Molar mass of Fe2O3 = g/mol
Answers
Answer:
159.6882 grams
Explanation:
Related Questions
I would like to know if question number 3 is rite

Answers
The balanced reaction of this chemical reaction is as follows :
\(2HClO_4(aq)\text{ + Ba}(OH)_2(aq)\text{ }\Rightarrow Ba(ClO_4)_2(aq)+2H_2O(l)\text{ }\)• As we can see, this is a double-displament reaction
The half-life of cobalt-60 is 5 years. How old is a sample of cobalt-60 if only one-eighth of the original sample is still cobalt-60?.
Answers
\(\tt \large{\boxed{\bold{Nt=No.(\frac{1}{2}) ^{\frac{T}{t1/2} } }}\)
Nt/No = 1/8
T = duration of decay
t 1/2 = half-life
\(\tt \dfrac{1}{8}=\dfrac{1}{2}^\dfrac{T}{5}}\\\\(\dfrac{1}{2})^3=\dfrac{1}{2}^\dfrac{T}{5}}\\\\3=\dfrac{T}{5}\rightarrow T=15~years\)
Place the particles in order from smallest to largest.
1Electron
2Molecule
3Neutron
4Nucleus
5 atom
Answers
Answer:
smallest to largest:
Electron, Neutron, Atom, Molecule, Nucleus
Explanation:
sorry if it's not right
Electron--Neutron--Nucleus-- atom--Molecule is the order from smallest to largest.
Why electron is smaller?Electron is the smallest subatomic particles having the radius is about 9.1× 10−31 kg while on the other hand, the size of neutrin is 1.7×10−15 meters. Both neutron and proton are present in the nucleus so nucleus is bigger than neutron. Nucleus is present inside an atom so atom is bigger than nucleus whereas atom is smaller than molecule.
So we can conclude that Electron--Neutron--Nucleus-- atom--Molecule is the order from smallest to largest.
Learn more about particles here: https://brainly.com/question/11066673
What is the H3O+ concentration in 1.26 M NH4+ (aq)? (Ka of NH4+=5.6*10^-10).
a. 7.1*10^-10
b.1.2*10^-9
c.2.7*10^-5
d.3.36*10^-5
Answers
The approximate concentration of H3O+ in the 1.26 M NH4+ (aq) solution is 7.1 × 10⁻¹⁰ M.
What is the concentration of H3O+ in the solution?The dissociation of ammonium ions in water is:
NH4+ (aq) + H2O (l) ⇌ NH3 (aq) + H3O+ (aq)
Also given that, Ka = 5.6 × 10⁻¹⁰
Calculate H3O+ in the solution usingKa = 5.6 × 10⁻¹⁰:
Ka = [H3O+][NH3] / [NH4+]
Since NH3 and H3O+ concentrations are in the same reaction:
5.6 × 10⁻¹⁰ = [H3O+][H3O+] / 1.26 M
Solve for [H3O+]:
[H3O+] = 7.056 × 10⁻¹⁰ M
[H3O+] = 7.1 × 10⁻¹⁰ M
Therefore, the concentration of H3O+ is 7.1 × 10⁻¹⁰ M.
Option A) 7.1 × 10⁻¹⁰ M is the correct answer.
Learn more about ammonium ion here: https://brainly.com/question/32795930
#SPJ1
What would happen if a small amount of base were added to a buffered solution?
OA. The pH would remain about the same.
OB. The pH would remain neutral.
OC. The pH would decrease.
OD. The pH would increase.
Answers
In the reaction 2Li(s) + 2H2O(I) -> 2LiOH(aq) + H2(g), which compound is in the aqueous state?
A. Li
B. H2O
C. LiOH
B. H2

Answers
The substance that is in aqueous state in the reaction is LiOH from the equation shown.
What is an aqueous state?An aqueous state is a state in which a substance is dissolved in water. This implies that the substance is in solution.
Thus, the substance that is in aqueous state in the reaction is LiOH from the equation shown.
Learn more about aqueous state:https://brainly.com/question/14959096
#SPJ1
Answer:
C
Explanation:
What region of the periodic table contain atoms that form covalent bonds?
