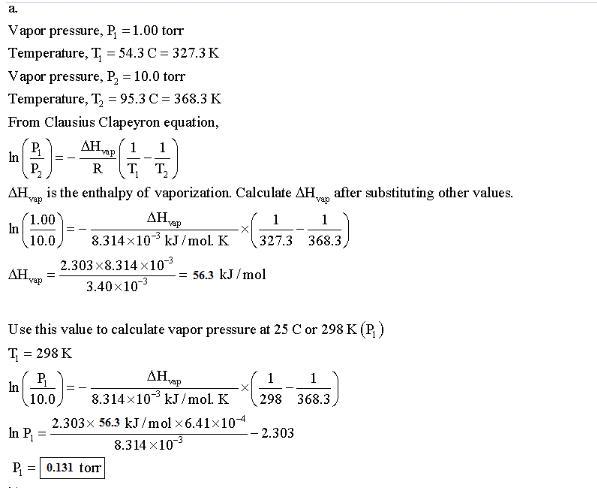
Methyl salicylate, C₈H₈O₃, the odorous constituent of oil of wintergreen, has a vapor pressure of 1.00 torr at 54.3°C and 10.0 torr at 95.3°C. (a) What is its vapor pressure at 25°C?
Answers
Its vapor pressure at 25°C is P1= 0.131 torr.
Vapour pressure is a degree of the tendency of cloth to exchange into the gaseous or vapor country, and it increases with temperature. The temperature at which the vapor stress at the surface of a liquid turns into identical to the pressure exerted with the aid of the environment is known as the boiling factor of the liquid.
it's far important to observe that when a liquid is boiling, its vapor stress is identical to the external stress. for example, as the water boils at the sea stage, its vapor stress is 1 ecosystem because the external strain is also 1 environment.
Vapor pressure or equilibrium vapor pressure is described because of the strain exerted by a vapor in thermodynamic equilibrium with its condensed stages at a given temperature in a closed system. The equilibrium vapor pressure is an indication of a liquid's evaporation rate.
Learn more about vapor pressure here https://brainly.com/question/15195906
#SPJ4
Related Questions
what will be the final pressure of the system after the reaction is complete? (neglect the volume of the ammonium chloride formed.)
Answers
The final pressure of the system is equal to 0.960 atm after the reaction is complete.
What is the ideal gas equation?The state of an ideal gas is described in terms of its pressure, volume, and temperature. The ideal gas law can be represented in the terms of the product of the volume and pressure is equal to the multiplication of the absolute temperature, moles of gas, and the universal gas constant.
An ideal gas of mathematical equation can be written as follows:
PV = nRT
The balanced chemical equation of ammonia and HCl:
\(NH_3 (g) + HCl (g) \longrightarrow NH_4Cl(s)\)
Given the mass of ammonia, m = 5.00 g
The number of moles of ammonia = 5/17 = 0.294 mol
The number of moles of HCl, n = PV/RT
\(n = \frac{1.68 atm\times 2L}{0.082 \times 298 K}\)
n = 0.137 mol
The number of moles of NH₃ did not react, n = 0.294 - 0.137 = 0.157 mol
The final pressure of the system after the reaction is complete:
\({\displaystyle {P = \frac{nRT}{V}\)
\({\displaystyle {P = \frac{0.157 \times 0.082 \times 298}{4}\)
P = 0.960 atm
Learn more about ideal gas equation, here:
brainly.com/question/3637553
#SPJ1
Your question was incomplete, but most probably the complete question was,
Ammonia and hydrogen chloride react to form solid ammonium chloride: Two 2.00-L flasks at 25 degrees C are connected by a valve, as shown in the drawing. One flask contains 5.00 g ammonia, and the other contains HCl(g) at 1.68 atm. When the valve is opened, the gases react until one is completely consumed. What will be the final pressure of the system after the reaction is complete? (Neglect the volume of the ammonium chloride formed.)
How many moles of NaOH
would you have in a
sample that weighed 10g?
Answers
Answer: There are 0.25 moles of NaOH in a sample that weighed 10g.
Explanation:
Given: Mass of NaOH = 10 g
Molar mass of NaOH is 40 g/mol.
Number of moles is the mass of substance divided by its molar mass.
Therefore, moles of NaOH are calculated as follows.
