Melting is a phase change in which the molecules involved experience _ in kinetic energy and _ on potential energy.
a) no change; no change
b) no change; an increase
c) a decrease; no change
Answers
Answer:
b) no change; an increase
Explanation:
Kinetic energy does not change but potential energy increases at melting point.
Related Questions
True or false? The subscripts in a chemical formula do not change for a given compound.
Answers
True. The subscripts in a chemical formula represent the relative number of atoms of each element in a compound. They indicate the ratio of atoms present and remain constant for a given compound.
Changing the subscripts would alter the composition and stoichiometry of the compound.
forces between the negatively charged electron and the positively charged nucleus, allowing the electron to be completely removed from the atom. It is typically measured in units of electron volts (eV) or kilojoules per mole (kJ/mol). Ionization energy is influenced by factors such as the atomic structure, electron shielding, and the effective nuclear charge experienced by the outermost electrons. The ionization energy generally increases as you move across a period in the periodic table due to increased nuclear charge and decreased atomic radius. It also decreases as you move down a group due to increased electron shielding and atomic size. Ionization energy plays a crucial role in understanding chemical reactions, electron configurations, and the reactivity of elements.
Learn more about chemical here:
https://brainly.com/question/29240183
#SPJ11
The Lyman series results from excited state hydrogen atoms transiting to
the ground state by emitting electromagnetic radiation.
a. In total, how many spectral lines will result if a sample of hydrogen is excited into the fourth
excited state? (Hint: draw a small energy level diagram and use arrows to represent
transitions from higher to lower energy states.
b. How many of the lines from the previous answer belong to the Lyman series?
c. Calculate the energy for each of the lines in the Lyman series that you identified in the last
answer. (Recall that the energy of the light emitted represents the difference between two
energy states of the atom.)
Answers
Answer:
I can't draw diagrams on this web site but I can do with numbers I think. So an electron is moved from n = 1 to n = 5. I'm assuming I've interpreted the problem correctly; if not you will need to make a correction. I'm assuming that you know the electron in the n = 1 state is the ground state so the 4th exited state moves it to the n = 5 level.
n = 5 4th excited state
n = 4 3rd excited state
n = 3 2nd excited state
n = 2 1st excited state
n = 1 ground state
Here are the possible spectral lines.
n = 5 to 4, n = 5 to 3, n = 5 to 2, n = 5 to 1 or 4 lines.
n = 4 to 3, 4 to 2, 4 to 1 = 3 lines
n = 3 to 2, 3 to 1 = 2 lines
n = 2 to 1 = 1 line. Add 'em up. I get 10.
b. The Lyman series is from whatever to n = 1. Count the above that end in n = 1.
c.The E for any level is -21.8E-19 Joules/n^2
To find the E for any transition (delta E) take E for upper n and subtract from the E for the lower n and that gives you delta E for the transition.
So for n = 5 to n = 1, use -Efor 5 -(-Efor 1) = + something which I'll leave for you. You could convert that to wavelength in meters with delta E = hc/wavelength. You might want to try it for the Balmer series (n ending in n = 2). I think the red line is about 650 nm.
Explanation:
Select the correct scientific notation form of this numeral using only 2 significant figures. 5,489.6654
Answers
Answer:
5.4 × 10 3
Explanation:
Answer:
5.4 × 10^3.
Explanation:
Not 5.49 × 10^3, 5.4 × 10^7, or 5.5 × 10^3.
If an eletric dicharge produce 300L of ozone(O3), how many liter of oxygen(o2) are required?
Answers
If an electric discharge produce 300 L of ozone(O₃), the liter of oxygen(O₂) are required is 450 L.
The reaction is as follows :
3O₂ ---> 2O₃
The volume of the Ozone = 300 L
The mole ratio is given as :
3 moles of the oxygen that is O₂ will produce the 2 moles of the ozone that is O₃.
The number of the moles is as follows :
The number of moles = mass / molar mass
The 300 L of the ozone will required the oxygen is as :
To produce 300 L of ozone = ( 3/2 ) × 300 L of the oxygen required
= 1.5 × 300 L
= 450 L
Thus, the 450 L of the oxygen is required to produce the 300 mL of the ozone .