A. Right side
B. Bottom left side
C. Middle
D. Top left side
Answers
Answer:
A. the right side
Explanation:
Taking into account the definition of covalent bond, the correct answer is option A.) right side of the periodic table contain atoms that form covalent bonds.
The covalent bond is the chemical bond between atoms where electrons are shared, forming a molecule. Covalent bonds are established between non-metallic elements. These elements have many electrons in their outermost level (valence electrons) and have a tendency to gain electrons to acquire the stability of the electronic structure of noble gas. The shared electron pair is common to the two atoms and holds them together.
In other words, a covalent bond is a force that joins two atoms of non-metallic elements to form a molecule. Atoms share pairs of electrons from their valence shell to achieve the stability of the molecule formed. The tendency of the elements to reach a stable configuration is known as the octet rule.
Finally, since the nonmetallic elements are found on the right side of the periodic table, the correct answer is option A.) right side of the periodic table contain atoms that form covalent bonds.
Learn more:
https://brainly.com/question/9921593?referrer=searchResultshttps://brainly.com/question/12661797?referrer=searchResultshttps://brainly.com/question/10777799?referrer=searchResultshttps://brainly.com/question/12663276?referrer=searchResultsWhat are the atomic numbers of elements whose outermost electrons are represented by (1) 3s^2 (2)2s^2 2p^3 (3) 3s^2 3p^5.
Answers
Answer:
1) 3s² - Magnesium 12
2)2s² 2p³ - Nitrogen 7
3) 3s² 3p⁵ - Chlorine 17
ionic bond formation by correctly pairing these terms: cation, anion, electron gain, and electron loss.
Answers
Answer:
cation - electron loss hence positive charge
anion - electron gain hence negative charge
The given terms are correctly paired thus: anion is to electron gain while cation is to electron loss.
What is ionic bond formation?Ionic bond formation is defined as the formation of an ionic bond during a chemical reaction whereby an atom losses electrons while another gains electrons through transfer of these electrons.
For example in the formation of the compound NaCl. Sodium is the element that donates an electron from its outermost shell to chloride. Therefore, the sodium atom is the cation.
Also the chloride element would accept electron from the sodium, therefore, it is called the anion.
Learn more about ionic bond here:
https://brainly.com/question/28894350
#SPJ1
How many moles are in a 13.7g block of calcium?
Answers
0.342 mol Ca
General Formulas and Concepts:Math
Pre-Algebra
Order of Operations: BPEMDAS
Brackets Parenthesis Exponents Multiplication Division Addition Subtraction Left to RightChemistry
Atomic Structure
Reading a Periodic TableStoichiometry
Using Dimensional AnalysisExplanation:Step 1: Define
13.7 g Ca
Step 2: Identify Conversions
Molar Mass of Ca - 40.08 g/mol
Step 3: Convert
Set up: \(\displaystyle 13.7 \ g \ Ca(\frac{1 \ mol \ Ca}{40.08 \ g \ Ca})\)Multiply/Divide: \(\displaystyle 0.341816 \ mol \ Ca\)Step 4: Check
Follow sig fig rules and round. We are given 3 sig figs.
0.341816 mol Ca ≈ 0.342 mol Ca
Dana has experienced a number of unpredictable short periods in which she suddenly feels terrified and thinks she is going to faint. Her heart races a
Answers
If she suddenly feels terrified and thinks she is going to faint, it sounds like Dana may be experiencing panic attacks.
Panic attacks can be triggered by a variety of things, such as stress, anxiety, or even physical sensations. During a panic attack, the body's fight or flight response is activated, causing symptoms such as a rapid heart rate, shortness of breath, and a feeling of impending doom.
It's important for Dana to seek medical attention to rule out any underlying medical conditions, and to speak with a mental health professional to address any anxiety or stress that may be contributing to her panic attacks. There are treatments available, such as therapy and medication, that can help manage and reduce the frequency of panic attacks.
More on panic attacks: https://brainly.com/question/31465561
#SPJ11
Question 4 "That oil sands executive is greedy and heartless and therefore can't be trusted when she claims to want to improve her company's environmental record."
O False dilemma
O Ad hominem attack
O Straw man
O Appeal to authority
Question 5 "There is no proof that humans are causing climate change so it must natural causes
O False dilemma
O Appeal to ignorance
Strawman
O Appeal to authority
Answers
That oil sands executive is greedy and heartless and therefore can't be trusted when she claims is Ad hominem attack. So, Option B is correct.