\(No. of moles = \frac{mass}{molarmass}\\= \frac{10g}{40 g/mol}\\= 0.25 mol\)
Thus, we can conclude that there are 0.25 moles of NaOH in a sample that weighed 10g.
HELPP MEE PLEASEE !!!!
At what group # does the ionic radius suddenly increase dramatically from the lowest to the highest? Why?
Answers
Explanation:
Relations kmsksodnnwjsieneoe
Question 1 of 10 Which of the following battery is NOT rechargeable? A. alkaline B. lithium-ion C. lead-acid D. nickel-cadmium Reset Selection 1 Points
Answers
The following battery is not rechargeable is A. alkaline.
Alkaline batteries are primary batteries that have a very long life span and are commonly used in households, these batteries are designed for one-time use only and cannot be recharged. Zinc, manganese oxide, and potassium hydroxide are the primary constituents of alkaline batteries. Because of their long shelf life and relatively high capacity, they are widely used in low-drain and high-drain devices. Lithium-ion batteries, lead-acid batteries, and nickel-cadmium batteries, on the other hand, are all rechargeable.
Lithium-ion batteries are used in a variety of electronic devices, including cellphones, laptops, and cameras. Lead-acid batteries are often used in automobiles and boats. Nickel-cadmium batteries, on the other hand, are used in power tools and portable electronic devices, among other things. So therefore the correct answer is A. Alkaline batteries are not rechargeable.
Learn more about lithium-ion batteries at:
https://brainly.com/question/31115504
#SPJ11
cual es la longitud de una taza con aceite???? AYUDA ES URGENTE!!!
Answers
Answer:
1 taza = 250 ml eso creo
Explanation: Eso creo jejejejeje.
What are the spectator ions in the equation below? (Choose all that apply) 2 LiOH(aq) + BaCl2(aq) → 2 LiCl(aq) + Ba(OH)2(s)
Answers
Answer:
Li and CL
Explanation:
I took the test
melanie needs to mix a 10% acid solution with a 60% acid solution to create 100 milliliters of a 45% solution. how many milliliters of each solution must melanie use?
Answers
70 ml of 10% of fungicide solution to be mixed with,30 ml of 60% of fungicide solution to get 100 ml,of 25% of fungicide solution.
A fungicide solution is what?Pesticides known as fungicides work to eradicate or stop the development of fungi & their spores.They are effective against rusts, mildews, and other plant-damaging fungi.They could also be applied elsewhere to manage mold and mildew.
What fungicide is the most effective?According to Mueller, the most popular class of fungicides worldwide is the triazole family.These locally systemically fungicides are not absorbed by the leaf but rather travel up and down the plant.Triazole pesticides (Folicur, Domark) block an enzyme involved in the formation of fungal sterol.
to know more about fungicide solution visit:
https://brainly.com/question/4390444
#SPJ4
¿Qué bacterias existen según su
nutrición?
Answers
Answer:
autotrofas estrictas son aquellas bacterias incapaces de crecer usando materia orgánica como fuente de carbono. Mixotrofas son aquellas bacterias con metabolismo energético litotrofo (obtienen energía de compuestos inorgánicos), pero requieren sustancias orgánicas como nutrientes para su metabolismo biosintético.
In the equation for the orbital radii in hydrogen-like atoms, a0 is called the:
Select the correct answer below:
A. energy level
B. Bohr radius
C. nuclear charge
D. orbital constant
Answers
:)
how many electrons does fluorine have in its outer shell
Answers
Fluorine (F) has seven electrons in its outermost shell. This shell is referred to as the valence shell.
Fluorine is the element with the atomic number 9, which means it has nine protons in its nucleus. It has the electron configuration of 1s22s22p5, with two electrons in the first energy level, two electrons in the second energy level, and five electrons in the third energy level.
The outermost electrons in the third energy level, that is, 2s2p5, are referred to as valence electrons. They participate in chemical bonding and determine the chemical properties of the element.
Fluorine requires one more electron to complete its octet, which is a stable configuration of eight valence electrons. This is why it is highly reactive and tends to gain an electron from other elements to form the fluoride ion (F-) in chemical reactions.
Learn more about nucleus
brainly.com/question/23366064
#SPJ11
Find the speed of the ATV that goes 60 miles in 2 hours._______________________ m/hr
Answers
Given parameters:
Distance of ATV = 60miles
Time = 2hrs
Unknown:
Speed = ?