To learn more about ozone here
https://brainly.com/question/22172775
#SPJ4
A coffee cup calorimeter with a heat capacity of 6. 70 J/∘ C was used to measure the change in enthalpy of a precipitation reaction. A 50. 0 mL solution of 0. 360 M AgNO3 was mixed with 50. 0 mL of 0. 540 M KSCN. After mixing, the temperature was observed to increase by 4. 06∘C. Calculate the enthalpy of reaction, ΔHrxn, per mole of precipitate formed (AgSCN). Assume the specific heat of the product solution is 4. 11 J / (g⋅∘C) and that the density of both the reactant solutions is 1. 00 g/mL. Calculate the theoretical moles of precipitate formed from AgNO3 and KSCN. Moles of precipitate formed from AgNO3: mol moles of precipitate formed from KSCN: mol Calculate the heat change experienced by the calorimeter contents, ????contents. ????contents= J Calculate the heat change expierenced by the calorimeter contents, ????cal. ????cal= J Calculate the heat change produced by the solution process, ????solution. ????solution= J Calulate ΔHsolution for one mole of precipitate formed. ΔHsolution= kJ/mole
Answers
A coffee cup temperature with a heat capacity of 6. 70 J/∘ C was used to measure the change in enthalpy of a precipitation reaction.The value of ΔHrxn was found to be 61.9 kJ/mol.
Calculate the enthalpy of reaction, ΔHrxn, per mole of precipitate formed (AgSCN). Assume the specific heat of the product solution is 4. 11 J / (g⋅∘C) and that the density of both the reactant solutions is 1. 00 g/mL.1. Calculation of Moles of precipitate formed from AgNO3:To find the value of ΔHrxn, we used the formula ΔHrxn = Qsolution/n, where Qsolution is the heat change produced by the solution process and n is the number of moles of AgSCN formed.
To find the value of n, we first calculated the number of moles of AgNO3 and KSCN used in the reaction using the formula n = M × V.To find the heat change produced by the solution process, we used the formula
Q = m × c × ∆T,
where Q is the heat change, m is the mass of the product solution, c is the specific heat capacity of the product solution, and ∆T is the change in temperature of the solution.The value of ΔHrxn was found to be 61.9 kJ/mol.
To know more about temperature Visit;
https://brainly.com/question/29072206
#SPJ11
The number of grams of helium in a balloon at a pressure of 99.8 kPa, a temperature of 301 K, and a volume of 0.785 L would be
Options:
814 g
0.125 g
0.278 g
337 g
Answers
pV=nRT
in formula n=m/M
you have to find m
P=99.8/101.325
v=0.785
R=0.0821
T=301
The number of grams of helium in a balloon at a pressure of 99.8 kPa, a temperature of 301 K, and a volume of 0.785 L would be 0.125g.
How to calculate mass?The mass of a substance can be calculated by multiplying the number of moles by its molar mass.
However, the number of moles of helium must be calculated as follows:
PV = nRT
Where;
P = pressureV = volumen = no of molesR = gas law constantT = temperature0.985 × 0.785 = n × 0.0821 × 301
0.773 = 24.7n
n = 0.773 ÷ 24.7 = 0.031moles
mass of He = 0.031 × 4 = 0.125g
Therefore, the number of grams of helium in a balloon at a pressure of 99.8 kPa, a temperature of 301 K, and a volume of 0.785 L would be 0.125g.
Learn more about mass at: https://brainly.com/question/19694949
#SPJ6
pleasee helpppp
i have posted this many times m=but no one has helped :(
will mark brainliest!! :)

Answers
Answer:
its 1 transferd electrons 2 shared electrons 3 metal only
Explanation:
Answer:
1 transferd electrons 2 shared electrons 3 metal only
Explanation:
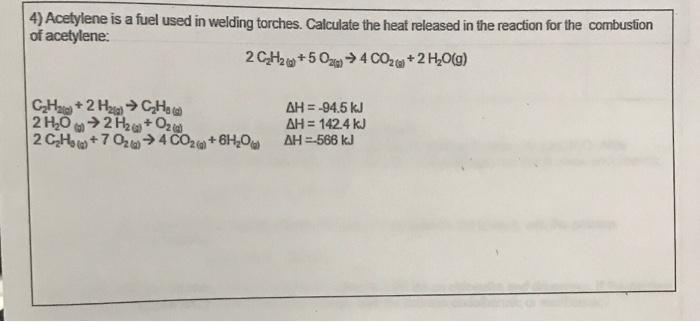
acetylene is a fuel used in welding torches. calculate the heat released in the reaction for the combustion of acetylene
Answers
Acetylene and oxygen are converted throughout the process into carbon dioxide and water vapour, producing 566 kJ of heat for each mole of acetylene burned.