4- The argument in question 4 is an example of an ad hominem attack. This is due to the argument's focus on the character of the oil sands executuive rather than the actual problem, which is how to improve the company's environmental record.
The argument holds that the executive cannot be believed when she says she wants to improve the company's environmental record because she is avaricious and callous. This is an error in logic, though, as the executive's character may not necessarily be related to the company's environmental policies.
5- The argument in question 5 is an example of an appeal to ignorance. This is because the argument states that there is no proof that humans are causing climate change, so it must be natural causes. Just because there is no conclusive proof that humans are causing climate change, it does not mean that they are not.
The argument assumes that just because there is no evidence to the contrary, the argument must be true. This is a logical fallacy.
So, Option B is correct.
Learn more about climate change -
brainly.com/question/27170698
#SPJ11
A gas sample is collected in a 2. 2-L tank when the temperature is 2°C. What is the kelvin temperature of the gas?
Answers
When the temperature is 2°C, a gas sample is collected in a 2. 2-L tank. the Kelvin temperature of the gas is 275.15K
To convert a temperature from Celsius to Kelvin, simply multiply it by 273.15 degrees Celsius. So, in this instance, we have:
Celsius temperature + 273.15 K Kelvin temperature = 2°C + 273.15 K Kelvin temperature = 275.15 K
As a result, the gas's Kelvin temperature is 275.15 K.
The Kelvin temperature unit is used in the scientific community. It is an absolute temperature scale, with 0 Kelvin representing the absolute coldest temperature possible.
One Kelvin degree is the same size as one Celsius degree, but the zero point on the Kelvin scale is 273.15 degrees different from the Celsius scale. To convert a temperature from Celsius to Kelvin, simply multiply it by 273.15 degrees Celsius. Kelvin temperatures are widely used in scientific and engineering applications, especially thermodynamics and materials science.
learn more about Kelvin here:
https://brainly.com/question/29082662
#SPJ4
What would happen if a TLC plate was placed In a jar where the solvent level was above the level of the origin (sample spot)? What could you do if you inadvertently spotted your plate too low (aside from consulting your TA)?
Answers
If a TLC plate is placed in a jar where the solvent level is above the level of the origin (sample spot), the solvent will reach and dissolve the sample before it has a chance to form a spot. This can cause the sample to spread out and interfere with the accuracy of the results.
If you inadvertently spotted your TLC plate too low, one solution would be to let the solvent front reach the bottom of the plate, let the solvent evaporate, and then re-develop the plate with fresh solvent. This will help to avoid any interference from previous runs and ensure accurate results. Another solution would be to scrape off the low spots and re-spot the sample higher up on the plate. It's also a good practice to make a note of the error in your lab notes so that you can take it into account when interpreting your results
A TLC plate, or Thin Layer Chromatography plate, is a type of chromatography used in analytical chemistry to separate and identify components in a mixture. It consists of a solid substrate, usually made of glass, plastic, or aluminum, coated with a thin layer of adsorbent material, such as silica gel or aluminum oxide
Here you can learn more about A TLC plate
brainly.com/question/29414463
#SPJ4
Which of the following are not potential "chemical hazards"? antibiotic traces O pesticides animal feces oven cleaner
Answers
Answer:
antibiotic traces because they can always put more of something and less of something
Explanation:
PLS ANSWER QUICKLY
Directions: Determine whether the following chemical reactions are synthesis (S), decomposition (D), single replacement (SR), double replacement (DR), or combustion (C). 1. CO, → C + O2
2. NaCl + AgNO3 > NaNO, + AgCl
3. S + Cl2 → SCI,
4. BaCl, + 2NaOH 2NaCl + Ba(OH)2
5. C,H, + 50, -300, +41,0 CH3
6. Zn + CuSO4 → ZnSO4 + Cu
7. 2KI + Br, 2KBr + +
8. 3CaBr, + 2Na, P Ca P, +6NaBr : - 2
9. 2NaF → 2Na+ F
10. CH.0+ 02 →CO, + H2O
Answers
2. DR
3. SR
4. DR
5. C
6. SR
7. S
8. D
10. C
A beaker contains 217 grams osmium (III) fluoride (OsF3= 247.224 amu) in 0.0673 liters of solution. What is the molarity?
