x^2\)
\(1.14 * 10^-5 - 1.754 * 10^-5x = x^2\\x^2 + 1.754 * 10^-5x - 1.14 * 10^-5 = 0\)
x = 0.003 M
pH = -log(0.003)
= 2.5
2) Kb = \(x^2\)/0.35 - x
\(1.8 * 10^-5 (0.35 - x) = x^2\\6.3 * 10^-6 - 1.8 * 10^-5x = x^2\\x^2 + 1.8 * 10^-5x - 6.3 * 10^-6 = 0\)
x = 0.003 M
pOH = -log (0.003)
= 2.5
pH = 14 - 2.5 = 11.5
3) Hydrogen ion concentration = Antilog (-11.5)
= 3.2 * 10^-12 M
\(4.9 * 10^-10 = ( 3.2 * 10^-12)^2/x \\4.9 * 10^-10x = ( 3.2 * 10^-12)^2\\x = ( 3.2 * 10^-12)^2/4.9 * 10^-10\\x = 2.1 * 10^-14 M\)
Learn more about pH:https://brainly.com/question/29766400
#SPJ1
What is the calculated value of the cell potential at 298K for an electrochemical cell with the following reaction, when the Hg2 concentration is 7.36E-4 M and the Al3 concentration is 1.05 M
Answers
Answer:
Explanation:
Concentration of Hg⁺² = 7.36 x 10⁻⁴ M
Concentration of Al⁺³ = 1.05 M
2Al + 3Hg⁺² = 2Al⁺³ + 3Hg .
E = E₀ + RT / nF ln [ Al⁺³]² / [ Hg⁺² ]³
E₀ = reduction potential of Hg⁺² minus reduction potential of Al⁺³
= 0.92 V - ( - 1.66 V )
= 2.58 V
E = 2.58 + .059 /n log [ Al⁺³]² / [ Hg⁺² ]³
n = 6 , [Al⁺³] = 1.05 M ; [Hg⁺²] = 7.36 x 10⁻⁴ M
E = 2.58 + .059 /6 log [ 1.05]² / [ 7.36 x 10⁻⁴ ]³
= 2.58 + .059 /6 log 27.65 x 10⁸ .
= 2.58 + .059 /6 [8+ log 27.65 ].
= 2.58 + .059 /6 [8+ log 27.65 ].
= 2.58 + .09
= 2.67 V .
How many grams of ammonia are present in 5.0 L of a 0.050 M solution
Answers
Answer:
4.25g
Explanation:
Molar concentration or molarity can be calculated thus;
Molarity (M) = number of moles (n) ÷ volume (V)
According to the provided information in this question, Volume = 5.0L, M = 0.050 M
number of moles = molarity × volume
n = 0.050 × 5
n = 0.25moles
Number of moles in a substance = mass (M) ÷ molar mass (MM)
Molar mass of ammonia (NH3) = 14 + 1(3)
= 17g/mol
Mass = moles × molar mass
Mass (g) = 0.25 × 17
Mass = 4.25g
Help me please.
This is Organic Chemistry

Answers
The condensed structural formulas as a bond-line formula are given in the attachment:
1. (CH3)2CHCH2CH3
2. CH2=C(CH2CH3)2
3. CH3CHCICH2CH(CH3)2
What is a bond-line formula?A bond-line formula, also known as a line-angle formula or skeletal formula, is a shorthand representation of a molecule in which the carbon and hydrogen atoms are implied and only the bonds between the atoms are shown.
In this representation, each vertex or endpoint of a line represents a carbon atom, and each line segment represents a bond between two adjacent atoms. The hydrogen atoms are not shown explicitly, but are assumed to be attached to the carbon atoms to satisfy the valence requirements.
Learn more about bond-line formula at: https://brainly.com/question/28454387
#SPJ1

If the amount of radioactive phosphorus-32 in a sample prepared for treatment of leukemia decreases from 1.2g to 0.30g in 28.6 days, what is the half-life of phosphorus-32? Express the answer in 2 significant figures.
Answers
The half-life of the phosphorus-32, given the data is 14.3 days
What is half-life?Half-life is the time taken for half a material to decay.
