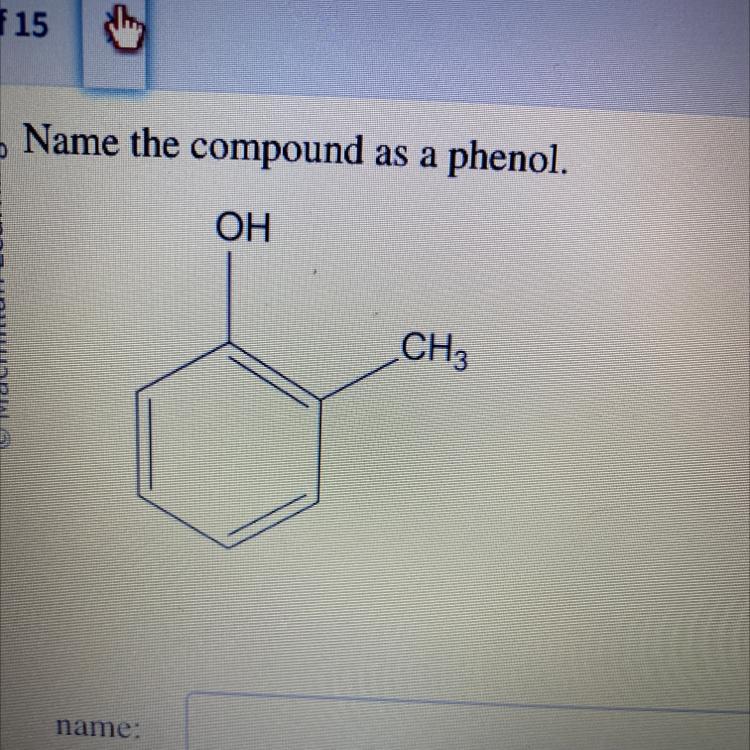
Answers
The name of the compound given in the question above is 2-methylphenol
How do I name the organic compound as phenol?The naming of the organic compound as phenol can be obtained by following the steps given below:
Identify the functional group in the compound. In this case it is phenol since the compound is a benzene ring with an OH group.Identify the substituent group attached to the compound. In this case, the substituent group attached is methyl (CH₃)Give the substituent group the lowest count. In this case the substituent group is located at carbon 2.Thus, the name of the compound is: 2-methylphenol
Learn more about IUPAC name:
https://brainly.com/question/16180944
#SPJ1
Related Questions
How many formula units are in 4.52 moles of H3SO3?
Type your answer
Answers
Answer:
The answer is 98.07848. We assume you are converting between grams H2SO4 and mole. You can view more details on each measurement unit: This compound is also known as Sulfuric Acid. The SI base unit for amount of substance is the mole. 1 grams H2SO4 is equal to 0.010195916576195 mole.
Quick conversion chart of moles H2SO3 to grams
1 moles H2SO3 to grams = 82.07908 grams
2 moles H2SO3 to grams = 164.15816 grams
3 moles H2SO3 to grams = 246.23724 grams
4 moles H2SO3 to grams = 328.31632 grams
5 moles H2SO3 to grams = 410.3954 grams
6 moles H2SO3 to grams = 492.47448 grams
7 moles H2SO3 to grams = 574.55356 grams
8 moles H2SO3 to grams = 656.63264 grams
9 moles H2SO3 to grams = 738.71172 grams
10 moles H2SO3 to grams = 820.7908 grams
Which option identifies the major method scientists use to share their research findings with other scientists?
(Select ALL that apply)
newspaper articles
Internet videos
peer-reviewed journals
conference presentations
Answers
which type of rock is rhyolite A.Intusive igneous B.Sedimentary C.Extrusive igneous D.Metamorphic
Answers
Answer:
C - Extrusive igneous
Explanation:
Rhyolite is an extrusive igneous due to the high silica content, the lava is very dangerous.
Answer:
Its C
Explanation:
A P E X
How many moles are in 30 mL water sample
Answers
Answer:
moles of water in 1 ml = 1/18 mol.
take 1 ml and calculate it by 30
1 ml x 30= 30 ml
Explanation:
What is another term for anode?
Answers
Answer:positive electrode
Explanation:
Anode can also be referred to as positive electrode in a cell
What’s crystallisation?
Answers
Can water molecules form ions by
themselves?
Answers
Escape room winter wonderland
Answers
The Escape room winter wonderland is described as an immersive experience for students during the winter time.