Solution:
Speed is a scalar quantity. It has magnitude but no direction. Mathematically, speed is distance divided by time.
Speed = \(\frac{distance}{time}\)
Input the parameters;
Speed = \(\frac{60}{2}\) = 30miles/hr
The speed of the ATV is 30miles/hr
which of these is used to determine the age of an object? question 8 options: palynology taphonomy radiocarbon paleontology
Answers
Radiocarbon dating is used to determine the age of an object.
Radiocarbon dating is a method used to estimate the age of organic materials based on the decay of radioactive carbon-14 isotopes. This technique is widely employed in archaeology, geology, and other scientific fields. When living organisms, such as plants or animals, are alive, they maintain a ratio of carbon-14 to stable carbon-12 isotopes.
However, once they die, the carbon-14 begins to decay at a known rate. By measuring the remaining carbon-14 and comparing it to the initial ratio, scientists can calculate the time that has passed since the organism's death. This method is particularly useful for dating objects that are up to around 50,000 years old. Palynology is the study of pollen grains, taphonomy focuses on the process of decay and fossilization, and paleontology deals with the study of fossils but not specifically dating methods.
To learn more about Radiocarbon dating click here
brainly.com/question/12693872
#SPJ11
Based on your understanding of the pH scale, which of the following statements are TRUE? (Mark all the statements that are true)
A.
A neutral pH of 7 indicates that the concentration of (H+) ions is equal to the concentration of (OH-) ions.
B.
The lower the pH, the higher the concentration of (OH-) ions compared to the concentration of (H+) ions.
C.
The pH of an unknown solution changed from pH 5 to pH 4. This means that the concentration of (H+) ions increased by ten times.
D.
The lower the pH, the more acidic the solution.
E.
Seawater is an acid.
F.
The pH of seawater would become more acidic if the concentration of (H+) ions increased.
G.
The pH of seawater since the Industrial Revolution has changed from 8.2 to 8.1. This means the concentration of (H+) ions has decreased.
Answers
The chloride of a trivalent element X is examined in a mass spectrometer and peaks are obtained at 180, 182, 184, and 186 amu. Assuming that chloride has two isotopes of mass numbers 35 and 37, show that only one isotope of X is involved in this chloride and calculate it's mass number
Answers
Explanation:
Since the element X is trivalent it has 3 valence electrons. Since the Cl ion has a charge of -1, the chloride of this element is of the form \(\text{XCl}_3\).
Assuming that Cl has two isotopes of mass numbers 35 and 37, it follows that \(\text{Cl}_3\) has four possible mass numbers:
35 + 35 + 35 = 105 amu35 + 35 + 37 = 107 amu35 + 37 + 37 = 109 amu37 + 37 + 37 = 111 amuNotice that these four possible mass numbers all differ by exactly 2 -- the same difference observed in the peaks of the mass spectrometer.
This means that only one isotope of X is involved. If there were more than one isotope of X involved, we would expect to see more than four peaks (since there would be more than four possible combinations of \(\text{Cl}_3\) with X).
Finally, to calculate the mass number of X, we subtract the observed peak data from the known mass numbers of \(\text{Cl}_3\):
186 - 111 = 75 amu184 - 109 = 75 amu182 - 107 = 75 amu180 - 105 = 75 amu(In fact, we only really needed to calculate one of these since we already concluded that there is only one isotope of X involved!).
So, the mass number of X is 75 amu.
let a be an invertible × matrix, and let b be an × matrix. explain why 1 can be computed by row reduction:
Answers
Row reduction provides a method to compute the inverse of an invertible matrix.
The computation of the inverse of a matrix can be achieved through row reduction (also known as Gaussian elimination) because row operations preserve the invertibility of a matrix and can transform it into its reduced row echelon form.When we perform row reduction on a matrix A, we apply a series of elementary row operations, such as swapping rows, multiplying rows by a scalar, or adding a multiple of one row to another. These operations can be represented by elementary matrices.If we perform the same row operations on the augmented matrix [A | I], where I represents the identity matrix of the same size as A, we can transform the left side of the augmented matrix into the reduced row echelon form. The right side will then contain the inverse of A.This is possible because row operations correspond to left multiplication by elementary matrices. Since elementary matrices are invertible, their left multiplication preserves the invertibility of the original matrix.In essence, row reduction transforms the given matrix A into the identity matrix I through a series of elementary row operations. Consequently, the right side of the augmented matrix becomes the inverse of A.
for more such questions on reduction
https://brainly.com/question/21851295
#SPJ11
A tank contains a mixture of 3.00 mol N₂, 2.00 mol O₂, and 1.00 mol CO₂ at 25 °C and a total pressure
of 10.0 atm. Calculate the partial pressure of each gas in the mixture.
