List the extensive and intensive
properties for the example below.
Alan's liquid sample was
translucent and yellow.
It had an awful smell.
It was very viscous and
heavy. It weighs 152
grams and it had a
volume of nearly a liter.
The liquid would stick
to wood but not metal.

Answers
Answer:hi ths not abl
Related Questions
this question is giving me a hard time

Answers
Answer:
options 3rd is the correct answer
Which kind of energy is stored in the chemical bonds of molecules found in
wood?
A. Mechanical energy
B. Potential energy
C. Gravitational energy
D. Kinetic energy
Answers
Answer:
B. Potential energy
Explanation:
It's not mechanical energy, because it is not doing work
It's not kinetic energy because it;s not moving
It's not gravitational energy because it is not above the ground
It is potential energy because it is stored energy
diagram 5 water molecules showing polarity and hydrogen bonding define and give an example of
Answers
A water molecule consists of one oxygen atom and two hydrogen atoms. The oxygen atom is electronegative, meaning it attracts electrons towards itself more strongly than the hydrogen atoms do. This creates a partial negative charge on the oxygen atom and partial positive charges on the hydrogen atoms, resulting in a polar molecule.
When five water molecules come together, they can form hydrogen bonds with each other. A hydrogen bond is a type of intermolecular force that occurs when the partially positive hydrogen atoms of one molecule are attracted to the partially negative oxygen atoms of another molecule.
To diagram five water molecules showing polarity and hydrogen bonding, you can draw five water molecules with the oxygen atoms represented by a red circle and the hydrogen atoms represented by white circles. The partial positive charges on the hydrogen atoms can be indicated by a small plus sign, while the partial negative charge on the oxygen atom can be indicated by a small minus sign. You can then draw dashed lines between the partially positive hydrogen atoms and the partially negative oxygen atoms of adjacent water molecules to represent the hydrogen bonds.
An example of hydrogen bonding in water is its high boiling point. Because of the strong hydrogen bonds between water molecules, a lot of energy is required to break these bonds and turn water into steam. This is why water boils at a relatively high temperature compared to other liquids.
To know more about hydrogen bonding visit:
https://brainly.com/question/31139478
#SPJ11
what is the formula to calculate rate of reaction
Answers
Answer:
Explanation:
rate = Δ[C]/Δt,
Reaction rate is calculated using the formula rate = Δ[C]/Δt, where Δ[C] is the change in product concentration during time period Δt. The rate of reaction can be observed by watching the disappearance of a reactant or the appearance of a product over time.
Complete the following radioactive decay problem. Please help

Answers
Answer:
230 90Th
Explanation:
A careful observation of the equation given in question shows that 234 92U is undergoing alpha decay. This means that the resulting daughter nuclei will have a decrease of 4 in the mass number and a decrease of 2 in the atomic number.
Please see attached photo for further details.