The Escape room winter wonderland allows the students to demonstrate their knowledge of logic, weather, precipitation, and dichotomous keys in a fun and engaging way.
Materials required for Escape room winter wonderland?There are some materials which are mandatory and some which are optional and they include:
Required Materials:
- All printed materials (included)
- Manila envelopes (used for each of the puzzles)
- Hairdryer or heat source
- Fraxion pens
- UV pen/light
Optional Materials
- One Lockout Hasp
- Three, 4-digit locks
- Two, 3-digit locks
- One storage box that includes a place for the lockout hasp
Learn more about lockout hasp at:
https://brainly.com/question/24573204
#SPJ1
A student completed a lab report. Which correctly describes the difference between the “Question” and “Hypothesis” sections of her report? “Question” states what she is asking, and “Hypothesis” states the result of her experiment. “Question” states what she is asking, and “Hypothesis” states what she thinks the answer to that question is in “if . . . then . . . because” format. “Question” describes what she is trying to find out, and "Hypothesis" states the procedures and methods of data collection. “Question” describes what she is trying to find out, and “Hypothesis” states any additional information or prior knowledge about the question.
Answers
The question in a investigation is the problem that we are asked to find and the hypothesis states what we thinks the answer to that question.
What is a scientific question?In a scientific experiment, the problem on which we work to solve or the question addressing an unknown thing is called a scientific question. The scientific question have to be precise and experientable.
A scientific hypothesis is a predictive statement describing the possible answer of the problem under study based on the observations and some previous scientific reports.
A hypothesis must be rectifiable, testable and falsifiable. We are checking whether our hypothesis is true or false through an experiment. Hence, second option is correct.
Find more on scientific hypothesis:
https://brainly.com/question/2681563
#SPJ1
Approximately what mass of CuSO4*5H20 (250 g mol^-1) is required to prepare 250 mL of 0.10 M copper(ll)
sulfate solution?
A) 4.0 g
B
6.2 g
С
34 g
D
85 g
E
140 g
Answers
Mass of CuSO₄.5H₂0 = 6.25 g (B)
Further explanationMW CuSO₄.5H₂0 =250 g/ mol
mol of CuSO₄
\(\tt 0.25\times 0.1=0.025\)
mass of CuSO₄ (MW=159.61 g/mol)
\(\tt 0.025\times 159.61=3.99~g\)
\(\tt mass~CuSO_4=\dfrac{159.61}{250}\times mass~CuSO_4.5H_2O\\\\mass~CuSO_4.5H_2O=\dfrac{3.99\times 250}{159.61}=6.25~g\)
A constant electric current deposited 365 mg of Ag in 216 minutes from an aqueous Silver trioxonitrate (v). What is the Current?
Answers
The electric current is 0.025 A
Electric current refers back to the go with the flow of energy in an electronic circuit and to the amount of strength flowing through a circuit. it's far measured in amperes (A). the bigger the cost in amperes, the more energy is flowing within the circuit.
Ag+ + e¯ →Ag
1F deposits 107.87 g/mol (molecular mass) of silver
1F = 96500 C
Let, 107.87 g/mol needed = 96500 C
Number of coulombs required to deposit 0.3650 g of silver =(96500/107.87) 0.3650
Q = 326.5 C
According to Faraday’s law, Q = I x t
I = 326.5 C / (216 x 60 s) = 0.025 A
Learn more about electric current here:-https://brainly.com/question/2984202
#SPJ9
All alkali metals react with water to produce hydrogen gas and the corresponding alkali metal hydroxide. A typical reaction is that between lithium and water: 2Li(s) + 2H2O(1) 2LiOH(aq) + H2(g) How many grams of Li are needed to produce 9.89 g of H₂ ?
Answers
Answer:
69.23g
Explanation:
Find out how many moles is in 9.89g of H2.
number of moles = mass(g) / molar mass
1 is the molar mass of hydrogen (to the nearest whole)
relative molecular mass of H2: 2*1 = 2
number of moles of H2 = 9.89/2 = 4.945
1 mol of H2 is produced from 2 mol of Li
so
4.945 mol of H2 produces 9.89 mol of Li
mass(g) = number of moles * molar mass
7 is the molar mass of Lithium (to the nearest whole)
mass = 9.89 * 7 = 69.23
69.23 grams of Li are needed to produce 9.89 of H2
help please help please help please

Answers
4. The process which leads to transition from the above task are as follows:
Ocean - Atmosphere: Evaporation Atmosphere - Clouds: Condensation Clouds - Snow: Condensation Glacier ( river ice ) - River: Melting Cloud - Soil: Precipitation5. The two processes that cause transition from each given below:
Ocean - Cloud is: Evaporation and transpiration Cloud - Glacier is: Evaporation and precipitation6. The major reason why water shortages are the problems for many people around the world is simply because of climatic change which alters the weather of a given place at a period of time, thereby leading to water insufficiency.