The heat released throughout the process may be calculated using the change in enthalpy, often known as the heat of reaction or the enthalpy of reaction. Using the enthalpy values of the products and reactants, one can determine the change in enthalpy, which is determined by the difference between the enthalpies of the products and the reactants. The balanced chemical equation for the burning of acetylene is 2 C2H2 + 5O2 4 CO2 + 2 H2O, and the change in enthalpy, or heat released, is -566 kJ/mol. This indicates that the process transforms acetylene and oxygen into carbon dioxide and water vapour, releasing 566 kJ of heat for every mole of acetylene burnt.
learn more about acetylene here:
https://brainly.com/question/28916568
#SPJ4
what is the percent composition of potassium phosphate? show your work
Answers
Answer:
35.62%
Mass percent composition of K = 0.3562 x 100% Mass percent composition of K = 35.62%
Explanation:
a 90 g samole of naoh is dissolved in water and the solution is diluted to give a final volume of 3 liters the moarity of the final solution is
Answers
When a 90 g of NaOH is dissolved in water and the solution is diluted to give a final volume of 3 liters the molarity of the final solution is 0.75 M.
To find the molarity of the final solution, we need to first calculate the number of moles of NaOH present in the solution.
The formula for calculating the number of moles of a substance is:
moles = mass / molar mass
The molar mass of NaOH is 40.00 g/mol, so the number of moles of NaOH present in the 90.0 g sample can be calculated as:
moles = 90.0 g / 40.00 g/mol
moles = 2.25mol
Given , volume = 3 L
Now we can use the formula:
Molarity = moles / volume
to calculate the molarity of the concentrated NaOH solution:
Molarity = 2.25 mol / 3.00 l
Molarity = 0.75 M
Therefore, the molarity of the final NaOH solution is 0.75 M.
To know more about molarity here
https://brainly.com/question/31473692
#SPJ4
Why is Hawaii made of volcanoes?
Answers
Answer:
Explanation:
The Hawaiian Islands were literally created from lots of volcanoes—they're a trail of volcanic eruptions. In the case of the Hawaiian Islands, the Pacific Plate is continually moving to the northwest over the Hawaiian hot spot. This movement caused the Hawaiian chain of islands to form.
How are plant and animal cells similar? How are they different? To answer these questions, make a list of the different organelles in each cell. Explain how each organelle is vital to the life and function of a plant or animal.
Answers
Answer:
A plant cell contains a large, singular vacuole that is used for storage and maintaining the shape of the cell. In contrast, animal cells have many, smaller vacuoles. Plant cells have a cell wall, as well as a cell membrane. In plants, the cell wall surrounds the cell membrane.
what do you mean by chemical reaction
Answers
Answer:
Chemical reaction, a process in which one or more substances, the reactants, are converted to one or more different substances, the products.
A chemical reaction rearranges the constituent atoms of the reactants to create different substances as products.
Chemical reactions are an integral part of technology, of culture, and indeed of life itself. Burning fuels, smelting iron, making glass and pottery, brewing beer, and making wine and cheese are among many examples of activities incorporating chemical reactions that have been known and used for thousands of years. Chemical reactions abound in the geology of Earth, in the atmosphere and oceans, and in a vast array of complicated processes that occur in all living systems.
Explanation:
hope it help
plss brainlys me
Answer:
Chemical reaction, a process in which one or more substances, the reactants, are converted to one or more different substances, the products
Explanation:
Substances are either chemical elements or compounds. A chemical reaction rearranges the constituent atoms of the reactants to create different substances as products.
Which option is a use of geothermal energy?
Responses
fueling vehicles
capturing solar power
powering hybrid vehicles
generating electricity
Answers
Generating electricity from the given list option is the use of geothermal, therefore the correct option is A .
What is a nuclear power plant ?It is a type of power plant in which the power is generated with the help of a nuclear reactor involving a nuclear which could be either nuclear fission or fusion reaction.
Fueling vehicles, capturing solar power, and powering hybrid vehicles do not use geothermal energy.
Generating electricity from the given list option is the use of geothermal, therefore the correct option is A.
To learn more about Nuclear power plants here, refer to the link ;
brainly.com/question/4246037
#SPJ1
Which equation is derived from the combined gas law?

Answers
Answer:
First one. V1/T1=V2/T2
If the pressure is the same.
Explanation:
Answer:
A on edge2021
What is a key property of crystals?
Answers
Answer: Crystals differ in physical properties, i.e., in hardness, cleavage, optical properties, heat conductivity, and electrical conductivity.
These properties are important since they sometimes determine the use to which the crystals are put in industry.
hope this helps :)
Answer:
A crystals key properties are hardness, cleavage, optical properties, heat conductivity, and electrical conductivity.
