List 4 kinds of hazards that are caused by Volcanoes.
Answers
Four kinds of hazards that are caused by the volcanoes are lava flow, floods or tsunami, landslides, and volcanic earthquakes.
Volcanoes emits the hot, dangerous gases, ash, lava, and rocks which can be powerfully destructive. People have been dying from the volcanic blasts. Volcanic eruptions results in the additional menances to the health, such as floods, power outages, water contamination, and wildfires.
Ash exposure can be harmful to infants, elderly people, and people with respiratory conditions such as asthma, emphysema, and other chronic lung diseases.
Hazards such as -
Earthquakes such as preparing for, surviving, and recovering from an earthquake. Floods such as making sure food and water are safe, cleaning up, and emergency supplies. Landslides and Mudslides such as protective measures to take before, during, and after a landslide or debris flow. Power outages such as carbon monoxide poisoning, alternative heat and energy sources, downed power lines, and food and water safety. Wildfires such as smoke inhalation and other wildfire hazards.
Sources
To learn more about volcano hazards,
brainly.com/question/3809876
#SPJ1
Related Questions
It is given that the probability of requiring ICU care for patients hospitalized for COVID-19 is 5%. What is the probability of observing at least one ICU admissions, among the next six patients hospitalized for COVID-19?| Answer: a. 0.26491 b. 0.73509 c. 0.03125 d. 0.98437 e. 0.23213
Answers
The probability of observing at least one ICU admission, among the next six patients hospitalized for COVID-19 is 0.26491. Option A is correct.
The probability of not observing any ICU admission among the next six patients hospitalized for COVID-19:
Probability of not observing ICU admission = 1 - Probability of observing ICU admission
= 1 - 0.05
= 0.95
Probability of no ICU admission among the next six patients hospitalized for COVID-19:
Probability of no ICU admission in one trial = 0.95
Probability of no ICU admission in six trials (patients)
= 0.95 × 0.95 × 0.95 × 0.95 × 0.95 × 0.95
= 0.73509
Now, we can calculate the probability of observing at least one ICU admission among the next six patients hospitalized for COVID-19:
Probability of at least one ICU admission= 1 - Probability of no ICU admission
= 1 - 0.73509
= 0.26491
Therefore, the answer is A.
Learn more about patients -
brainly.com/question/31288405
#SPJ11
a 16.20 g sample contains 4.80 g f, 4.90 g h, and 6.50 g c. what is the percent composition of carbon in this sample?
Answers
The percent composition of carbon in the given sample is 40.12%.
To calculate the percent composition of carbon, we need to determine the mass of carbon in the sample and divide it by the total mass of the sample, then multiply by 100.
Given:
Mass of fluorine (F) = 4.80 g
Mass of hydrogen (H) = 4.90 g
Mass of carbon (C) = 6.50 g
Total mass of the sample = 16.20 g
Mass of carbon in the sample = 6.50 g
Percent composition of carbon = (mass of carbon / total mass of the sample) * 100
Percent composition of carbon = (6.50 g / 16.20 g) * 100 ≈ 40.12%
Therefore, the percent composition of carbon in the sample is approximately 40.12%.
Learn more about composition from this link:
https://brainly.com/question/32502695
#SPJ11
ILL GIVE YOU BRIANLIEST! PLEASE HELP: Every compound is a molecule, but every molecule is not a compound. Why is this? Give an example with your answer.
Answers
Answer:
A molecule can be made up of two atoms of the same kind:)
Explanation:
hope that helps:))
Answer:
Explanation:
A compound is a makeup of molecules.
Every organism is made up of an atom.Combined atoms can create a moleculeCombined molecules can create a compound.When you make a compound, you are combining molecules. But when you make a molecule, you are not combining compounds, you are combining atoms.