What is meant by melting?Melting refers to a chance of state of matter which refers to the process whereby a solid substance changes to liquid.
From the context of the above task, river ice melts into river in a process known as melting.
In conclusion, we can now deduce from the explanation given above that transition is a process which involves the change in form of matter.
Read more on melting:
https://brainly.com/question/40140
#SPJ1
The half life for the radioactive decay of carbon- to nitrogen- is years. Suppose nuclear chemical analysis shows that there is of nitrogen- for every of carbon- in a certain sample of rock. Calculate the age of the rock. Round your answer to significant digits. g
Answers
Answer:
Age of rock = 6.12 × 10³ years
Note: The question is incomplete.A similar but complete question is given below.
The half-life for the radioactive decay of carbon-14 to nitrogen-14 is 5.73 x 10^3 years. Suppose nuclear chemical analysis shows that there is 0.523mmol of nitrogen-14 for every 1.000 mmol of carbon-14 in a certain sample of rock.
Calculate the age of the rock. Round your answer to 2 significant digits.
Explanation:
The half-life of a radioactive material is the time taken for half the atoms in the atomic nucleus of a material to disintegrate.
The half-life for the radioactive decay of carbon-14 to nitrogen-14 is given as 5.73 x 10³ years. This means that given 1 mole of carbon-14 is present initially, after one half-life, 0.5 moles of carbon-14 would remain.
Number of millimoles of carbon-14 remaining = 1 - 0.523 = 0.477 mmol
Number of half-lives that the carbon-14 has undergone is determined as follows:
Amount remaining = (1/2)ⁿ
where nnis number of half-lives
0.5 mmol = one half-life
0.5 = (1/2)¹
O.477 = (1/2)ⁿ = (0.5)ⁿ
㏒₀.₅(0.477) = n
n = ㏒(0.477)/㏒(0.5)
n = 1.067938829
Age of the rock = number of half-lives × half-life
Age of rock = 1.067938829 × 5.73 × 10³ years
Age of rock = 6.12 × 10³ years
write the structural formula for 2-bromo-3-chloro-4,4-dimethylpentanal
Answers
Answer:
Br-CH2-CH(CH3)2-C(Cl)H-CH(CH3)2-CHO
Explanation:
The molecule has a total of 14 carbon atoms, 13 hydrogen atoms, and 1 bromine atom. The carbon atoms are arranged in a chain with a methyl group attached to the second carbon atom, a chlorine atom attached to the third carbon atom, and two methyl groups attached to the fourth carbon atom. The fifth carbon atom has a carbonyl group attached to it.
The molecule is an aldehyde, which means that it has a carbonyl group (C=O) at the end of the chain. The carbonyl group is polar, and the oxygen atom has a partial negative charge. The hydrogen atom has a partial positive charge. This polarity makes the aldehyde group susceptible to nucleophilic attack.
The bromine and chlorine atoms are both electrophilic, which means that they have a partial positive charge. This makes them susceptible to nucleophilic attack.
The methyl groups are non-polar and do not have any significant reactivity.
The molecule is a chiral molecule, which means that it has a mirror image that is not superimposable on itself. This is because the carbon atom with the carbonyl group is attached to four different groups.
The molecule is a liquid at room temperature and has a strong odor. It is used in a variety of products, including perfumes, flavorings, and plastics.
14. Which statement gives you enough information to say that the atom is electrically neutral?
A. The atom has 15 neutrons and 15 electrons.
B. The atom has 19 electrons and 19 neutrons.
C. The atom has 4 neutrons and 4 protons.
D. The atom has 7 protons and 7 electrons
Answers
The statement which gives you enough information to say that the atom is electrically neutral is: The atom has 7 protons and 7 electrons.
One of the hydrates of CoCl2 is cobalt(II) chloride dihydrate .