Answers
The partial pressure of N₂ is 3.75 atm, the partial pressure of O₂ is 2.50 atm, and the partial pressure of CO₂ is 1.25 atm in the given mixture at 25 °C and a total pressure of 10.0 atm.
To calculate the partial pressure of each gas in the mixture, we can use the concept of Dalton's law of partial pressures. According to this law, the total pressure exerted by a mixture of gases is equal to the sum of the partial pressures of each individual gas.
First, we need to find the mole fraction of each gas in the mixture. The mole fraction of a gas is the ratio of the number of moles of that gas to the total number of moles in the mixture. We can calculate the mole fraction using the following formula:
Mole fraction (X) = Moles of gas / Total moles of all gases
For N₂:
Mole fraction of N₂ (X_N₂) = 3.00 mol / (3.00 mol + 2.00 mol + 1.00 mol) = 0.375
For O₂:
Mole fraction of O₂ (X_O₂) = 2.00 mol / (3.00 mol + 2.00 mol + 1.00 mol) = 0.250
For CO₂:
Mole fraction of CO₂ (X_CO₂) = 1.00 mol / (3.00 mol + 2.00 mol + 1.00 mol) = 0.125
Next, we can use the mole fractions to calculate the partial pressures of each gas. The partial pressure of a gas is equal to the mole fraction of that gas multiplied by the total pressure of the mixture.
Partial pressure of N₂ (P_N₂) = X_N₂ * Total pressure = 0.375 * 10.0 atm = 3.75 atm
Partial pressure of O₂ (P_O₂) = X_O₂ * Total pressure = 0.250 * 10.0 atm = 2.50 atm
Partial pressure of CO₂ (P_CO₂) = X_CO₂ * Total pressure = 0.125 * 10.0 atm = 1.25 atm
For more such questions on partial pressure visit:
https://brainly.com/question/19813237
#SPJ8
2. Complete the table below to organize the information from this section. Jupiter Saturn Uranus Neptune Pluto ORDER FROM THE SUN 5th 7th Usually, 9th THE OUTER PLANETS ATMOSPHERE Thick; hydrogen and helium Thick methane MOONS 1, Charon , including At least 31, including Titan Science online Visit blue.msscience.com to access your textbook, interactive including including
Answers
We can see here that organizing the information, we have:
Order from the Sun:
Jupiter (5th)Saturn (6th), Uranus (7th)Neptune (8th)Pluto (9th)Atmosphere: Jupiter and Saturn have thick atmospheres composed mainly of hydrogen and helium, while Uranus and Neptune have thick atmospheres containing methane.
Moons: Jupiter has 79 moons, Saturn has 82 moons, Uranus has 27 moons, Neptune has 14 moons, Pluto has 1 moon (Charon).
What is planet?A planet is a celestial body that orbits around a star, is spherical in shape, and has cleared its orbit of other debris. To be considered a planet, the object must be massive enough for its own gravity to pull it into a roughly spherical shape and it must dominate its orbit, meaning it has cleared its orbit of other debris.
In our solar system, there are eight officially recognized planets: Mercury, Venus, Earth, Mars, Jupiter, Saturn, Uranus, and Neptune.
Learn more about planet on https://brainly.com/question/1286910
#SPJ1
A student reported to her instructor that her unknown contained salt, salicylic acid, and sand. In reality the unknown contained only the first two components, but no sand. What might have led the student to believe sand was present in the unknown? How could the substance be tested to determine if it was actually sand?
Answers
Answer:
Explanation:
From the information given:
The unknown contained salt, salicylic acid, and sand.
It is okay for the student to believe that the sand is present in the unknown, but if we carry how a scientific experiment, we will confirm such a hypothesis if it is right or wrong.