Examples:
Atoms:
\(\mathrm{H}\) (hydrogen) and \(\mathrm{N}\) (nitrogen)
Molecules:
\(\mathrm{H_2}\) and \(\mathrm{N_2}\)
Compounds:
\(\mathrm{H_2SO_2}\) and \(\mathrm{NaCL}\)
Take a look at the Compounds and Molecules section. The \(\mathrm{H_2}\) in the example of a molecule is in one of the examples of the compounds, but the \(\mathrm{H_2SO_2}\) (THE WHOLE THING) is not in the molecule. This shows how every compound is a molecule, but every molecule is not a compound.
Rutherford's model of atom could not explain:
Select one:
a.
Intensive properties
b.
Physical properties
c.
Chemical properties
d.
Extensive properties
Answers
Rutherford's model of atom could not explain chemical properties as it did not make any mention as to how chemical changes take place.
What are chemical properties?These properties are defined as those properties which become evident during or after a chemical reaction after the identity of the substance is changed during chemical reaction.
These properties cannot be determined externally just by viewing the substance ,these change immensely after a substance undergoes a chemical change.These are used for identification of unknown substances and for building up chemical classifications.
The major chemical properties are flammability,toxicity,reactivity,acidity and heat of combustion.For a chemical property to be apparent , it is necessary that the structure of the substance is altered.
Learn more about chemical properties,here:
https://brainly.com/question/5186976
#SPJ1
when 0.224 g of sodium metal is added to an excess of hydrochloric acid, 2330 j of heat are produced. what is the enthalpy of the reaction as written? 2na(s) 2hcl(aq)⟶2nacl(aq) h2(g)
Answers
The enthalpy of the reaction as written is approximately 239,306 J/mol.
To calculate the enthalpy of the reaction, we need to use the heat released (2330 J) and the amount of sodium reacted (0.224 g) to determine the heat released per mole of sodium reacted.
The molar mass of sodium (Na) is 22.99 g/mol.
First, we need to calculate the number of moles of sodium reacted:
Number of moles of Na = Mass of Na / Molar mass of Na
Number of moles of Na = 0.224 g / 22.99 g/mol ≈ 0.00974 mol
Next, we can calculate the enthalpy change (ΔH) per mole of sodium reacted:
ΔH = Heat released / Number of moles of Na
ΔH = 2330 J / 0.00974 mol ≈ 239306 J/mol
Enthalpy (H) is a thermodynamic property that represents the total heat content of a system at constant pressure. It includes the internal energy of the system plus the product of pressure and volume. Enthalpy is often used to describe heat changes in chemical reactions, where the difference in enthalpy between the reactants and products determines whether a reaction is exothermic (releases heat) or endothermic (absorbs heat). Enthalpy is typically measured in joules (J) or kilojoules (kJ).
To know more about enthalpy refer here
https://brainly.com/question/5642818#
#SPJ11
An isotope of an element has a different number of _____.
neutrons
protons
electrons
Answers
I believe that the answer is
A) Neutrons
I hope this helps you ^-^
Answer:
neutrons
Explanation:
2C2H6 + 7O2 ------>4CO2 + 6H2O
If you are given 5.00 grams of C2H6 and an excess amount of O2 how many grams of H2O would you make?
____
Right answer gets brainliest
Answers
Since we are told that O2 is the excess reactant, we need only to focus on C2H6, which will be our limiting reactant. Convert the mass of C2H6 to moles by dividing the mass by the molar mass of C2H6:
(5 g C2H6)(30.069 g/mol) = 0.1663 mol C2H6
Since C2H6 is the limiting reactant, its quantity will determine how much of each product is formed. We are asked to find the number of grams (the mass) of H2O produced. The molar ratio between H2O and C2H6 per the balanced equation is 6:2. That is, for every 6 moles of H2O that is produced, 2 moles of C2H6 is used up (intuitively, then, the number of moles of H2O produced should be greater than the number of moles of C2H6 consumed, specifically 3 times greater).
So, the number of moles of H2O produced would be (0.1663 mol C2H6)(6 mol H2O/2 mol C2H6) = 0.499 mol H2O. We multiply by the molar mass of H2O to convert moles to mass: (0.499 mol H2O)(18.0153 g/mol) = 8.987 g H2O.