A 56.2 gram sample of CoCl2 2 H2O was heated thoroughly in a porcelain crucible, until its weight remained constant. After heating, how many grams of the anhydrous compound remained?
Answers
Answer:
The molar mass of CoCl2 2H2O is 165.87 g/mol.
This can be calculated by adding the molar masses of each component:
Co: 58.93 g/mol2
Cl: 2 x 35.45 g/mol = 70.90 g/mol
2H2O: 2 x 18.02 g/mol = 36.04 g/mol
To find the mass of the anhydrous compound, we need to subtract the mass of the water that was driven off during heating. The difference in weight between the original sample and the final, heated sample is equal to the weight of the water that was lost.
In using the Haber process in the formation of ammonia, what mass of hydrogen is needed to produce 51.0 grams of ammonia? 3 H₂(g) + N2 (g) → 2 NH3(g).
Answers
The mass of hydrogen needed to produce 51.0 grams of ammonia is ≈ 9.07 grams.
To determine the mass of hydrogen required to produce 51.0 grams of ammonia (NH3) using the Haber process, we need to calculate the stoichiometric ratio between hydrogen and ammonia.
From the balanced chemical equation:
3 H₂(g) + N₂(g) → 2 NH₃(g)
We can see that for every 3 moles of hydrogen (H₂), we obtain 2 moles of ammonia (NH₃).
First, we need to convert the given mass of ammonia (51.0 grams) to moles. The molar mass of NH₃ is 17.03 g/mol.
Number of moles of NH₃ = Mass / Molar mass
= 51.0 g / 17.03 g/mol
≈ 2.995 moles
Next, using the stoichiometric ratio, we can calculate the moles of hydrogen required.
Moles of H₂ = (Moles of NH₃ × Coefficient of H₂) / Coefficient of NH₃
= (2.995 moles × 3) / 2
≈ 4.493 moles
Finally, we can convert the moles of hydrogen to mass using the molar mass of hydrogen (2.02 g/mol).
Mass of H₂ = Moles × Molar mass
= 4.493 moles × 2.02 g/mol
≈ 9.07 grams
Therefore, approximately 9.07 grams of hydrogen is needed to produce 51.0 grams of ammonia in the Haber process.
Know more about the mass of hydrogen here:
https://brainly.com/question/14083730
#SPJ8
What are molecules, and how are the properties of molecules different from the atoms they come from? Give an example.
Answers
Answer:
Molecules are the smallest particle of a compound or element which can be found in nature as a free entity and made up of atoms bonded together. The molecules can not take part in the chemical reaction on their own directly.
An atom is on other hand is the smallest particle that does not exist free in nature and bonded together to form molecules and involves in the chemical reactions directly.
Therefore we can say that the molecules are formed by atoms while the atom exists as an entity in nature.
Which of the following statements is not true of the process of fusion?
O A. Two nuclei are pulled far apart.
O B. The strong nuclear force overcomes the electrostatic force.
O C. It takes a lot of energy for two nuclei to get close enough to fuse.
O D. Fusion produces more energy than fission.
Answers
Answer: A
Explanation:
everything else is true
What are aliphatic aldehydes? Class 12
Answers
Answer:
Explanation: The aldehydes in which the aldehydic functional group (−CHO) is attached to a saturated carbon chain are called Aliphatic aldehydes.
To form a 1:1 ratio through ionic bonding, an element in group 1A would need to react with an element in group 7A.
True
False
When Aluminum reacts with oxygen, it would take 3 aluminums and 1 oxygen atoms to become stable by all atoms in that compound having filled outer shells.
True
False
Answers
First group metals forms 1:1 compounds with 17th group or group 7 A. Thus, the statement is true. Aluminum forms its oxides by 2 Al and 3 oxygen atoms. Hence, the statement is false.
What is ionic bonding ?Ionic bonds are formed between metals and non metals. Metals are electron rich and easily loss one or more electrons to electron deficient nonmetals.
First group elements are called alkali metals. They have one valance electron. Group 7A are halogens with 7 valence electrons. They need one more electron to achieve octet.
Thus, alkali metals donate their valence electron to halogens and forms 1:1 compound. Therefore, the statement is true.
Aluminum reacts with oxygen to form its oxide Al₂O₃. Thus, 2 Al and 3 Os are combined to form covalent bond. Thus, the statement is false.