For a test containing salt, salicylic acid, and sand.
We know that the salt is soluble in water, but salicylic acid is sparingly soluble i.e., lightly soluble, and sand is insoluble in water.
So, we will add the unknown mixture into the water. The salt will eventually dissolve first, and then the salicylic acid will dissolve lightly.
Afterward, we will heat the mixture to evaporate the salicylic acid to evaporate, leaving us with the salt.
If there is a positive result of her claim that there is some presence of sand in the evaporated salt sample, that might result from impurities.
I need some help with this I would really appreciate if you could help out. Thank You

Answers
The type of chemical reaction occuring in the table above is as follows:
DecompositionCombination CombustionSingle replacementDouble displacementWhat is a chemical reaction?A chemical reaction is a process involving the breaking or making of interatomic bonds, in which one or more substances are changed into others.
There are several types of chemical reactions as illustrated in the equation given in the above table. They are as follows:
Decomposition reaction; This involves the breaking down of a large substance into its subsequent parts. Combination reaction: This involves the joining of two or more chemical elements to become one compound. Combustion reaction: It is a process wherein a fuel is combined with oxygen, usually at high temperature, releasing heat.Displacement reaction: In this reaction, one or more element is replaced in a compound by another in a chemical reaction.Learn more about chemical reaction at: https://brainly.com/question/22817140
#SPJ1
calculate the molar solubility of ca(oh)2 in 0.10 m ca(no3)2 (ksp= 1.3x10^-6) in pure water
Answers
Ca(OH)₂⇒ Ca²⁺ + 2OH⁻
s s 2s
Ksp = [Ca²⁺][OH⁻]²
Ca(NO₃)₂ ⇒ Ca²⁺ + 2NO₃⁻
0.1 M 0.1 0.2
Input in Ksp
1.3 x 10⁻⁶ = 0.1 . 4s²
s² = 3.25 x 10⁻⁶
s = 1.8 x 10⁻³
1.8 x 10⁻³ is the molar solubility. Solubility is the amount of a substance that can be dissolved in a liquid to form a solution.
What is solubility?Solubility is the amount of a substance that can be dissolved in a liquid to form a solution; it is typically represented as grammes of solute every litre of liquid. One fluid's (liquid or gas) solubility in another can be entire (e.g., methanol and water are completely miscible) or partial (e.g., oil and water barely mix). Generally speaking, "like dissolves like" (for instance, the aromatic hydrocarbons dissolves in one another but not in water).
A material's solubility in two solvents is measured by the distribution coefficient, which is used in some separation techniques (such as absorption and extraction). In general, as temperature rises, so do the dissolution rates of solids in liquids, while they fall as temperature rises and rise with pressure for gases.
Ca(OH)₂⇒ Ca²⁺ + 2OH⁻
s s 2s
Ksp = [Ca²⁺][OH⁻]²
Ca(NO₃)₂ ⇒ Ca²⁺ + 2NO₃⁻
0.1 M 0.1 0.2
1.3 x 10⁻⁶ = 0.1 . 4s²
s² = 3.25 x 10⁻⁶
s = 1.8 x 10⁻³
Therefore, 1.8 x 10⁻³ is the molar solubility.
To know more about solubility, here:
https://brainly.com/question/14366471
#SPJ2
No2 (g) +CO()NO)CO2(g) calculate the order of the reaction with respect to the following reactants according to the following experimental data: Experiment INO2lo (M) ICOlo (M) Initial Rate-AINO2VAt (M/s) 1 0.263 0. 826 1.44 x 10^-5 2 0.263 0. 413 1.44 x 10^-5 3 0.526 0.413 5.76 x 10^-5 Order of the reaction with respect to NO2: _____Order of the reaction with respect to CO: ______
Answers
The order of the reaction with respect to NO2 is x = 1, and the order of the reaction with respect to CO is y = 0.5.