Answers
To calculate the molarity of a solution, you need to know the number of moles of the solute (OsF3) and the volume of the solution in liters.
First, let's calculate the number of moles of OsF3:
Molar mass of OsF3 = 247.224 g/mol
Mass of OsF3 in the beaker = 217 grams
Number of moles of OsF3 = Mass of OsF3 / Molar mass of OsF3
= 217 g / 247.224 g/mol
Next, we need to calculate the volume of the solution in liters:
Volume of the solution = 0.0673 liters
Now we can calculate the molarity:
Molarity = Number of moles of solute / Volume of solution
Substituting the values, we get:
Molarity = (217 g / 247.224 g/mol) / 0.0673 L
Calculating this expression, we find the molarity of the OsF3 solution.
Learn more about molarity on:
https://brainly.com/question/2817451
#SPJ1
What is this help ASAP
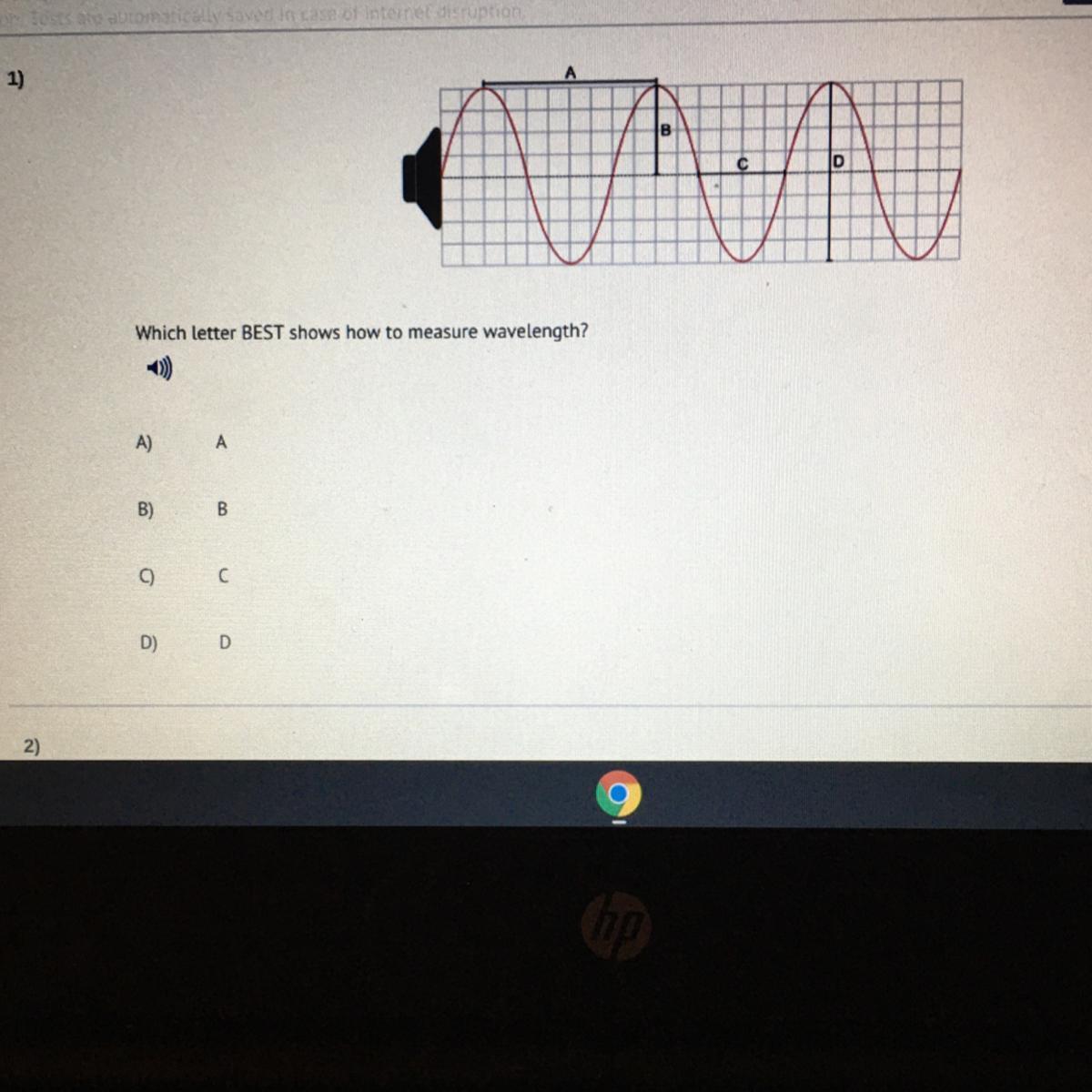
Answers
Answer:
A, im gonna say. Hopefully this helps!