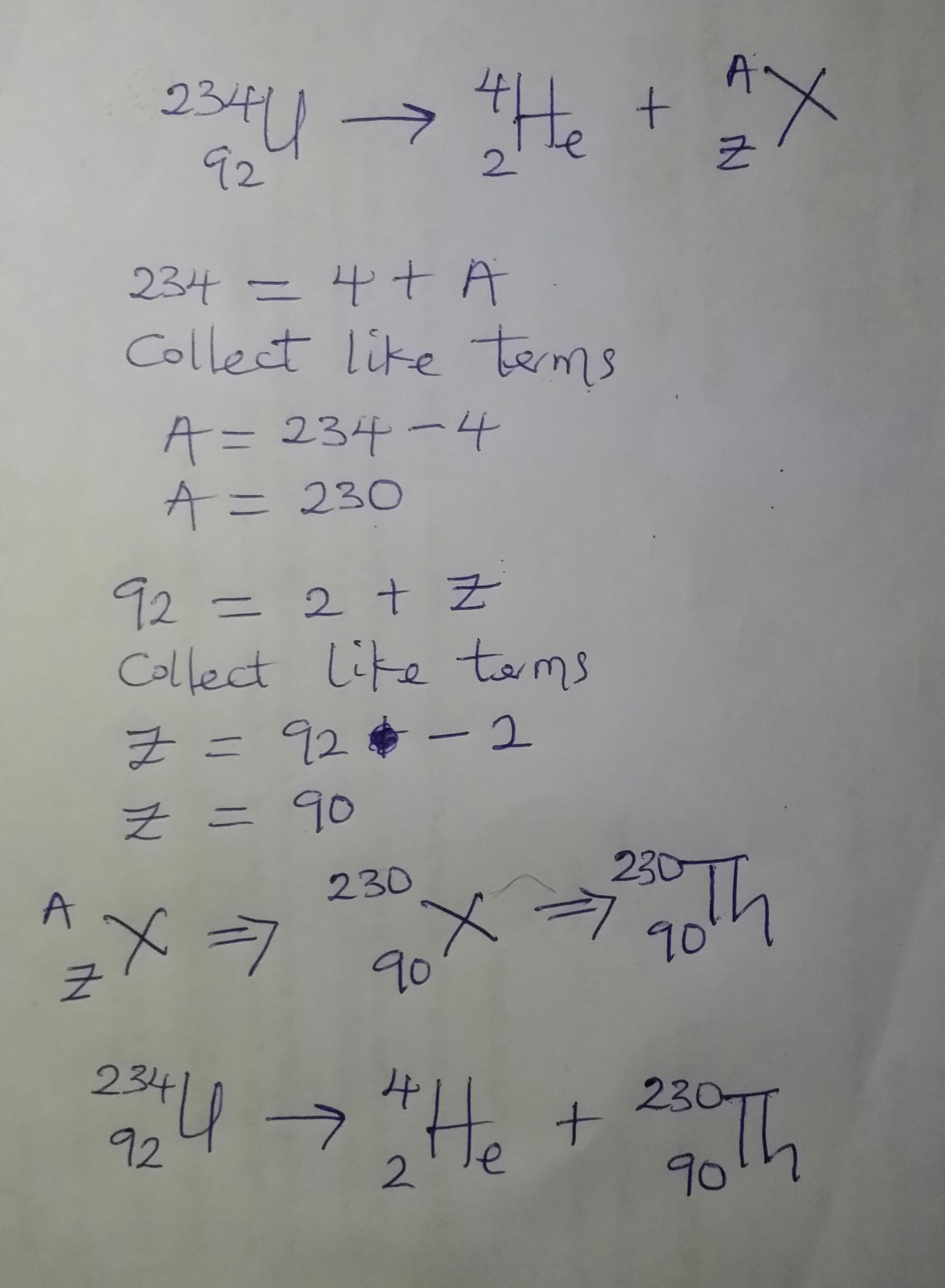
when enzyme action stops due to a buildup of end product this control is called A. negative feedback. B. competitive inhibition. C. enzyme induction. D. enzyme repression.
Answers
The control mechanism you're referring to, where enzyme action stops due to a buildup of end product, is called A. negative feedback.
Negative feedback occurs when the accumulation of an end product inhibits the initial enzyme responsible for its production. This process helps maintain the optimal levels of substances within the cell and prevents overproduction. Here's a step-by-step explanation:
1. An enzyme catalyzes a reaction, leading to the formation of a product.
2. As the product accumulates, it reaches an optimal level within the cell.
3. When the optimal level is reached, the end product binds to the enzyme or its regulatory site, decreasing the enzyme's activity.
4. As a result, the production of the end product slows down, maintaining a balance within the cell.
This process ensures that resources and energy are not wasted in producing excess product and helps maintain homeostasis within the cell.
Learn more about Negative feedback here:
brainly.com/question/31105398
#SPJ11
What is the weight of an object that has the area of 74.6 m² and exerts a pressure of 1500 N/m^2
Answers
111900g is the weight of an object that has the area of 74.6 m² and exerts a pressure of 1500 N/m².