No2 (g) + CO(g) → NO(g) + CO2(g) is the given chemical reaction to calculate the order of the reaction with respect to the following reactants according to the given experimental data as mentioned below:
Let's understand this in detail:
Order of reaction with respect to NO2:
We know that the rate of reaction is given by the formula as follows,
Rate = k[NO2]^x [CO]^yWhere,
k = Rate constant
[NO2] = Concentration of NO2
[CO] = Concentration of CO
x and y = Order of reaction with respect to NO2 and CO, respectively. The first experiment data is taken into account for calculating the order of reaction with respect to NO2 as follows:
1.44 x 10^-5 = k [0.263]^x [0.826]^y......(i)
The second experiment data is taken into account for calculating the order of reaction with respect to NO2 as follows:1.44 x 10^-5 = k [0.263]^x [0.413]^y......(ii)
Now, dividing equation (i) by equation (ii), we get
[0.826]^y/[0.413]^y = 1 => (2)^(2y) = 2 => 2y = 1 => y = 0.5
Substituting the value of y in equation (i), we get
1.44 x 10^-5 = k [0.263]^x [0.826]^0.5=> k = 0.015
Therefore, the order of the reaction with respect to NO2 is x = 1.
Order of reaction with respect to CO:
The first experiment data is taken into account for calculating the order of reaction with respect to CO as follows:
1.44 x 10^-5 = k [0.263]^x [0.826]^y......(i)
The third experiment data is taken into account for calculating the order of reaction with respect to CO as follows:
5.76 x 10^-5 = k [0.526]^x [0.413]^y......(ii)
Now, dividing equation (i) by equation (ii), we ge
t[0.826]^y/[0.413]^y = 2 => 2y = 1 => y = 0.5
Substituting the value of y in equation (i), we get1.44 x 10^-5 = k [0.263]^x [0.826]^0.5=> k = 0.015
Therefore, the order of the reaction with respect to CO is y = 0.5. Hence, the order of the reaction with respect to NO2 is x = 1, and the reaction with respect to CO is y = 0.5.
Learn more about the order of reaction: What is the order of reaction with respect to a and b for a reaction that obeys the rate law: rate = k[a]4[b]5? https://brainly.com/question/28179168
#SPJ11
Why does salt increase the boiling point?
Answers
in order for the reaction shown to occur, the procedure calls for the addition of sulfuric acid to nitric acid. what might be the consequence of adding nitric acid to the sulfuric acid instead? the nitration would occur exclusively at the meta position. the nitration would occur exclusively at the ortho position. there are no regioselectivity or safety consequences. the exothermic reaction could cause the concentrated acid to boil.
Answers
Adding nitric acid to sulfuric acid could cause the concentrated acid to boil, potentially leading to safety hazards.
The result of adding nitric corrosive to sulfuric corrosive rather than the opposite way around is that the exothermic response could make the concentrated corrosive bubble, possibly prompting security risks. Nitration responses regularly require a combination of sulfuric corrosive and nitric corrosive as the nitrating specialist, as the sulfuric corrosive goes about as an impetus to produce the electrophilic nitrating species. Assuming that nitric corrosive were added first, the concentrated sulfuric corrosive might actually bubble, which could bring about the deficiency of reactants, decline in yield, and posture wellbeing perils because of the potential for arrival of harmful exhaust and additionally blast.
To learn more about nitration, refer:
https://brainly.com/question/14920790
#SPJ4
CHCl3 has how many double bonds?
Answers
Answer:
It has two sigma bonds ( the single bonds between each H and C) plus one pi bond and one sigma bond that consitute the double bond between C and O. It contains three covalent bonds, one . Hydrogen Bond Donor Count: 0: Computed by Cactvs 3.4.
Explanation:
Hope it helps you!
how to sold equation with periodic table of elements?
Answers
Answer:
6w6288shbdmdkdhckcncjciicjcididjdn dmxixisk ddjdidjjd
Explanation:
dnnxjxkxjxjndnfncjcshndnsbsnnsnssbsnsnsnsndndndnx
How many of the following species are diamagnetic?
K Zr2⁺ Al3⁺ Zn2⁺
A) 1 B) 3 C) 0 D) 2 E) 4
Answers
A species is diamagnetic or not, we need to consider its electron configuration and the presence of unpaired electrons. Diamagnetic species have all their electrons paired, while paramagnetic species have unpaired electrons. 2 out of the 4 species listed are diamagnetic (Al³⁺ and Zn²⁺). Therefore, the correct answer is D) 2.
Let's examine each species:
1. K (potassium): The electron configuration of K is [Ar] 4s¹. It has one unpaired electron, so it is paramagnetic.
2. Zr²⁺ (zirconium ion): Zr²⁺ has the electron configuration [Kr] 4d². Since it has two unpaired electrons, it is paramagnetic.