Explanation:
These properties are important since they sometimes determine the use to which the crystals are put in industry.
the molality of hydrochloric acid, hcl, in an aqueous solution is 7.29 m. what is the mole fraction of hydrochloric acid in the solution?
Answers
To find the mole fraction of hydrochloric acid (HCl) in the solution, we need to use the definition of mole fraction, which is the ratio of moles of the solute to the total moles in the solution.
The mole fraction (X) of HCl can be calculated as follows:
Mole fraction of HCl = Moles of HCl / Total moles in the solution
Given that the molality (m) of HCl is 7.29 m, we can determine the moles of HCl per kilogram of solvent (water) using the molality formula:
Moles of HCl = Molality × Mass of solvent (in kg)
To calculate the mole fraction, we need to know the mass of the solvent used. Let's assume we have 1 kilogram (1000 grams) of water as the solvent.
Moles of HCl = 7.29 m × 1000 g
= 7290 moles of HCl
Total moles in the solution = Moles of HCl
Therefore, the mole fraction of HCl in the solution is:
Mole fraction of HCl = 7290 moles of HCl / (7290 moles of HCl)
= 1
The mole fraction of HCl in the solution is 1, indicating that the entire solution is composed of HCl.
To know more about mole fraction, click here:
https://brainly.com/question/30724931
#SPJ11
what are standard conditions when working with gases?
Answers
Answer:
STP in chemistry is the abbreviation for Standard Temperature and Pressure. STP most commonly is used when performing calculations on gases, such as gas density. The standard temperature is 273 K (0° Celsius or 32° Fahrenheit) and the standard pressure is 1 atm pressure.
What is the percentage of Aluminum in Aluminum Sulfate?
Answers
Answer:
342. 15 g/mol is the mass
Al Aluminium 26.98g 2 atoms per mole = 15.7716%
S Sulfur 32.06g 3 atoms per mole = 28.1151%
O Oxygen 15.99g 12 atoms per mole = 56.1133%
Explanation:
Hope this helps
Answer:
15,7%
Explanation:
What is the percentage of Aluminum in Aluminum Sulfate?
Aluminum Sulfate
is Al2 (SO4)3
its molar mass is
(2 X 27) + (3 X 96) = 54 + 288 = 344
THE % Al IS (54 /344) X 100 = 15,7%
All flowers have colorful petals and smell wonderful.
True
False
Answers
Answer:
false i am pretty sure because dead flowers.
How many chiral carbon atoms are present in a molecule of 2,3,4-trichloropentane?
Answers
Answer:
The answer is 2.
Explanation:
Zelda noticed a puddle outside her front door. She saw that the puddle got smaller every day, until the 3rd day when it was completely gone. The next week, she noticed the puddle again. This time the puddle was gone the next day. Since the sun was out the second week but not the first week, Zelda hypothesized that the heat from the sun was the reason for the water evaporating at a faster rate. If she were to set up two containers with equal amounts of water, what would be the best way for Zeldato test her hypothesis\
Answers
Answer: Zelda should place one container of water in sunlight (by a window or outdoors) and the other container in a dark room (closet) away from the sun.
Explanation: This would allow Zelda to test two different settings (sun and no sun) so she can test her hypothesis.
a sample of oxygen occupies 47.2 liters under a pressure of 1240 torr at 25oc. what volume would it occupy at 25oc if the pressure were decreased to 730 torr?
Answers
The volume of 80.175 liters occupy at 25 degree c if the pressure were decreased to 730 torr. It is calculated from the expression of Boyle's Law.
According to the Boyle's Law, an inverse relationship exists between pressure and volume. if the number of molecules (n) and the temperature (T) are both constant. Boyle's Law is used to predict the result of introducing a change in volume and pressure only and only to the initial state of a fixed quantity of gas.
The relationship for Boyle's Law can be expressed as follows,
P1V1 = P2V2
where P1 and V1 are the initial pressure and volume values, and P2 and V2 are the values of the pressure and volume of the gas after change.
A sample of oxygen occupies 47.2 liters under a pressure of 1240 torr at 25oc.
P1 = 1240 torr
V1 = 47.2 liters
P2 = 730 torr
V2 = P1 V1 / P2
putting all values we get,
V2 = 1240 * 47.2 / 730
= 80.175 liters
To learn more about Boyle's law please visit:
https://brainly.com/question/1696010
#SPJ4
Another question.... Please

Answers
Answer:
2 mol KOH
Explanation:
KOH + HNO3 ---> KNO3 + H2O
from reaction 1 mol 1 mol
given x mol 2 mol
x = 1*2/1 = 2 mol
Suppose 50.0 grams of compound AB reacts completely with 100.0 grams of compound CD, how many grams of products will be formed, according to the Law of Conservation of Matter?
a. 50
b. 100
c. 150
d. 200
Answers
Answer:
Option C = 150
Explanation:
First of all we will understand what is law of conservation of mass.