Note: If significant figures must be considered, then the answer would be 8.99 g H2O.
Tides are caused by the pull of gravity by the _____ and the _____.
(fill in the blanks)
Answers
tides are caused by the gravitational force of the sun and the moon
When water is lost, but electrolytes are retained, the first thing that happens is that
O both the ECF and the ICF become more dilute.
O there is an increase in the volume of the ECF.
O the osmolarity of the ECF falls.
O osmosis moves water from the ICF to the ECF.
O aldosterone is secreted.
Answers
When water is lost, but electrolytes are retained, the first thing that happens is that moves water from the ICF to the ECF.
About Intracellular Fluid (ICF)The fluid inside of cells, also called the cytoplasm or cytosol, makes up about 60% of the water in the human body, totaling about 7 gallons. Organelles like the nucleus, endoplasmic reticulum, mitochondria, lysosomes, and Golgi apparatus are suspended in and supported by the ICF. Also found in the ICF are cellular building blocks like sugars, proteins, carbohydrates, and lipids.
About Extracellular Fluid (ECF)ECFs are any body fluids that are not inside cells. The two main components of ECF are plasma and interstitial fluid (IF). The balance consists of cerebrospinal fluid, lymph, the synovial fluid in the joints, pleural fluid in the pleural cavities (lungs), pericardial fluid around the heart, peritoneal fluid in the peritoneal cavity (abdomen), and the aqueous humor of the eye. In mammals, milk is also considered an extracellular fluid.
The Movement of Solutes Between CompartmentsThe ICF has higher amounts of potassium, phosphate, magnesium, and protein compared to the ECF. The plasma has high concentrations of sodium, chloride, and bicarbonate, but lower levels of protein as compared to the ICF. While water moves passively via osmosis, sodium and potassium ions move in and out of cells using active transport ion pumps. The pumps are powered by adenosine triphosphate (ATP) to provide the energy to move the ions against their concentration gradients (i.e. sodium moves out of the cell and potassium is pumped in) and maintain the gradients inside and outside the cell.
learn more about ICF and ECF at https://brainly.com/question/17312247.
#SPJ4
Please answer this, im too lazy to do the experiment.
1. What happened to the heavier materials in a mixture when left undisturbed?
2. How can we separate liquid and heavier particles in a mixture?
3. What materials are attracted by the magnet?

Answers
Explanation:
(1) heavier materials sink to the bottom of the mixture
(2)use a filter funnel and filter paper (filtration method)
(3) ferromagnetic materials (typically metals)
how many total atoms of each element are presented in the following formula

Answers
Answer:
Aluminium (Al): (3*2)+(5*2)=16
Sulphor (S): (3*1)=3
Oxygen (O): (4*3)+(3*1)=15
If you have 500.0 g of Aluminum Sulphate, how many moles of the compound do you have?
Answers
Answer:
500 moles
Explanation: moles=grams
balance the equation:2Na+3H2O-2NaOH+H2
Answers
Answer:
2Na + 2H2O → 2NaOH + H2
Explanation:
A balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge are the same for both the reactants and the products.
The wool in a sweater is the result of a physical change? true or false
Answers
Answer:
false
Explanation:
still wool. the sweater is wool
Answer:
yes it is
Explanation:
Table Salt or NaCl is composed of sodium and chlorine. While the individual elements of sodium and chlorine are very reactive, together they form a popular cooking and baking ingredient. In order to form a salt, an electron must be transferred from one element to the other. Which element (Na or Cl) is more likely to steal an outer electron from the other? Why?
Answers
Answer:
Chlorine is more likely to steal a valence electron from sodium.
Explanation:
Sodium is number 11 on the periodic table with one valence electron. Belonging to the first group, it's one of the alkali metal, which are known to be highly reactive. Chlorine is number 17 with seven valence electrons, and it's in the second-to-last group of halogens--also very reactive.