Find more on ionic bonding :
https://brainly.com/question/11527546
#SPJ1
Using Boyle's Law solve the following: An unknown gas has a volume of 200.0 mL and a pressure of 350.0 torr, pressure were increased to 700.0 torr, what is the resulting volume?
Answers
Answer:
400 mL
Explanation:
Boyle's Law: \(P_1*V_1 = P_2*V_2\)
Let x = the resulting volume
350 (200) = 700 (x)
x = 400 mL
Be sure to answer all parts. Ammonia is a principal nitrogen fertilizer. It is prepared by the reaction between hydrogen and nitrogen. 3H2(g) + N2(g) → 2NH3(g) In a particular reaction, 4.00 moles of NH3 were produced. How many moles of H2 and how many moles of N2 were reacted to produce this amount of NH3?
Answers
moles of H2
Mole ratio of H2 to NH3 is 3: 2
if 3: 2
? 4
= 4*3/2= 6
6moles of H2
moles of N2
mole ratio of N2 to NH3 is 1: 2
if 1 : 2
? 4
4*1/2 = 2
2 moles of N2
combustion always result in to formation of water. what other type of reactions may result into formation of water? examples of these reactions
Answers
As combustion always result into the formation of water, the other type of reactions that may result into formation of water are Acid-Base Neutralization Reactions and Hydrogen and Oxygen Reaction.
Acid-Base Neutralization Reactions:
A neutralisation reaction is a chemical process in which an acid and a base combine to produce salt and water as the end products.
H⁺ ions and OH⁻ ions combine to generate water during a neutralisation reaction. Acid-base neutralisation is the most common type of neutralisation reaction.
Example: Formation of Sodium Chloride (Common Salt):
HCl + NaOH → NaCl + H₂O
Hydrogen and Oxygen Reaction:
Water vapour is created when hydrogen gas (H₂) and oxygen gas (O₂) are combined directly. This reaction produces a lot of heat and releases a lot of energy.
Example: 2 H₂ + O₂ → 2 H₂O
Learn more about reactions:
https://brainly.com/question/25769000
Which is for which? Here is the image to my question. Please help god bless.

Answers
Answer:
1 - Gravitational.
2 - Normal
3 - Tension
4 - Frictional
5 - Centripetal
Explanation:
1. If you drop something, gravity pulls it down to the Earth, So falling towards the earth is gravity.
2. Pushing back on another object is normal, Newton's law: Every action has an equal and opposite reaction.
3. When two forces are pulled on opposite sides, the object must stretch which creates tension. Think of a rubber band. If it is pulled more than the object can stretch, it will tear. Tensile strength refers to how much pulling force an object can withstand before it tears.
4. When objects or molecules rub against other objects or molecules they create friction.
5. Last two options go together.
In the following experiment, a coffee-cup calorimeter containing 100 mL
of H2O is used. The initial temperature of the calorimeter is 23.0 ∘C
. If 6.60 g of CaCl2 is added to the calorimeter, what will be the final temperature of the solution in the calorimeter? The heat of solution ΔHsoln of CaCl2 is −82.8 kJ/mol
.
Assume that the specific heat of the solution formed in the calorimeter is the same as that for pure water: Cs=4.184 J/g⋅∘C
.
Express your answer with the appropriate units.
Answers
In the following experiment, a coffee-cup calorimeter containing 100 mL of \(H_{ 2} O\) is used. The initial temperature of the calorimeter is 23.0 ∘C. If 6.60 g of \(CaCl_{2}\) is added to the calorimeter, Final temperature of the solution in the calorimeter = 11.
The first step in solving this problem is to calculate the number of moles of \(CaCl_{2}\\\) added to the calorimeter.
Moles of \(CaCl_{2}\) = mass of \(CaCl_{2}\) / molar mass of \(CaCl_{2}\)
Moles of\(CaCl_{2}\) = 6.60 g / 110.98 g/mol (molar mass of \(CaCl_{2}\)
Moles of\(CaCl_{2}\) = 0.0594 mol
We can use the equation for heat transfer to find the change in temperature of the solution. q = mCsΔT, where q is the heat transferred, m is the mass of the solution, Cs is the specific heat of the solution, and ΔT is the change in temperature.