Weight being a force The SI unit for weight is Newton (N), which also happens to be the same as the SI unit for force. When we look at how weight is expressed, we can see how it depends on both mass as well as the acceleration caused by gravity; while the mass might not vary from one location to another, the acceleration caused by gravity does.
Pressure = thrust/ area
= weight/ area
1500 = weight/ 74.6
weight = 111900g
To know more about weight, here:
https://brainly.com/question/30176113
#SPJ1
draw the major 1,2- and 1,4-addition products formed when this diene reacts with hcl. do not include any byproducts formed
Answers
The reaction can proceed by either 1, 2-addition or 1, 4-addition, depending on the conditions.
The major product formed in this reaction depends on the stability of the intermediate and the nature of the reaction conditions.
When a diene reacts with HCl, the major 1, 2, and 1, 4-addition products are formed.
The reaction is an example of an electrophilic addition reaction.
The addition products formed in this reaction are named according to the positions of the added chlorine atoms.
1, 2-addition product: The 1, 2-addition product is formed when the HCl molecule adds to the carbon atoms on either side of the double bond.
It forms a cyclic intermediate, and the product is a single compound containing two chlorine atoms. This product is called a dichloride.
1, 4-addition product: The 1, 4-addition product is formed when the HCl molecule adds to one of the carbon atoms on the diene, and then the other chlorine atom is added to the other carbon atom on the diene.
The product is a mixture of two different compounds.
One compound has two chlorine atoms on the same carbon atom, and the other compound has one chlorine atom on each of the two carbon atoms.
The product is called a chlorohydrin.
The reaction of a diene with HCl is an example of an electrophilic addition reaction.
The reaction involves the addition of an electrophile (H+) to the diene, which creates a carbocation intermediate.
The intermediate then reacts with the chloride ion to form the addition product.
The reaction can proceed by either 1, 2-addition or 1, 4-addition, depending on the conditions.
The major product formed in this reaction depends on the stability of the intermediate and the nature of the reaction conditions.
To know more about atoms, visit:
https://brainly.com/question/1566330
#SPJ11
Precipitation reactions occur when an insoluble salt is formed. Precipitation reactions can be used in industry to...
a. remove metals from their ores
b. remove unwanted impurities from metals
c. remove some substances from waste water
d. split large molecules into smaller ones
Answers
Precipitation reactions are commonly employed in industry to remove specific substances from wastewater. Hence option C) is the correct answer.
Option c. remove some substances from wastewater. Precipitation reactions are commonly employed in industry to remove specific substances from wastewater. In these reactions, insoluble salts are formed when certain ions in the wastewater react with specific reagents or chemicals. The formed precipitate can then be separated from the solution, effectively removing the targeted substances. This process is particularly useful for removing heavy metals, such as lead, mercury, or cadmium, from wastewater. By introducing a precipitating agent that forms insoluble metal salts, the heavy metal ions can be converted into solid precipitates. These precipitates can be filtered or settled out, allowing for the separation and disposal of the heavy metal contaminants. Overall, precipitation reactions offer an effective method for the removal of certain substances, including heavy metals, from wastewater, helping to mitigate environmental pollution and safeguard water quality.
For more question on Precipitation
https://brainly.com/question/23499164
#SPJ11
select the components found in most energy drinks. multiple select question.mineralsunsaturated fats stimulants like caffeine and citicoline food additives nutrient macromolecules vitamins
Answers
Components found in most energy drinks include stimulants like caffeine and citicoline, food additives, vitamins, and nutrient macromolecules.
Energy drinks are drinks that are marketed as providing mental and physical stimulation, as well as increased stamina. Caffeine, taurine, ginseng, vitamins, and various forms of sugar are often present in energy drinks. There are a few substances in energy drinks that are commonly found such as:
Stimulants: Energy drinks include several stimulants that are intended to increase alertness and energy levels. These stimulants are usually caffeine, taurine, and guarana extracts.