Determination of the number of half-lives that has elapsedWe'll begin by obtaing the number of half-lives that has elapsed. This can be obtained as follow:
Original amount (N₀) = 1.2 gAmount remaining (N) = 0.3 gNumber of half-lives (n) =?2ⁿ = N₀ / N
2ⁿ = 1.2 / 0.3
2ⁿ = 4
Express 4 in index form
2ⁿ = 2²
n = 2
Thus, 2 half-lives has elapsed.
How to determine the half-lifeNumber of half-lives (n) = 2Time (t) = 28.6 daysHalf-life (t½) =?The half-life of the phosphorus-32 can be obtained as follow:
t½ = t / n
t½ = 28.6 / 2
t½ = 14.3 days
Thus, the half-life of the phosphorus-32 14.3 days
Learn more about half life:
https://brainly.com/question/26374513
#SPJ1
2. Experimental data for a simple reaction showing the rate of
change of reactant with time are given to Table 5.13.
Table 5.13 Experimental
data for a simple reaction.
Time
(min)
Concentration
(kg·m−3)
0 16.0
10 13.2
20 11.1
35 8.8
50 7.1
Show that the data gives a kinetic equation of order 1.5 and determine the rate constant.
Answers
The kinetic equation for the given reaction is first-order with respect to the reactant, and the rate constant is zero.
To determine the kinetic equation and rate constant for the given data, we need to analyze the relationship between the concentration of the reactant and time.
The general form of a first-order reaction is given by the equation:
Rate = k[A]^n
Where:
Rate is the rate of the reaction
k is the rate constant
[A] is the concentration of the reactant
n is the order of the reaction with respect to the reactant
By analyzing the given data, we can calculate the reaction rate and determine the order of the reaction and the rate constant.
Let's first calculate the reaction rate using the initial and final concentrations and the corresponding time intervals:
Rate = (Change in concentration) / (Change in time)
For the first time interval (0 to 10 min):
Rate = (13.2 kg·m^(-3) - 16.0 kg·m^(-3)) / (10 min - 0 min) = -2.8 kg·m^(-3)·min^(-1)
Similarly, we can calculate the rates for the other time intervals:
10 to 20 min: Rate = (11.1 kg·m^(-3) - 13.2 kg·m^(-3)) / (20 min - 10 min) = -2.1 kg·m^(-3)·min^(-1)
20 to 35 min: Rate = (8.8 kg·m^(-3) - 11.1 kg·m^(-3)) / (35 min - 20 min) = -2.3 kg·m^(-3)·min^(-1)
35 to 50 min: Rate = (7.1 kg·m^(-3) - 8.8 kg·m^(-3)) / (50 min - 35 min) = -1.7 kg·m^(-3)·min^(-1)
By observing the rates for different time intervals, we can see that the rate of change in concentration does not remain constant. This suggests that the reaction is not first-order with respect to the reactant.
To determine the order of the reaction, we can examine how the rate changes with the concentration. Let's calculate the rate ratios for the different time intervals:
Rate ratio (10/0) = (-2.8 kg·m^(-3)·min^(-1)) / (-2.8 kg·m^(-3)·min^(-1)) = 1
Rate ratio (20/10) = (-2.1 kg·m^(-3)·min^(-1)) / (-2.8 kg·m^(-3)·min^(-1)) ≈ 0.75
Rate ratio (35/20) = (-2.3 kg·m^(-3)·min^(-1)) / (-2.1 kg·m^(-3)·min^(-1)) ≈ 1.10
Rate ratio (50/35) = (-1.7 kg·m^(-3)·min^(-1)) / (-2.3 kg·m^(-3)·min^(-1)) ≈ 0.74
By observing the rate ratios, we can see that they are not constant, indicating that the reaction is not a simple integer order (e.g., first-order or second-order). However, we can approximate the order of the reaction by calculating the average rate ratio:
Average rate ratio = (1 + 0.75 + 1.10 + 0.74) / 4 ≈ 0.897
The order of the reaction can be approximated as the exponent that gives this average rate ratio. In this case, the order is approximately 0.897, which we can round to 1. Therefore, the kinetic equation for the reaction is:
Rate = k[A]^1.5
Now, to determine the rate constant (k), we can choose any set of data points and solve for k. Let's use the first data point at time = 0 min:
16.0 kg·m^(-3) = k * (0 min)^1.5
Since (0 min)^1.5 is zero, the right side of the equation is zero. Therefore, k must be zero as well.
For more such questions on kinetic equation visit;
https://brainly.com/question/22855016
#SPJ8