Law of conservation of mass:
This law stated that, mass can neither be created nor destroyed in a chemical equation.
This law was given by French chemist Antoine Lavoisier in 1789. According to this law mass of reactant and mass of product must be equal, because masses are not created or destroyed in a chemical reaction.
For example:
In given photosynthesis reaction:
6CO₂ + 6H₂O + energy → C₆H₁₂O₆ + 6O₂
there are six number of carbon atoms, eighteen oxygen atoms and twelve hydrogen atoms on the both side of chemical equation so this reaction followed the law of conservation of mass because mases are same on both side.
In given chemical reaction:
AB + CD → X
50 g + 100 g = 150 g
Thus option c is correct.
What is the number of moles of glucose (C₆H₁₂O₆) in 0.500 L of a 0.40 M solution?
Answers
Answer:
0.20 moles
Explanation:
In order to solve this problem it is necessary to keep in mind the definition of molarity:
Molarity = moles / litersIf we input the data given by the problem we're left with:
0.40 M = moles / 0.500 LMeaning that we can proceed to calculate the number of moles:
moles = 0.40 M * 0.500 Lmoles = 0.20 molesThe number of moles of glucose (\(C_6H_{12}O_6\)) in 0.500 Liters of a 0.40 M solution is 0.2 moles.
Given the following data:
Molarity of solution = 0.40 MVolume of solution = 0.500 LTo determine the number of moles of glucose (\(C_6H_{12}O_6\)) in 0.500 Liters of a 0.40 M solution:
Mathematically, the molarity of a solution is given by the formula:
\(Molarity = \frac{number\;of\;moles}{Volume \;in\;liters}\)
Making number of moles the subject of formula, we have:
\(Number\;of\;moles = Molarity \times Volume\)
Substituting the given parameters into the formula, we have;
\(Number\;of\;moles = 0.40 \times 0.500\)
Number of moles = 0.2 moles.
Read more: https://brainly.com/question/13750908
Guys pls help me ill give 10 points
Why are important documents stored in an environment without air from the atmosphere?
Answers
Answer:
Save earth, and universe
When sample X'is passed through a filter paper a
white residue, Y, remains on the paper and a clear
liquid, Z, passes through. When liquid Z is
vaporized, another white residue remains. Sample
X is best classified as

Answers
Sample X is best classified as a heterogeneous mixture
Homogenous mixture has the same uniform composition and appearance through out the reaction and it is difficult to separate the substrates present in it.
A heterogeneous mixture contains of visibly different phases or substances. Here separating the mixture becomes easy.
In the given question When sample X is passed through the filter paper we see that a white residue is remaining which is Y. This itself suggests that the the mixture is heterogeneous mixture.
As we initially got the residue it suggests that the solution is a homogenous mixture as we can now differentiate two substances
To know more about heterogenous mixtures
https://brainly.com/question/24898889
#SPJ1
In 2007, the population of Canada was 1.26 × 108, the population of Mexico was 10.6 × 107, the population of Brazil was 13.6 × 107, and the population of China was 1.86 × 109. Which two countries have the highest population?
A. Canada and China
B. Mexico and China
C. China and Brazil
D. Brazil and Mexico
Answers
which line shows the growth of an obligate aerobe incubated anaerobically? A) a B) b C) c
Answers
Aerobic organisms must have oxygen in order to develop, breathe, metabolize, and use other vital routes. At the end of the electron transport chain during respiration in these organisms.
oxygen acts as an electron acceptor. Hence, when an obligatory aerobe is incubated in anaerobic conditions, they are unable to grow because there is no oxygen present, which in turn restricts its respiration and metabolism.
An electron is a subatomic particle with a negative charge that orbits the nucleus of an atom. It has a relative mass of 9.109 x 10⁻³¹ kg, which is about 1/1836th the mass of a proton. Electrons are responsible for the chemical behavior of atoms, as they are involved in the formation of chemical bonds between atoms. They also play a crucial role in electricity and magnetism, as they are the carriers of electrical charge and can produce magnetic fields. Electrons exist in energy levels around the nucleus, and they can absorb or emit energy to move between energy levels, giving rise to the emission and absorption of light and other electromagnetic radiation. Electrons are also used in various technologies, including electronics, telecommunications, and medical imaging.
Learn more about electron here:
https://brainly.com/question/11134441
#SPJ4