Considering that elements with one valence electron are just about 100% likely to give up electrons to reach a stable state, sodium would be the element that is more likely to lose its valence electron to chlorine. In other words, chlorine would be the electron thief.
If an object has a kinetic energy of 250 J and a mass of 5 kg, what is its velocity?
Answers
Answer:
v = 10 m/s
Explanation:
Given: An object has a kinetic energy of 250 J and a mass of 5 kg
To find: Its velocity
Formula: \(KE = \frac{1}{2} mv^2\)
Solution: In classical mechanics, kinetic energy (KE) is equal to half of an object's mass (\(\frac{1}{2}\) × m) multiplied by the velocity squared. In this case, an object with a mass of 5 kg (m = 5 kg) is moving at a velocity of 10 meters per second (v = 10 m/s), the kinetic energy is equal to 250 Joules, or (\(\frac{1}{2}\) × 5 kg) × 10 m/s².
Therefore, the velocity is v = 10 meters per second.
why would an electric vehicle be allowed to travel in the high occupancy vehicles lands with only one driver ?
Answers
Answer:
because it could self drive
Explanation:
idrk but thats my answer
what two chemical groups are found at the end of either side of the fatty-acid carbon chain?
Answers
Answer:
COOH and/or CH3
Hope this helped
__H2 + __SO2 —> __H2S + __H2O identify the proper coefficients
Answers
..................
Electric lights will not come on unless their electrical circuit is a?
Answers
Answer:
closed circuit
Explanation:
Hope this helps- Good luck! ^w
Electric lights do not come on unless their electrical circuit is closed circuit.
What is an electrical circuit?An electric circuit can be described as a path through which electric current flows. An electric circuit is a closed path in which the ends are joined making a loop. The flow of electric current can be only possible because of the closed circuit.
If an electric circuit is open circuit in which the flow of electrons is cut because the circuit will be broken. Electric current will not flow in an open circuit.
In a series circuit, there is one path for the flow of electrons and the entire circuit is closed or open. No current flow in case of a circuit break because the entire circuit is open.