Nutrient Macromolecules: Energy drinks are often high in carbohydrate and amino acid levels. They are required for both metabolism and energy production.
Food Additives: Food additives, such as flavouring, sweeteners, preservatives, and artificial colours, are often found in energy drinks. These additives are not harmful to your health, but they can cause symptoms like stomach upset, headaches, and allergic reactions.
Vitamins: Energy drinks contain various vitamins, such as vitamins B6 and B12, which are essential for energy production and neurological function. However, consuming more vitamins than you require might lead to negative side effects.
For more such questions on stimulants, click on:
https://brainly.com/question/28452820
#SPJ11
Which area of chemistry best links the use of titanium and plastics in artificial bone and joint replacements?
Answers
The question is incomplete, the complete question is;
Which area of chemistry best links the use of titanium and plastics in artificial bone and joint replacements? A) environmental chemistry
B) materials chemistry
C) agricultural chemistry
D) physical chemistry
Answer:
materials chemistry
Explanation:
Chemistry is the study of the nature of matter as well as the interaction between the particles that compose matter.
There are several branches of chemistry such as;
Physical chemistry
Organic chemistry
Biochemistry
Polymer chemistry and so on.
Materials chemistry is that branch of chemistry that deals with the design and production of diverse materials which can be used for various purposes.
Hence, the area of chemistry which best links the use of titanium and plastics in artificial bone and joint replacements is materials chemistry.
Answer:
B
Explanation:
took the test
Solid Magnesium reacts with a Iron (II) Chloride solution ->
Answers
Answer:
Magnesium + iron chloride → iron + magnesium chloride
Explanation:
It is the single replacement reaction.
Single replacement:
It is the reaction in which one elements replace the other element in compound.
AB + C → AC + B
Molecular equation:
Magnesium + iron chloride → iron + magnesium chloride
Chemical equation:
Mg(s) + FeCl₂(aq) → MgCl₂(aq) + Fe(s)
Ionic equation:
Mg(s) + Fe²⁺(aq) + 2Cl⁻(aq) → Mg²⁺(aq) + 2Cl⁻(aq) + Fe(s)
Net ionic equation:
Mg(s) + Fe²⁺(aq) → Fe(s) + Mg²⁺(aq)
plastic don't react with
Answers
Answer:
Most plastic is chemically inert and will not react chemically with other substances -- you can store alcohol, soap, water, acid or gasoline in a plastic container without dissolving the container itself.
Explanation:
Answer:
Most plastic is chemically inert and will not react chemically with other substances -- you can store alcohol, soap, water, acid or gasoline in a plastic container without dissolving the container itself.
Explanation:
Hope it helps u
FOLLOW MY ACCOUNT PLS PLS
The sun causes water in the pond to evaporate
1.conduction
2.convection
3.radiation
Answers
Answer:
The answer is Radiation
Answer:
Convectional
Explanation:
It is the movement of heat through fluid
While radiation travels though a vacuum and conduction accrues between atoms / solids
give a brainliest if it desires it plzzzz
An organic compound, which has the empirical formula C4H9 has an approximate molar mass of 114 g/mol. What is its probable molecular formula
Answers
The probable molecular formula of the organic compound is C8H18. To determine the probable molecular formula of the organic compound with the empirical formula C4H9 and a molar mass of approximately 114 g/mol, we need to calculate the empirical formula's empirical formula mass and compare it to the given molar mass.
The empirical formula mass can be calculated by summing the atomic masses of all the atoms in the empirical formula. For C4H9:
(4 × atomic mass of carbon) + (9 × atomic mass of hydrogen)
Using the atomic masses from the periodic table:
(4 × 12.01 g/mol) + (9 × 1.01 g/mol) = 48.04 g/mol + 9.09 g/mol = 57.13 g/mol
The empirical formula mass is found to be 57.13 g/mol.
To find the probable molecular formula, we divide the given molar mass (114 g/mol) by the empirical formula mass (57.13 g/mol):
114 g/mol / 57.13 g/mol ≈ 2
This means that the molecular formula likely consists of two times the number of atoms present in the empirical formula.