We know that the initial temperature of the calorimeter is 23.0 ∘C and the mass of the solution is 100 g (since the density of water is 1 g/mL). We can solve for ΔT: ΔT = q / mCs
To find q, we can use the enthalpy change of solution (ΔHsoln) and the number of moles of\(CaCl_{2}\)added: q = ΔHsoln x moles of\(CaCl_{2}\)
q = -82.8 kJ/mol x 0.0594 mol
q = -4.92 kJ
Now we can solve for ΔT: ΔT = (-4.92 kJ) / (100 g x 4.184 J/g⋅∘C)
ΔT = -11.8 ∘C
We can find the final temperature of the solution by adding the change in temperature to the initial temperature: Final temperature = 23.0 ∘C - 11.8 ∘C =11 ∘C.
Learn more about calorimeter here:
https://brainly.com/question/4802333
#SPJ1
What will happen to the equilibrium system when adding HCl to aqueous solution of Na2SO4?
Answers
When HCl is added to aqueous solution of sodium sulphate aqueous solution, sulfuric acid is formed, and the solution's chemical makeup and acidity get changed.
Thus, several chemical reactions happen when HCl is introduced to a sodium sulphate aqueous solution. While sodium sulphate breaks down into 2 Na+ ions and SO4^2- ions, HCl splits into H+ and Cl- ions. When the H+ ions from HCl interact with the SO4^2- ions, sulfuric acid, a more potent acid, is created.
The solution's H+ ion concentration rises as a result of this reaction, altering the equilibrium in favor of the products. As a result, the system's equilibrium is upset, which causes sulfuric acid to form. The solution's chemical makeup and acidity ultimately alter as a result of the addition of HCl.
Learn more about the sulfuric acid here:
https://brainly.com/question/1107054
#SPJ1
Given that equilibrium in the acid-base reaction below lies to the left, which acid is the weaker of the two acids involved in the reaction?
HC₂O₄⁻(aq) + NH₄⁺(aq) ⇄ NH₃(aq) + H₂C₂O₄(aq)
A.) H₂C₂O₄
B.) HC₂O₄⁻
C.) NH₃
D.) NH₄⁺
Answers
The weaker acid involved in the given reaction is HC₂O₄⁻. Option B is correct.
The position of equilibrium in an acid-base reaction can provide information about the relative strength of the acids and bases involved. In the given reaction, if the equilibrium lies to the left, it indicates that the forward reaction is not favored and the reverse reaction is favored.
This means that the products NH₃(aq) and H₂C₂O₄(aq) have a tendency to react and form the reactants HC₂O₄⁻(aq) and NH₄⁺(aq).
Therefore, the acid dissociation constant of HC₂O₄⁻(aq) is K_a1 = 5.9 × 10⁻². The acid dissociation constant of H₂C₂O₄(aq) is K_a2
= 5.9 × 10⁻⁵.
Since the equilibrium lies to the left, it means that the concentration of HC₂O₄⁻(aq) is higher than that of H₂C₂O₄(aq) at equilibrium. This suggests that HC₂O₄⁻(aq) is the weaker acid, as it does not dissociate as much as H₂C₂O₄(aq) does.
Hence, Option B is correct.
To know more about acid-base reaction here
https://brainly.com/question/15686955
#SPJ1
What is the oxidation state of N in NaNOz?

Answers
The oxidation state of nitrogen (N) in NaNO3 is +5. option B
To determine the oxidation state of nitrogen (N) in sodium nitrate (NaNO3), we need to assign oxidation numbers to each element in the compound.
In NaNO3, we know that the sodium ion (Na+) has a +1 oxidation state because it is an alkali metal. Oxygen (O) typically has an oxidation state of -2 in compounds, and there are three oxygen atoms in NaNO3. Since the compound is neutral, the sum of the oxidation states must be zero.
Let's assume that the oxidation state of nitrogen is x. Therefore, we can set up the equation:
(+1) + x + (-2) * 3 = 0
Simplifying the equation:
+1 + x - 6 = 0
x - 5 = 0
x = +5
Therefore, the oxidation state of nitrogen (N) in NaNO3 is +5.
The oxidation state of an element indicates the number of electrons it has gained or lost in a compound. In this case, the nitrogen atom in NaNO3 has gained five electrons to achieve a stable oxidation state of +5.
It is important to note that oxidation states are formal charges and do not necessarily represent the actual distribution of electrons in a compound. They are assigned based on a set of rules and can be useful in understanding the reactivity and behavior of elements in chemical reactions.
Option B
For more such questions on oxidation state visit:
https://brainly.com/question/25551544
#SPJ8