Explanation:
Length is horizontal, and A is horizontal too.
What solution turns litmus paper red?
A. acidic
B. neutral
C. none
D. basic
Answers
Answer:
The answer is A please mark brainliest
Explanation:
which method of expressing concentration should the hydrologist use to describe the concentration of lead in the water?
Answers
Option A is the way of representing concentration that the hydrogeologist should employ to describe the density of lead in the water.
Ppm or ppb. The following details should be taken into account Ppm stands for parts per million. Ppb stands for parts per billion. • We know that one part in a million equals one part in a billion. • The same method should be employed for water concentration. 1 Water concentrations can also be expressed in fractions of a million (ppm) or parts for every billion (parts per billion) (ppb). This relationship can be used to convert between parts per million and parts per billion: 1 part in a thousand equals 1,000 parts in a billion.
Learn more about concentrations here:
https://brainly.com/question/10725862
#SPJ4
The complete question is:
Which method of expressing concentration should the hydrologist use to describe the concentration of lead in the water?
A) ppm or ppb
B) volume percent
C) mass-volume percent
D) mass percent
chemical reactions generally reach equilibrium because one of the reactants is used up.T/F
Answers
False. Chemical reactions reach equilibrium when the rates of the forward and reverse reactions become equal. This means that the amount of reactants and products present in the system remain constant over time.
While it is true that some reactions may appear to have reached equilibrium because one of the reactants is used up, this is not always the case. In some cases, the reaction may continue to occur, but at a slower rate, until equilibrium is reached. Additionally, some reactions may involve multiple reactants or products, making it more complex to determine if one reactant being used up is the sole reason for equilibrium being reached.
False. Chemical reactions generally reach equilibrium not because one of the reactants is used up, but because the rates of the forward and reverse reactions become equal. At equilibrium, the concentrations of reactants and products remain constant, but they do not necessarily become zero. The reaction continues to occur in both directions, but the net effect is zero, as the formation of products and consumption of reactants occur at the same rate. Equilibrium is a dynamic state that reflects the balance between the forward and reverse reactions.
To learn more about equilibrium visit;
https://brainly.com/question/30268932
#SPJ11
A mixture of four gases exerts a total pressure of 960
mm Hg. Gase A each exert 120 mm Hg. Gas B exerts
50 mm Hg. What pressure is exerted by gas C?
Answers
Omitted question:I think you mean Gas A and D each exert 120 mm Hg, if Gas D has a different value , just lug it into he equation as shown below and solve.
Answer:Gas C= 670mmHg
Explanation:
According to Dalton law of partial pressure
The total pressure of a mixture of gases is the sum of the pressures of each gas and can be illustrated as
Pressure total=P1+P2+… +Pn.
Total Pressure = Gas A + Gas B + Gas C + Gas D + Gas...
Now we have
Total Pressure = 120 mm Hg + 50mm Hg + Gas C + 120 mm Hg
960mmHg = 120 mm Hg + 50mm Hg + Gas C + 120 mm Hg
960mmHg =290mmHg +Gas C
Gas C =960mmHg -290mmHg
Gas C= 670mmHg
How does tension affect the speed of a wave in a rope
Answers
I don’t know
but maybe next time
Please help ASAP I will give Brainliest
How many grams of nitrogen gas are in a balloon with a volume of 35. 7 L at STP?
Answers
There are 44.62 grams of nitrogen gas in the balloon with a volume of 35.7 L at STP.
The volume of a balloon is 35.7 L at STP.