Multiplying the subscripts of the empirical formula by 2 gives us the probable molecular formula:
C4H9 × 2 = C8H18
Therefore, the probable molecular formula of the organic compound is C8H18.
learn more about organic compound here
https://brainly.com/question/13508986
#SPJ11
how much energy is needed to convert 120g of ice at -35°C to steam at 150°C?
Answers
Identify the stronger acid in each pair.
A. HCN or H3O+
HCN
H3O+
They are the same in acidic properties.
B. H2SO4 or HCN
H2SO4
HCN
They are the same in acidic properties.
C. HS? or H2S
HS?
H2S
They are the same in acidic properties.
Answers
A. In this pair, H3O+ is the stronger acid because it can donate a proton more easily than HCN.
B. H2SO4 is the stronger acid in this pair because it is a strong acid, while HCN is a weak acid.
C. HS? is the stronger acid in this pair because it has a greater positive charge on the sulfur atom, making it more acidic than H2S.
The stronger acid in each pair.
A. Between HCN and H3O+, the stronger acid is H3O+. H3O+ is a stronger acid due to its higher ability to donate a proton.
B. Between H2SO4 and HCN, the stronger acid is H2SO4. H2SO4 is a stronger acid because it has a higher degree of ionization and can donate more protons than HCN.
C. Between HS- and H2S, the stronger acid is H2S. H2S is a stronger acid because it more readily donates a proton compared to HS-.
Learn more about stronger acid
brainly.com/question/31147529
#SPJ11
Hydrogen bonding forces exist in which of the following compounds?
A.
CH3F
B.
All of these compounds.
C.
HCN
D.
CH3NH2
E.
H2S
Answers
Hydrogen bonding forces exist in compounds where hydrogen is bonded to highly electronegative atoms such as nitrogen, oxygen, or fluorine.
Among the given compounds, HCN, CH3NH2, and CH3F have hydrogen bonding forces since they all have hydrogen bonded to nitrogen or fluorine. H2S does not have hydrogen bonding forces as hydrogen is bonded to sulfur, which is not highly electronegative. Therefore, the correct answer is option B, "All of these compounds" because all the given compounds have hydrogen atoms bonded to highly electronegative atoms, except H2S. Hydrogen bonding forces exist in compound D, CH3NH2.
This is because hydrogen bonding occurs when hydrogen is bonded to a highly electronegative atom, such as nitrogen (N), oxygen (O), or fluorine (F). In CH3NH2, the hydrogen is bonded to nitrogen, enabling hydrogen bonding to take place. The other compounds do not meet the criteria for hydrogen bonding, as hydrogen is not bonded to N, O, or F in those molecules.
To know about bonding:
https://brainly.com/question/31358643
#SPJ11
Which reaction does not involve neutralization? A. H 2 SO 4 + 2NH 3 -------> (NH 4 ) 2 SO 4 B. H 2 SO 4 + BaCl 2 --------> BaSO 4 + 2HCl C. H 2 SO 4 + CuO -----> CuSO 4 + H 2 O D. H 2 SO 4 + 2NaOH ----> Na 2 SO 4 + 2H 2 O
Answers
Answer:
B. H2SO4 + BaCl 2 --------> BaSO4 + 2HCl
Explanation:
Neutralization reactions are characterized by their reactants of acids and bases reacting to form salt and water. All of the options except B, have formation of salt and water. Also, B is more likely a percipitation reaction (also a double displacement reaction) and not neutralization.
where is the atomic symbol located
Answers
Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus.