Parallel Circuit is defined as a parallel type of electric circuit, different parts of the circuit are connected across many branches. Hence, electron flow takes place in several parts. If in one path a circuit breaks, electric current still flows in other paths.
Learn more about electrical circuit, here:
https://brainly.com/question/29032441
#SPJ2
Convert 3.4 kg to grams
Please Explain your answer
I will give brainliest for the best explained answer.
WARNING: NO LINKS OR YOU WILL GET REPORTED
Answers
Answer:
3400g
Explanation:
3.4×1000
3400g -_-
Please solve this asap.
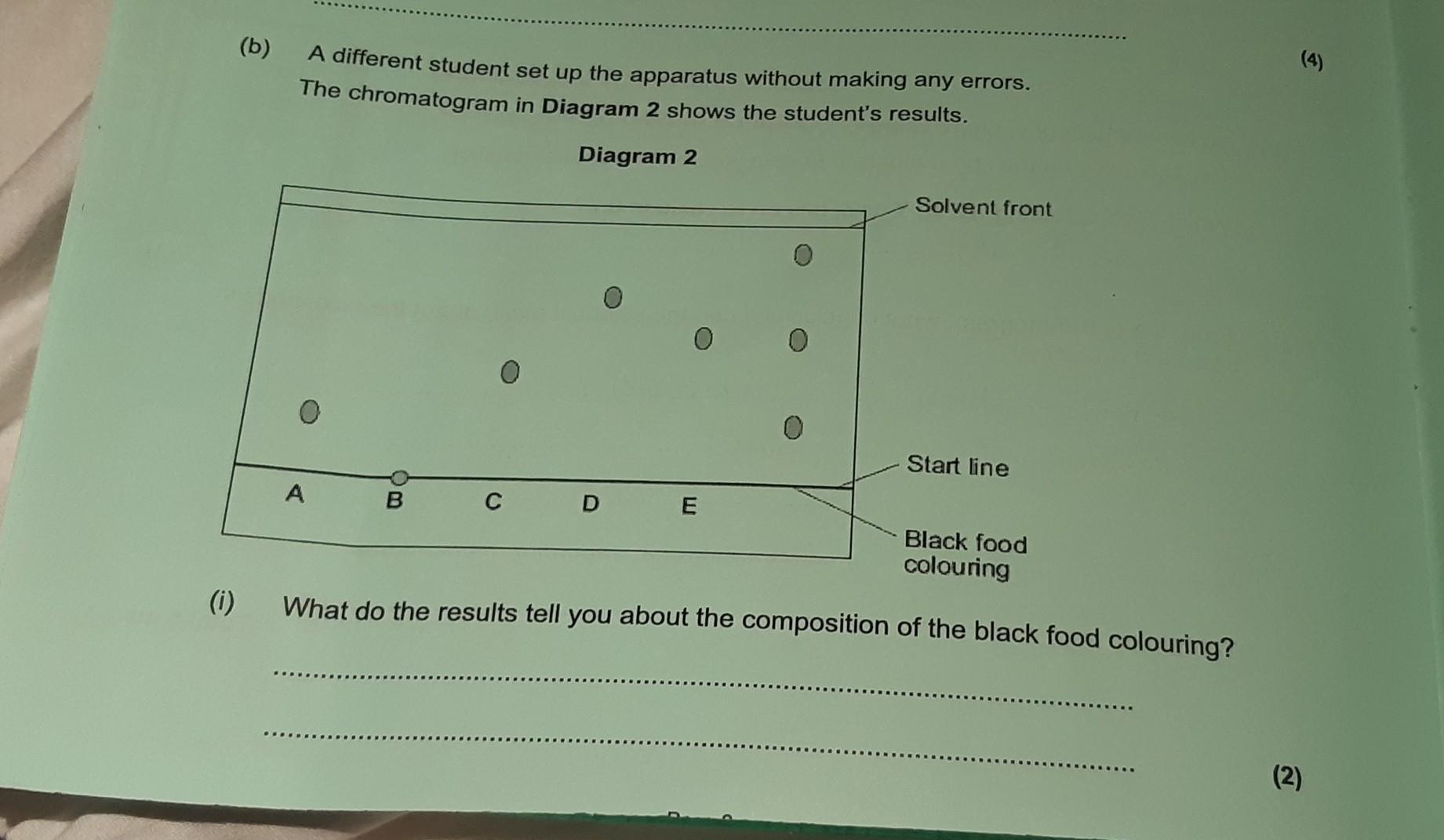
Answers
The results indicate that the black food colouring contains components A, B, C, D, and E.
What do the results tell you about the composition of the black food colouring?The results of Diagram 2 suggest that the black food colouring is composed of five components, labeled A, B, C, D, and E.\The start line of the chromatogram is at the far left, followed by component A, component B, component C, component D, and component E, in that order.Component E is the farthest right and is the component that contains the black food colouring.This indicates that component E is the component of the black food colouring that is most strongly attracted to the solvent and therefore has the highest polarity.The other components of the black food colouring, components A, B, C, and D, have lower polarities and are less strongly attracted to the solvent.This suggests that the black food colouring is composed of a mixture of components of varying polarities.To learn more about the black food colouring refer to:
https://brainly.com/question/30088961
#SPJ1
Balance the following equations
14)__C₂H4 +___O₂ ->_CO₂ +
15)___NaHCO3 -> Na₂CO3 +
16)__ _Cl₂ +
_Cl₂ +_NaBr ->
17)____Na₂S +
NaCl +
H₂O
H₂O + CO₂
Br2
HCI->_NaCl + H₂S

Answers
The balanced chemical equations are as follows:
14. C₂H₄ + 3 O₂ -> 2CO₂ + 2H₂O
15. 2NaHCO₃ -> Na₂CO₃ + H₂O + CO₂
16. 3Cl₂ + 2NaBr -> 2NaCl + Br₂
17. 3Na₂S + 2NaCl + 3H₂O -> 5NaCl + H₂S + 3O₂
What are balanced equations?Balanced equations are equations of chemical reactions that ensure that the law of conservation of mass is true.