STP is the abbreviation for Standard Temperature and Pressure, which means a temperature of 273.15 K and a pressure of 101.325 kPa.
The number of grams of nitrogen gas in a balloon of this volume is requested.
A mole of any element has 6.02 × 1023 atoms, and the atomic mass of nitrogen is 14.01 grams.
One mole of a gas is equal to its molar volume, which is 22.4 L at STP. 1 mole of N2 gas = 28.02 g of N2 gas
STP's molar volume is 22.4 L, and the balloon's volume is 35.7 L.
That is, there are 35.7/22.4 moles of N2 gas in the balloon.= 1.59 moles of N2 gas
Since one mole of N2 gas weighs 28.02 g, 1.59 moles of N2 gas will weigh:
28.02 g/mol × 1.59 mol = 44.62 g
Therefore, there are 44.62 grams of nitrogen gas in the balloon with a volume of 35.7 L at STP.
For such more questions on nitrogen gas
https://brainly.com/question/31324537
#SPJ8
gravity and magnetism are examples of what?
Answers
Answer:
hyposthesis
Explanation:
f Vx=7.70 units and Vy=−6.00 units, dotermine (a) the magnitude and (b)direction of V
Answers
The direction of V is 39.29° (approx) below the negative x-axis.Hence, the direction of V is 39.29° (approx) below the negative x-axis.
Given that Vx=7.70 units and
Vy=−6.00 units.
We need to determine the (a) the magnitude and (b) direction of V.
Step 1:Let's apply Pythagoras theorem to find the magnitude of V.
It is given that, Vx=7.70 units and Vy=−6.00 units.
By Pythagoras theorem, we have,
V = √Vx2 + Vy2V
= √(7.70)2 + (−6.00)2V
= √(59.29) V = 7.70 units (approx)
Therefore, the magnitude of V is 7.70 units (approx).
Step 2:Let's determine the direction of V.Since Vx=7.70 units is positive and Vy=−6.00 units is negative.
Therefore, the vector V lies in the fourth quadrant.In the fourth quadrant, tanθ = Vy/Vxtanθ
= (-6.00) / (7.70)tanθ
= - 0.77922θ
= tan-1 (-0.77922)θ
= -39.29°
The direction of V is 39.29° (approx) below the negative x-axis.Hence, the direction of V is 39.29° (approx) below the negative x-axis.
To know more about magnitude visit:
https://brainly.com/question/31022175
#SPJ11
25 POINTS! How does bond energy determine whether a chemical reaction is exothermic or endothermic?
Answers
What is an atom?
A An atom is a round spherical object found inside most objects made of
matter
B. An atom is a round spherical object found inside objects made of matter
C. An atom is a basic building block of matter.
D. An atom is a subunit of longer extended structures.
Answers
Explanation:
what is an atom?
An atom is a basic building block of matter.
Everyone has a laughing place, The trouble is most people don't take the time to look for it.
true
or
false
Answers
Answer:
True
Explanation:
Answer:
True
Explanation:
Most people stick to what they know and don't look for a laughing place.
why are protons (h+) pumped across the inner mitochondrial membrane?
Answers
Protons (H⁺) are pumped across the inner mitochondrial membrane as part of the process called electron transport chain, which is an essential step in cellular respiration.
The electron transport chain is responsible for generating adenosine triphosphate (ATP), the main energy currency of cells.
During cellular respiration, electrons are transferred from high-energy molecules (such as glucose) through a series of electron carriers embedded in the inner mitochondrial membrane. As electrons pass through the electron transport chain, energy is released and used to pump protons (H⁺) from the mitochondrial matrix to the intermembrane space.
There are some several reasons;
Establishing an Electrochemical Gradient; The pumping of protons across the inner mitochondrial membrane creates an imbalance of protons, resulting in a higher concentration of protons in the intermembrane space compared to the matrix.
Generation of ATP; The electrochemical gradient created by the proton pumping is utilized by ATP synthase, an enzyme complex embedded in the inner mitochondrial membrane.
Coupling Electron Transport with Proton Pumping; The pumping of protons across the inner mitochondrial membrane is coupled with the flow of electrons through the electron transport chain.
To know more about mitochondrial membrane here
https://brainly.com/question/31808640
#SPJ4