PLEASE HELP PLS PLS PLS
Methane, CH4(g), and oxygen gas, O2(g) react to produce carbon dioxide, CO2(g), and water H2O(g). What volume of methane is required to react with oxygen to produce 32.5 L of carbon dioxide? *
a) 65.0 L
b) 48.8 L
c) 16.3 L
d) 32.5 L
Answers
Answer:
D)
Explanation:
CH4 + 2O2 = CO2 + 2H2O
1 (L CO2)= 32.5(L CO2)
1 (CH4) = 32.5 (L CH4)
2. List and elaborate on at least two limitations of the 24-hour recall as a
method of diet assessment (Each item .5 pt for a total of 1 pt, support
for each response 5 pt for a total of 1 pt = 2 pts)
Answers
Answer:
The correct answer is - may not be typical, and participant burden.
Explanation:
The 24-hour recall is nothing but a retrospective method of diet assessment. In this method, an individual is interviewed about his or her diet consumption during the last 24 hours.
The disadvantages or limitations of this method include the inability of a single day's intake to describe the typical diet, multiple recalls to intake, cost and administration time; participant burden, have to recall to reliably estimate usual intake.
NEED ANSWERED ASAP
An ideal ga i at volume V at temperature T. If the volume i doubled at contant preure and the ame amount of ga particle, the temperature will be
unchanged
halved
doubled
four time
Answers
In the event that the volume I multiplied at contant preure and the ame measure of gas molecule, the temperature will be split
From ideal gas equation, PV = nRT
This implies n= PV/RT
Presently, strain and temperature both are multiplied and volume is split
In this way, obviously the number of moles will likewise turn out to be half
The observational connections among the volume, the temperature, the tension, and how much a gas can be joined into the ideal gas regulation, PV = nRT.For a gas to be "ideal" there are four overseeing suppositions: The gas particles have irrelevant volume. The gas particles are similarly estimated and don't have intermolecular powers (fascination or repugnance) with different gas particles. The gas particles move haphazardly in concurrence with Newton's Laws of Motion.The ideal gas equation is planned as: PV = nRT. In this equation, P alludes to the strain of the ideal gas, V is the volume of the ideal gas, n is the aggregate sum of ideal gas that is estimated as far as moles, R is the widespread gas steady, and T is the temperature.
Find out more about Ideal gas Equation
brainly.com/question/29986286
#SPJ4
What is the molar mass of sulfur dihydride?
Answers
Explanation
To find the molar mass of sulfur dihydride, you will need to find the sum of all the atomic masses making up sulfur dihydride.
sulfur dihydride is H2S.
Atomic mass of H = 1,00784 u
Atomic mass of S = 32,065 u
Molar mass = (1.00784 x 2) + 32,065 = 34.081 g/mol
Answer
34.081 g/mol
how many moles of sulfur dioxide are required to produce 5.0 moles of sulfur
Answers
Three moles of sulfur dioxide are required to produce 1 mole of sulfur, thus 15.0 moles of sulfur dioxide are required to produce 5.0 moles of sulfur.
The balanced equation is SO₂ + 2H₂S → 3S + 2H₂O. In this equation, three moles of sulfur dioxide react with two moles of hydrogen sulfide to produce three moles of sulfur and two moles of water. From the balanced equation, we can see that three moles of sulfur dioxide are required to produce 1 mole of sulfur.
Therefore, to calculate how many moles of sulfur dioxide are required to produce 5.0 moles of sulfur, we multiply the number of moles of sulfur by three. 5.0 moles of sulfur x 3 moles of SO₂/mole of S = 15.0 moles of sulfur dioxide. Thus, 15.0 moles of sulfur dioxide are required to produce 5.0 moles of sulfur.
Learn more about moles here:
https://brainly.com/question/30885025
#SPJ11
thank you so much for putting all the answers in there
Answers
Answer:
\(\huge\color{cyan}{\colorbox{magenta}{Answer}}\)
thank you
what carbonyl starting materials are needed to prepare the compound below using a directed aldol reaction?
Answers
In order to prepare the given compound through directed aldol reaction, cyclopentanone and propanal are required as carbonyl starting materials.
A directed aldol reaction is a particular kind of aldol reaction that takes place in the presence of a particular reactant. A reaction is known as an aldol reaction when an enolate or enol reacts with an aldehyde or ketone and forms a beta-hydroxy carbonyl compound. The carbonyl starting materials that are required for the directed aldol reaction of the given compound is cyclopentanone and propanal.