In a balanced equation, the number of atoms of each element on both sides of the equation is equal.
The given chemical equations are balanced as follows;
14. Place 3, 2, and 2 before O₂, CO₂, and H₂O respectively.
C₂H₄ + 3 O₂ -> 2 CO₂ + 2 H₂O
15. Place 2 in front of NaHCO₃.
2 NaHCO₃ -> Na₂CO₃ + H₂O + CO₂
16. Place 3, 2, and 2 in front of Cl₂, NaBr, and NaCl respectively.
3Cl₂ + 2NaBr -> 2NaCl + Br₂
17. Place 3, 2, 3, 5, and 3 in front of Na₂S, NaCl, H₂O, NaCl, and O₂ respectively.
3Na₂S + 2NaCl + 3H₂O -> 5NaCl + H₂S + 3O₂
Learn more about balancing equations at: https://brainly.com/question/11904811
#SPJ1
calculating the heat of reaction from molar reaction enthalpy and the mass of a reactant
Answers
1) Because ΔH is positive, the reaction is endothermic.
2) Yes, absorbed, because in endothermic reaction heat is absorbed.
3) Heat will be released 26.9 KJ
Enthalpy definitionEnthalpy depends only on the system's composition, temperature, and pressure; it is unaffected by the system's history. It is a quality or state function that resembles energy and has energy-like properties (and is thus measured in units of joules or ergs).
Given reaction is
2HgO(s) → 2Hg(l) + O₂(g) ΔH = 182KJ
1) Because ΔH is positive, the reaction is endothermic.
2) Yes, because heat is absorbed during endothermic reactions.
3) Heat will be released 26.9 KJ
According to the reaction,
2 moles HgO of release = 182 KJ heat
2×216.59 HgO release = 182 KJ heat
64 g HgO release = 182×64/2×216.59 KJ heat
64 g HgO release = 26.9 KJ.
To know more about enthalpy visit:
https://brainly.com/question/13996238
#SPJ4
Complete question is attached below

PLEAAAAAASE HELP IM SO CONFUSED...
What does water’s solubility of polar versus nonpolar substances do in cells? How does water’s solubility of polar versus nonpolar substances affect the ability to dissolve important biochemicals?
Answers
Answer:
Generally speaking, water is good at dissolving ions and polar molecules, but poor at dissolving nonpolar molecules. (A polar molecule is one that's neutral, or uncharged, but has an asymmetric internal distribution of charge, leading to partially positive and partially negative regions.)
Water's polarity allows it to dissolve other polar substances very easily. When a polar substance is put in water, the positive ends of its molecules are attracted to the negative ends of the water molecules, and vice versa.
By what factor is the rate of a reaction changed if an enzyme lowers the ea by 2. 0 kj/mol at 37°c?.
Answers
Answer:
10.2 times 7.0 times 15.1 times 32.9 times
Explanation:
True or false; A solution always contains only one solvent.
Answers
A solution is defined as a mixture of two or more substances, usually, a solute and a solvent, and the difference between these two are in quantity, solute represents the smallest amount and solvent will represent the highest amount, and while you can have more than one solute, you can only have one solvent for a solution. Therefore the statement is true
Which of the following is included in the measure of U.S. GDP?
Answers
the value of increase in business inventories.
Reptiles, amphibians, and fish are all cold-blooded animals. Their body temperatures are determined by their surroundings. How does a cold-blooded animal’s ability to digest food change in colder temperatures?
Answers
On the day of her students' chemistry final, Prof. Jackson removes the periodic table of elements from the classroom wall. Doing this is which of the following:
Extra-stimulus prompt
Reinforcement prompt
Stimulus fading
Prompt fading