3. Al³⁺ (aluminum ion): The electron configuration of Al³⁺ is [Ne]. It has no unpaired electrons, so it is diamagnetic.
4. Zn²⁺ (zinc ion): The electron configuration of Zn²⁺ is [Ar] 3d¹⁰. Since it has a completely filled d orbital with no unpaired electrons, it is diamagnetic.
To learn more about diamagnetic refer here:
https://brainly.com/question/30403646#
#SPJ11
PLEASE HELP !!!
Which two elements have the same number of dots around their chemical symbols in their electron dot diagrams.

Answers
The two elements have the same number of dots around their chemical symbols in their electron dot diagrams are: (C). P and As
Meaning of chemical symbolsChemical symbol can be defined as a representation of an element using letters.
Chemical symbol are given to each element distinctively and this is an easier way to represent an element.
Some examples of chemical symbols are; O for oxygen, Zn for zinc, and Fe for iron etc.
In conclusion, The two elements have the same number of dots around their chemical symbols in their electron dot diagrams are: (C). P and As
Learn more about Chemical symbol: https://brainly.com/question/15045637
#SPJ1
write a net ionic equation to show why solid potassium hydroxide, koh (s), forms a basic solution when it dissolves in water.
Answers
a net ionic equation to show why solid potassium hydroxide, koH (s), forms a basic solution when it dissolves in water
Equation
KOH (s) [H2O ] → K+(aq)+OH−(aq)
The Arrhenius theory, the Brnsted-Lowry theory, and the Lewis theory are the three ideas that have contributed to the definitions of acids and bases over time. According to Arrhenius, an acid is a chemical that, when ionised, releases protons (hydrogen ions) into the solution, whereas a base releases hydroxide ions.
According to Brnsted-Lowry, a base is a proton acceptor and an acid is a proton giver. According to Lewis, a base is an electron-pair donor, while an acid is an electron-pair acceptor
KOH satisfies the Arrhenius theory's definition of a base by producing hydroxide ions when ionised. The hydroxide ion is the base component from a Brnsted-Lowry perspective since it can accept a proton to create water. Although it is a little more difficult to understand from a Lewis perspective why this is a base, the oxygen in the hydroxide has three pairs of non-bonding electrons on it. When a proton (acid) is present, it lacks the electrons necessary to create a covalent link, therefore hydroxide donates an electron pair to the proton in order to build a coordinate covalent bond, which produces water. The three theoretical requirements for a base are thus satisfied by the aforementioned net ionic equation.
To know more about acid,
https://brainly.com/question/25148363
#SPJ4
An activated complex has
A. low potential energy and is stable.
B. high potential energy and is stable.
C. low potential energy and is unstable.
D. high potential energy and is unstable.
Answers
at a given temperature, different liquids will have different equilibrium vapor pressures because
Answers
At a given temperature, different liquids will have different equilibrium vapor pressures because the vapor pressure of a liquid depends on its intermolecular forces, molecular weight, and temperature. The equilibrium vapor pressure is a measure of the tendency of a liquid to evaporate and become a gas at a specific temperature.
Intermolecular forces, such as hydrogen bonding or London dispersion forces, play a significant role in determining the strength of attractions between molecules in a liquid. Liquids with stronger intermolecular forces will have lower vapor pressures because it requires more energy to overcome these forces and transition into the gas phase.
Molecular weight also influences vapor pressure. Generally, liquids with larger and heavier molecules will have lower vapor pressures compared to liquids with smaller and lighter molecules. This is because larger molecules have stronger intermolecular forces and require more energy to transition into the gas phase.
Furthermore, temperature affects the vapor pressure of a liquid. As the temperature increases, the average kinetic energy of the liquid molecules increases, resulting in more molecules with sufficient energy to overcome intermolecular forces and transition into the gas phase. Therefore, at higher temperatures, the vapor pressure of a liquid increases.
In summary, the equilibrium vapor pressure of a liquid is determined by the interplay of intermolecular forces, molecular weight, and temperature. Different liquids with varying intermolecular forces and molecular weights will exhibit different equilibrium vapor pressures at the same temperature.
To know more about the vapor pressures refer here :
https://brainly.com/question/25715932#
#SPJ11