In the directed aldol reaction, a particular enolate is preferred for the reaction and, as a result, specific reagents are used to form this enolate. The synthesis of the directed aldol reaction can be accomplished by the addition of a strong base such as LDA (lithium diisopropylamide) to the carbonyl compound. Afterward, a suitable electrophile is added to the enolate which reacts with it to form the desired product.
Learn more about aldol reaction here:
https://brainly.com/question/23970995
#SPJ11
SEP Construct an Explanation What challenges do the three industries have in making better batteries? What solutions are being suggested?
Answers
The three industries commonly associated with battery technology are the automotive, electronics, and renewable energy sectors. Each of these industries faces specific challenges when it comes to developing better batteries.
Automotive Industry:
Energy Density: One of the primary challenges for electric vehicles (EVs) is improving battery energy density, which refers to the amount of energy that can be stored per unit of volume or weight. Higher energy density batteries would allow for longer driving ranges and reduced charging times.Cost: Batteries constitute a significant portion of an electric vehicle's cost. Therefore, reducing the cost of battery production is crucial for making EVs more affordable and competitive with traditional internal combustion engine vehicles.Charging Infrastructure: The limited availability of charging stations and relatively longer charging times compared to refueling a conventional vehicle remain challenges. The industry is focusing on expanding charging infrastructure and developing fast-charging technologies to address this issue.Electronics Industry:
Power Density: Electronic devices, such as smartphones and laptops, require batteries with high power density to support their energy-intensive operations. However, increasing power density while maintaining safety and minimizing size is a challenge.Battery Lifespan: Consumers expect electronic devices to have a longer battery life before needing a recharge. Enhancing battery lifespan through improved materials, design, and management systems is an ongoing pursuit.Environmental Impact: The electronics industry is increasingly concerned about the environmental impact of batteries, particularly regarding the disposal and recycling of lithium-ion batteries. Developing sustainable and eco-friendly battery technologies is a suggested solution.Renewable Energy Industry:
Energy Storage Capacity: Renewable energy sources like solar and wind are intermittent, meaning they are not continuously available. Efficient energy storage solutions are needed to store excess energy produced during peak times and supply it during periods of low or no generation. Integration with the Grid: Integrating renewable energy sources with the existing electrical grid is a challenge due to fluctuations in supply and demand. Advanced battery technologies can help stabilize the grid by providing rapid response and balancing services.Durability and Longevity: Renewable energy projects, such as utility-scale installations, require long-lasting and durable batteries that can withstand frequent charge-discharge cycles without significant degradation. Enhancing battery life and reliability is a focus for the industry.For such more questions on technology
https://brainly.com/question/7788080
#SPJ8
plzzz help me i promise will mark u brainiest

Answers
Answer:
d) water
Explanation:
Answer:
C) sodium chloride
When ice melts, it becomes liquid.
Which statement correctly compares ice and liquid water?
O A. Both liquid water and ice have definite shapes.
B. Particles of liquid water have more energy than particles of ice.
ОО ОО
C. Liquid water has a lower temperature than ice.
D. Ice has a definite volume, but liquid water does not.
Answers
Given what we know about the energy of particles in each state, we can confirm that the best comparison between water and ice is that particles of liquid water have more energy than particles of ice.
Why do water particles have more energy than ice?This is due to the heat they receive. In order for water to stay in a liquid state, it requires energy in the form of heat. This heat energy excites the particles in the water, causing movement and higher internal kinetic energy.Water becomes ice when it loses this energy and is no longer able to maintain a higher temperature.Therefore, we can confirm that since the heat required to keep water in a liquid state excites the particles and gives them more energy, the correct comparison would be to say that particles of liquid water have more energy than particles of ice, option B is correct.
To learn more about heat energy visit:
https://brainly.com/question/1495272?referrer=searchResults
Answer:
b.
Explanation: